The Accumulation and Biosynthesis of Anthocyanin in Black, White, and Yellow Waxy Corns (Zea mays L. sinensis kulesh) during Kernel Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Extraction, Qualitative and Quantitative Analysis of Phenolic and Anthocyanin
2.3. In Vitro and Cell Antioxidant Activity Analysis
2.4. Analysis of Gene Expression during the Anthocyanin Biosynthesis Pathway by a Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Statistical Analysis
3. Results
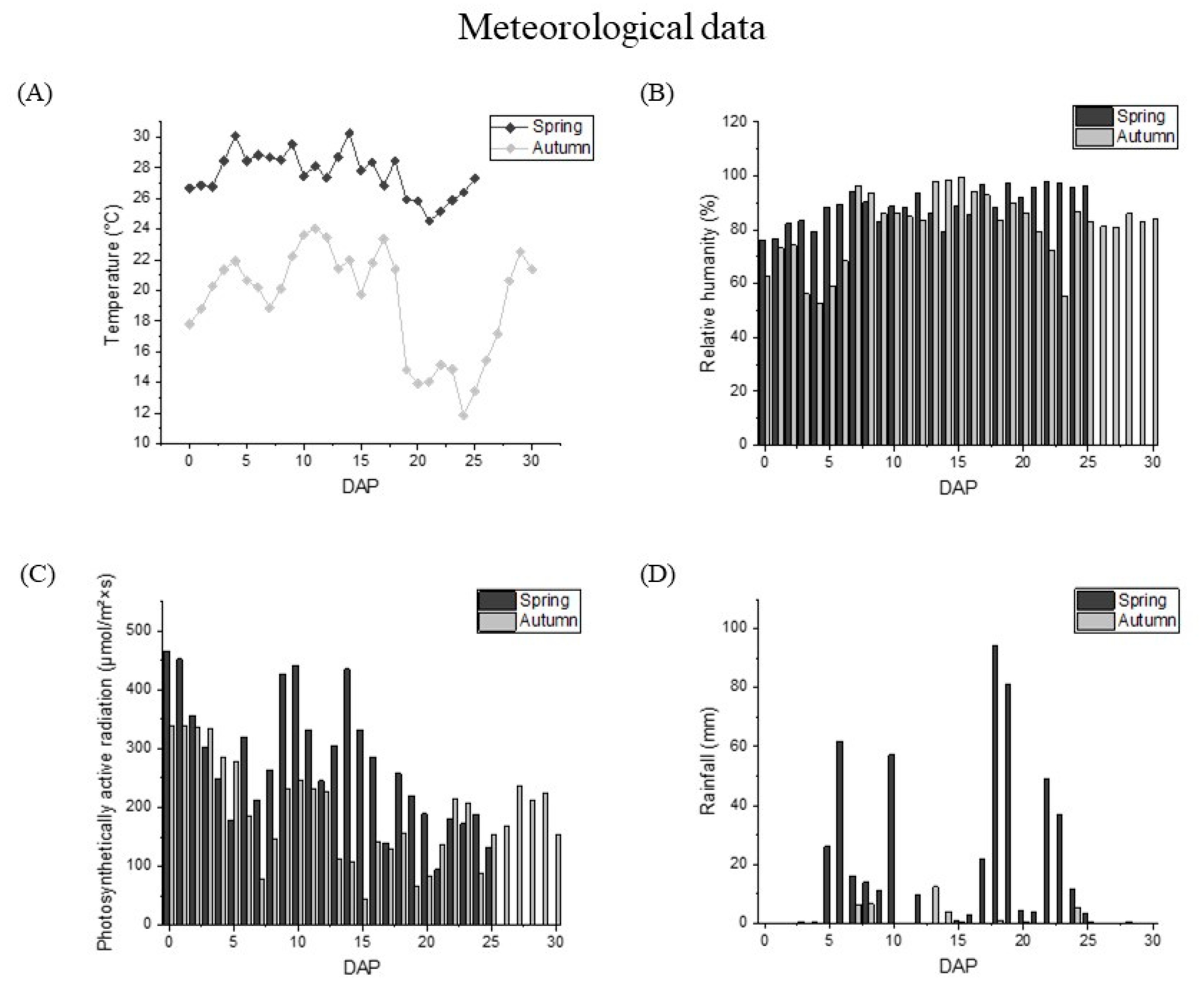
3.1. Weather Changes during the Maturation of Waxy Corn Kernels
3.2. Phenolic and Anthocyanin Profiles Tested by HPLC
3.3. Changes of Relative Gene Expression Levels during Kernel Maturation
3.4. In Vitro and Cell Antioxidant Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Inouchi, N.; Nishinari, K. Structural, thermal and viscoelastic characteristics of starches separated from normal, sugary and waxy maize. Food Hydrocoll. 2006, 20, 923–935. [Google Scholar] [CrossRef]
- Montilla, E.C.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple Corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, W.; Yang, H.; Dong, Q.; Ren, J.; Fan, H.; Zhang, X.; Zhou, Y. Comparative transcriptome analysis reveals differentially expressed genes related to the tissue-specific accumulation of anthocyanins in pericarp and aleurone layer for maize. Sci. Rep. 2019, 9, 2485. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Anthocyanin composition and changes during kernel development in purple-pericarp supersweet sweetcorn. Food Chem. 2020, 315, 126284. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Method 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Harakotr, B.; Suriharn, B.; Tangtuongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Food 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.; Tang, K.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2012, 60, 11050–11055. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A.; Hallmann, E. Effect of climate and roasting on polyphenols and tocopherols in the kernels and skin of six hazelnut cultivars (Corylus avellana L.). Agriculture 2020, 10, 36. