Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Sample Collection
2.3. Intramuscular Fat Content and Fatty Acid Composition Analysis
2.4. Nutritional Indexes
| PUFA: SFA = PUFAs/SFAs |
| DHA + EPA = (C22:6n-3 %) + (% C20:5n-3) |
| UI = (% monoenoics) × 1 + (% dienoics) × 2 + (% trienoics) × 3 + (% tetraenoics) × 4 + (% pentaenoics) × 5 + (% hexaenoics) × 6 |
| PI = (0.025 × percentage of monoenoic acid) + (1 × percentage of dienoic acid) + (2 × percentage of trienoic acid) + (4 × percentage of tetraenoic acid) + (6 × percentage of pentaenoic acid) + (8 × percentage of hexaenoic acid). |
| NVI = (C18:0 + C18:1n9)/(C16:0) |
| IA = (4 × C14:0 + C16:0)/(Σ MUFA + Σ PUFA) |
| IT = (C14:0 + C16:0 + C18:0)/[(0.5 × MUFA) + (0.5 × Σn-6 PUFA) + (3 × Σn-3 PUFA) + (Σn-3 PUFA/Σn-6 PUFA)] |
| HH ratio = (C18:1 + Σ PUFA)/(C14:0 + C16:0) |
| HPI = (Σ UFA)/(4 × C14:0 + C16:0) |
2.5. qRT-PCR
2.6. Statistical Analysis
3. Results
3.1. Intramuscular Fat