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional differences of phenolic compounds between black currant (Ribes nigrum L.) cultivars and their response to latitude and weather conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Zahoor, R.; Yang, X.; Wang, Y.; Zhou, Z.; Meng, Y. Cool temperature caused by late planting affects seed vigor via altering kernel biomass and antioxidant metabolism in cotton (Gossypium hirsutum L.). Field Crops Res. 2019, 236, 145–154. [Google Scholar] [CrossRef]
- Sparks, T.H.; Huber, K.; Croxton, P.J. Plant development scores from fixed-date photographs: The influence of weather variables and recorder experience. Int. J. Biometeorol. 2006, 50, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, L. Response of spring cereals to a deficit of atmospheric precipitation in the particular stages of plant growth and development. Agric. Water Manag. 2008, 95, 171–178. [Google Scholar] [CrossRef]
- Rodrigues, F.R.; Bispo, D.A.A.S.; Brandão, H.N.; Soares, T.L.; de Almeida, W.A.B.; de Santana, J.R.F. The impact of medium composition and photosynthetically active radiation level on the initial in vitro growth and production of flavonoids of Vernonia condensata Baker. Biocatal. Agric. Biotechnol. 2019, 18, 101063. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Tolic, M.T.; Krbavcic, I.P.; Vujevic, P.; Milinovic, B.; Jurcevic, I.L.; Vahcic, N. Effects of weather conditions on phenolic content and antioxidant capacity in juice of chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, R.H. Health benefits of whole grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef]
- Siyuan, S.; Tong, L.; Liu, R. Corn phytochemicals and their health benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Das, A.; Sreerama, Y.N.; Singh, V. Diversity in phytochemical composition and antioxidant capacity of dent, flint, and specialty corns. Cereal Chem. 2014, 91, 639–645. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Li, X.; Gong, K.; Liu, K. Phenolics and related in vitro functional activities of different varieties of fresh waxy corn: A whole grain. BMC Chem. 2021, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Nishihara, M.; Nakatsuka, T.; Yamamura, S. Flavonoid components and flower color change in transgenic tobacco plants by suppression of chalcone isomerase gene. FEBS Lett. 2005, 579, 6074–6078. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yun, C.-S.; Matsuda, F.; Sasaki, T.; Saito, K.; Tozawa, Y. Expression of bacterial tyrosine ammonia-lyase creates a novel p-coumaric acid pathway in the biosynthesis of phenylpropanoids in Arabidopsis. Planta 2010, 232, 209–218. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 15. [Google Scholar]
- Lou, Q.; Liu, Y.L.; Qi, Y.Y.; Jiao, S.Z.; Tian, F.F.; Jiang, L.; Wang, Y.J. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef]
- Jin, Z.P.; Grotewold, E.; Qu, W.Q.; Fu, G.X.; Zhao, D.X. Cloning and characterization of a flavanone 3-hydroxylase gene from Saussurea medusa. DNA Seq. 2005, 16, 121–129. [Google Scholar] [CrossRef]
- Petit, P.; Granier, T.; d’Estaintot, B.L.; Manigand, C.; Bathany, K.; Schmitter, J.-M.; Lauvergeat, V.; Hamdi, S.; Gallois, B. Crystal structure of grape dihydroflavonol 4-reductase, a key enzyme in flavonoid biosynthesis. J. Mol. Biol. 2007, 368, 1345–1357. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Tao, J.; Han, C.X.; Ge, J.T. Flower color diversity revealed by differential expression of flavonoid biosynthetic genes and flavonoid accumulation in herbaceous peony (Paeonia lactiflora Pall.). Mol. Biol. Rep. 2012, 39, 11263–11275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Chen, Y.Q.; Yuan, M.; Xue, Z.Y.; Jin, Q.J.; Xu, Y.C. Flower color diversity revealed by differential expression of flavonoid biosynthetic genes in sacred lotus. J. Am. Soc. Hortic. Sci. 2016, 141, 573–582. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, G.; Yue, W.; Zhang, S.; Wu, J. Transcriptome profiling reveals differential gene expression in proanthocyanidin biosynthesis associated with red/green skin color mutant of pear (Pyrus communis L.). Front Plant Sci. 2015, 6, 795. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Suzuki, T.; Harada, K.; Kobayashi, Y.; Dohra, H.; Ohno, H. Floral organ- and temperature-dependent regulation of anthocyanin biosynthesis in Cymbidium hybrid flowers. Plant Sci. 2019, 287, 110173. [Google Scholar] [CrossRef]


| (mg/100 g DW) | Spring | Autumn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAP | 10 | 15 | 20 | 25 | 15 | 20 | 25 | 30 | ||
| L1 | Cya-3-O-Glu | F | ND | ND | 0.3 ± 0.0 a | 0.7 ± 0.1 b | ND | 0.9 ± 0.1 a | 2.4 ± 0.1 c | 1.8 ± 0.3 b |
| Pal-3-O-Glu | F | ND | ND | 0.4 ± 0.1 c | 1.3 ± 0.1 d | ND | 0.6 ± 0.0 a | 0.9 ± 0.1 b | 1.0 ± 0.1 c | |
| Epicatechin | F | 74.8 ± 4.2 b | 72.6 ± 0.9 b | 41.5 ± 0.5 a | 38.5 ± 0.4 a | 247.0 ± 0.2 d | 140.1 ± 1.9 c | 91.5 ± 0.3 b | 87.0 ± 3.6 a | |
| Lutin | B | 30.5 ± 4.1 b | 14.4 ± 1.8 a | 13.5 ± 3.2 a | 15.1 ± 2.1 a | 92.1 ± 1.3 c | 37.8 ± 2.3 a | 36.2 ± 1.8 a | 50.8 ± 3.1 b | |
| Gallic acid | F | 41.2 ± 0.6 d | 21.0 ± 0.2 c | 15.7 ± 1.2 b | 12.4 ± 0.4 a | 43.1 ± 0.9 c | 20.3 ± 0.6 b | 18.8 ± 1.6 b | 15.3 ± 2.5 a | |
| B | 2.8 ± 0.5 b | 1.1 ± 0.1 a | 1.0 ± 0.2 a | 1.1 ± 0.0 a | 18.6 ± 0.7 c | 10.0 ± 0.3 a | 13.6 ± 0.3 b | 27.7 ± 1.6 d | ||
| T | 44.0 ± 0.2 d | 22.1 ± 0.2 c | 16.7 ± 1.1 b | 13.5 ± 0.4 a | 61.6 ± 1.6 c | 30.3 ± 0.8 a | 32.4 ± 1.5 a | 43.0 ± 1.4 b | ||
| p-Coumaric acid | F | 7.4 ± 0.4 c | 2.5 ± 0.1 b | 2.2 ± 0.1 ab | 1.9 ± 0.1 a | 7.3 ± 0.2 d | 2.4 ± 0.3 c | 1.1 ± 0.0 a | 1.6 ± 0.1 b | |
| B | 26.1 ± 3.8 c | 6.8 ± 0.2 a | 14.0 ± 2.4 b | 4.0 ± 0.2 a | 92.2 ± 3.3 c | 46.8 ± 7.5 b | 8.7 ± 0.5 a | 9.2 ± 1.0 a | ||
| T | 33.5 ± 3.4 c | 9.4 ± 0.4 a | 16.2 ± 2.4 b | 5.9 ± 0.2 a | 99.5 ± 3.3 c | 49.2 ± 7.4 b | 9.8 ± 0.5 a | 10.8 ± 1.0 a | ||
| Ferulic acid | F | 5.7 ± 0.2 c | 2.7 ± 0.1 b | 2.8 ± 0.1 b | 2.4 ± 0.1 a | 3.8 ± 0.1 c | 1.9 ± 0.1 b | 1.6 ± 0.1 a | 1.6 ± 0.1 a | |
| B | 249.4 ± 30.0 b | 158.6 ± 14.4 a | 259.9 ± 43.4 b | 234.9 ± 25.9 b | 1554 ± 35 b | 680.0 ± 55.7 a | 713.7 ± 64.2 a | 815.3 ± 108 a | ||
| T | 255.1 ± 30.0 b | 161.4 ± 14.5 a | 262.7 ± 43.4 b | 237.3 ± 26.0 b | 1558 ± 35 b | 681.9 ± 55.7 a | 715.3 ± 64.3 a | 817.0 ± 108 a | ||
| L2 | Epicatechin | F | 78.2 ± 1.1 d | 40.4 ± 0.6 c | 22.4 ± 1.6 b | 11.8 ± 0.5 a | 144.5 ± 8.9 b | 204.2 ± 4.5 c | 142.8 ± 3.2 b | 101.8 ± 9.5 a |
| Lutin | B | 23.8 ± 0.8 c | 22.3 ± 0.4 b | 19.1 ± 1.0 a | 20.0 ± 0.2 a | 47.9 ± 1.0 d | 25.5 ± 0.6 c | 22.3 ± 1.5 b | 14.9 ± 2.3 a | |
| Gallic acid | F | 48.1 ± 7.1 b | 20.3 ± 3.2 a | 19.9 ± 1.3 a | 14.65 ± 0.7 a | 27.2 ± 2.7 c | 22.6 ± 2.4 b | 14.9 ± 1.4 a | 15.4 ± 0.0 a | |
| B | 11.1 ± 0.7 b | 1.5 ± 0.1 a | 1.5 ± 0.2 a | 1.45 ± 0.1 a | 4.2 ± 0.3 b | 1.8 ± 0.2 a | 1.7 ± 0.0 a | 1.5 ± 0.2 a | ||
| T | 59.3 ± 7.8 b | 21.7 ± 3.1 a | 21.3 ± 1.0 a | 16.10 ± 0.7 a | 31.4 ± 1.8 c | 24.4 ± 2.3 b | 16.6 ± 1.4 a | 16.9 ± 0.2 a | ||
| p-Coumaric acid | F | 7.2 ± 0.2 d | 3.0 ± 0.1 c | 1.7 ± 0.0 b | 1.4 ± 0.0 a | 16.2 ± 2.6 c | 18.1 ± 0.2 d | 2.7 ± 0.0 b | 1.2 ± 0.1 a | |
| B | 20.8 ± 2.8 b | 27.3 ± 1.3 c | 6.2 ± 0.6 a | 5.3 ± 0.3 a | 61.8 ± 2.7 d | 14.3 ± 0.3 c | 12.0 ± 1.0 b | 4.3 ± 0.5 a | ||
| T | 28.0 ± 1.8 b | 30.3 ± 1.4 c | 7.9 ± 0.6 a | 6.7 ± 0.3 a | 78.1 ± 3.3 d | 32.3 ± 0.4 c | 14.8 ± 1.0 b | 5.4 ± 0.5 a | ||
| Ferulic acid | F | 7.0 ± 0.4 c | 3.3 ± 0.1 b | 2.2 ± 0.3 a | 2.4 ± 0.1 a | 5.6 ± 0.9 c | 2.0 ± 0.1 b | 1.5 ± 0.1 ab | 0.9 ± 0.1 a | |
| B | 192.8 ± 33.5 a | 176.9 ± 19.4 a | 184.2 ± 27.5 a | 190.8 ± 21.3 a | 606.9 ± 36.0 d | 405.0 ± 8.2 c | 344.3 ± 39.9 b | 198.3 ± 30.4 a | ||
| T | 199.9 ± 33.8 a | 180.2 ± 19.6 a | 186.4 ± 27.7 a | 193.2 ± 15.0 a | 612.4 ± 25.8 d | 407.0 ± 8.3 c | 345.8 ± 39.9 b | 199.2 ± 21.6 a | ||
| L3 | Epicatechin | F | 12.6 ± 1.3 c | 8.5 ± 0.1 b | 5.5 ± 0.4 a | 5.0 ± 0.7 a | 17.9 ± 0.5 c | 19.1 ± 1.1 c | 15.0 ± 0.8 b | 8.0 ± 0.4 a |
| Lutin | B | 37.0 ± 5.7 c | 20.9 ± 3.1 b | 11.4 ± 2.2 a | 11.9 ± 1.2 a | 25.8 ± 1.0 c | 14.0 ± 0.4 b | 10.2 ± 0.6 a | 10.5 ± 1.1 a | |
| Gallic acid | F | 37.6 ± 1.2 d | 18.3 ± 0.1 b | 13.4 ± 2.0 a | 21.3 ± 0.6 c | 44.7 ± 0.9 c | 30.9 ± 1.8 b | 14.5 ± 0.5 a | 17.0 ± 2.4 a | |
| B | 2.2 ± 0.0 c | 1.5 ± 0.1 a | 1.3 ± 0.1 a | 1.51 ± 0.1 ab | 1.6 ± 0.1 b | 0.6 ± 0.0 a | 0.5 ± 0.1 a | 0.5 ± 0.0 a | ||
| T | 39.9 ± 0.8 d | 19.7 ± 0.1 b | 14.7 ± 2.1 a | 22.79 ± 0.7 c | 46.2 ± 4.2 c | 31.5 ± 1.8 b | 15.0 ± 0.5 a | 17.6 ± 1.7 a | ||
| p-Coumaric acid | F | 7.9 ± 1.3 b | 2.3 ± 0.2 a | 1.8 ± 0.0 a | 1.8 ± 0.0 a | 11.3 ± 0.6 c | 3.0 ± 0.1 b | 1.5 ± 0.1 a | 1.3 ± 0.0 a | |
| B | 17.3 ± 1.5 d | 11.1 ± 0.5 c | 5.4 ± 0.9 a | 7.3 ± 0.3 b | 18.8 ± 0.7 c | 14.1 ± 0.8 b | 5.2 ± 1.0 a | 4.1 ± 0.4 a | ||
| T | 25.2 ± 2.8 c | 13.4 ± 0.6 b | 7.1 ± 0.7 a | 9.1 ± 0.3 a | 30.1 ± 1.2 c | 17.1 ± 0.9 b | 6.7 ± 0.9 a | 5.4 ± 0.4 a | ||
| Ferulic acid | F | 9.1 ± 0.4 c | 3.8 ± 0.0 b | 2.6 ± 0.2 a | 2.6 ± 0.3 a | ND | 3.2 ± 0.3 b | 1.9 ± 0.1 a | 1.7 ± 0.4 a | |
| B | 306.7 ± 38.9 a | 286.4 ± 20.8 a | 263.8 ± 23.8 a | 382.8 ± 8.9 b | 162.5 ± 14.0 b | 108.7 ± 8.9 a | 78.7 ± 10.3 a | 77.3 ± 21.9 a | ||
| T | 315.8 ± 39.0 b | 290.1 ± 20.8 ab | 266.4 ± 24.0 a | 385.4 ± 8.6 c | 162.5 ± 14.0 b | 111.9 ± 9.2 a | 80.63 ± 7.3 a | 78.97 ± 22.3 a | ||
| Anthocyanin (mg/kg DW) | Spring | Autumn | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 DAP | 15 DAP | 20 DAP | 25 DAP | 15 DAP | 20 DAP | 25 DAP | 30 DAP | |
| Cyanidin | ND | 14.7 ± 0.3 a | 23.8 ± 0.9 b | 74.5 ± 0.3 c | ND | 50.4 ± 0.2 a | 111.1 ± 0.9 c | 76.4 ± 0.3 b |
| Pelargonidin | ND | 17.3 ± 0.3 a | 25.8 ± 0.8 b | 97.6 ± 1.1 c | ND | 13.5 ± 0.5 a | 21.2 ± 0.8 c | 15.4 ± 0.3 b |
| Peonidin | ND | 3.7 ± 0.0 b | 3.6 ± 0.0 b | 3.4 ± 0.1 a | ND | 4.2 ± 0.2 b | 4.2 ± 0.0 b | 3.0 ± 0.1 a |
| Total | ND | 35.7 ± 0.6 a | 53.2 ± 0.7 b | 175.6 ± 1.2 c | ND | 68.1 ± 0.8 a | 136.5 ± 0.2 c | 94.8 ± 0.1 b |
| Spring | Autumn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAP | 10 | 15 | 20 | 25 | 15 | 20 | 25 | 30 | ||
| ORAC (μmol TE/g DW) | L1 | F | 38.8 ± 2.3 c | 29.9 ± 2.1 ab | 26.0 ± 1.7 a | 33.3 ± 4.3 b | 38.1 ± 1.5 c | 24.8 ± 0.5 b | 19.3 ± 0.1 a | 23.6 ± 0.8 b |
| B | 102.6 ± 8.1 c | 52.9 ± 0.6 a | 92.4 ± 3.0 c | 77.9 ± 1.6 b | 97.9 ± 4.7 d | 35.1 ± 0.6 a | 46.8 ± 3.8 b | 64.1 ± 2.9 c | ||
| T | 141.4 ± 9.4 c | 82.8 ± 11.9 a | 118.4 ± 4.4 b | 111.2 ± 3.6 b | 136.0 ± 5.5 c | 59.9 ± 0.1 a | 66.1 ± 4.2 a | 87.7 ± 3.6 b | ||
| L2 | F | 66.7 ± 0.3 b | 40.4 ± 13.4 a | 26.7 ± 0.3 a | 29.1 ± 5.9 a | 40.4 ± 0.2 d | 23.0 ± 2.5 c | 16.4 ± 2.1 c | 13.8 ± 0.8 c | |
| B | 92.6 ± 7.0 b | 87.0 ± 6.5 ab | 85.7 ± 0.9 ab | 77.3 ± 5.8 a | 193.6 ± 6.1 e | 117.2 ± 1.2 a | 86.0 ± 9.1 b | 56.1 ± 3.6 d | ||
| T | 159.2 ± 7.3 c | 127.3 ± 19.2 b | 112.4 ± 1.2 ab | 106.4 ± 0.1 a | 234.0 ± 5.9 d | 140.2 ± 2.6 a | 102.3 ± 9.3 a | 70.0 ± 4.3 c | ||
| L3 | F | 39.1 ± 2.0 c | 19.0 ± 3.1 ab | 17.7 ± 1.0 a | 22.1 ± 1.6 b | 44.3 ± 3.8 b | 20.7 ± 2.7 a | 21.2 ± 1.4 a | 16.4 ± 1.4 a | |
| B | 122.8 ± 8.6 c | 96.6 ± 10.4 ab | 94.8 ± 4.6 a | 115.2 ± 13.8 bc | 187.1 ± 11.0 c | 114.4 ± 8.5 b | 76.8 ± 1.1 a | 94.9 ± 14.0 a | ||
| T | 161.9 ± 10.5 c | 115.6 ± 12.1 a | 112.5 ± 3.7 a | 137.3 ± 15.0 b | 231.4 ± 10.6 c | 135.0 ± 9.2 b | 97.9 ± 2.5 a | 111.3 ± 15.2 a |
| DAP | 15 | 20 | 25 | 30 | ||
|---|---|---|---|---|---|---|
| L1 | CAA value (μmol QE/100 g DW, no PBS wash) | F | 17.3 ± 1.1 b | 14.9 ± 0.8 a | 19.6 ± 0.7 c | 15.5 ± 1.3 ab |
| B | 64.7 ± 2.9 c | 32.7 ± 0.8 b | 28.6 ± 1.8 a | 25.0 ± 1.8 a | ||
| T | 82.0 ± 2.0 c | 47.6 ± 1.3 b | 48.3 ± 2.2 b | 40.5 ± 0.7 a | ||
| CAA value (μmol QE/100 g DW, PBS wash) | F | 3.4 ± 1.1 a | 9.7 ± 0.7 b | 12.7 ± 0.5 c | 4.4 ± 0.4 a | |
| B | 31.2 ± 1.1 c | 6.4 ± 0.7 a | 6.3 ± 0.2 a | 8.3 ± 0.3 b | ||
| T | 34.6 ± 1.1 d | 16.0 ± 1.3 b | 19.0 ± 0.7 c | 12.7 ± 0.6 a | ||
| L2 | CAA value (μmol QE/100 g DW, no PBS wash) | F | 49.9 ± 2.8 c | 12.2 ± 0.2 b | 7.8 ± 0.8 a | 7.2 ± 0.4 a |
| B | 65.8 ± 2.7 d | 56.8 ± 1.7 c | 21.2 ± 2.1 b | 9.5 ± 0.2 a | ||
| T | 115.7 ± 2.3 d | 69.0 ± 1.7 c | 29.0 ± 2.1 b | 16.7 ± 0.6 a | ||
| CAA value (μmol QE/100 g DW, PBS wash) | F | 22.1 ± 0.9 c | 7.1 ± 0.5 b | 4.1 ± 0.8 a | 3.8 ± 0.2 a | |
| B | 21.8 ± 1.0 d | 14.9 ± 2.5 c | 5.4 ± 1.1 b | 2.1 ± 0.4 a | ||
| T | 43.9 ± 1.8 d | 22.0 ± 2.6 c | 9.6 ± 1.3 b | 5.9 ± 0.3 a | ||
| L3 | CAA value (μmol QE/100 g DW, no PBS wash) | F | 79.1 ± 2.4 b | 32.3 ± 1.9 a | 32. 7 ± 0.2 a | 33.8 ± 1.1 a |
| B | 71.9 ± 3.5 c | 29.9 ± 1.8 b | 19.9 ± 1.9 a | 17.6 ± 1.2 a | ||
| T | 151.0 ± 4.3 c | 62.1 ± 1.0 b | 52.6 ± 2.0 a | 51.4 ± 0.6 a | ||
| CAA value (μmol QE/100 g DW, PBS wash) | F | 58.6 ± 0.8 d | 15.2 ± 0.0 c | 10.2 ± 0.9 a | 13.9 ± 0.4 b | |
| B | 30.8 ± 1.5 c | 12.3 ± 1.8 b | 5.0 ± 0.6 a | 5.4 ± 0.8 a | ||
| T | 89.4 ± 2.2 d | 27.5 ± 1.8 c | 15.2 ± 1.4 a | 19.3 ± 1.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Liu, J.; Shan, Q.; Bai, S.; Li, W.; Wen, T.; Guo, X.; Hu, J. The Accumulation and Biosynthesis of Anthocyanin in Black, White, and Yellow Waxy Corns (Zea mays L. sinensis kulesh) during Kernel Maturation. Foods 2023, 12, 1486. https://doi.org/10.3390/foods12071486
Hu X, Liu J, Shan Q, Bai S, Li W, Wen T, Guo X, Hu J. The Accumulation and Biosynthesis of Anthocyanin in Black, White, and Yellow Waxy Corns (Zea mays L. sinensis kulesh) during Kernel Maturation. Foods. 2023; 12(7):1486. https://doi.org/10.3390/foods12071486
Chicago/Turabian StyleHu, Xiaodan, Jianhua Liu, Qiji Shan, Song Bai, Wu Li, Tianxiang Wen, Xinbo Guo, and Jianguang Hu. 2023. "The Accumulation and Biosynthesis of Anthocyanin in Black, White, and Yellow Waxy Corns (Zea mays L. sinensis kulesh) during Kernel Maturation" Foods 12, no. 7: 1486. https://doi.org/10.3390/foods12071486
APA StyleHu, X., Liu, J., Shan, Q., Bai, S., Li, W., Wen, T., Guo, X., & Hu, J. (2023). The Accumulation and Biosynthesis of Anthocyanin in Black, White, and Yellow Waxy Corns (Zea mays L. sinensis kulesh) during Kernel Maturation. Foods, 12(7), 1486. https://doi.org/10.3390/foods12071486







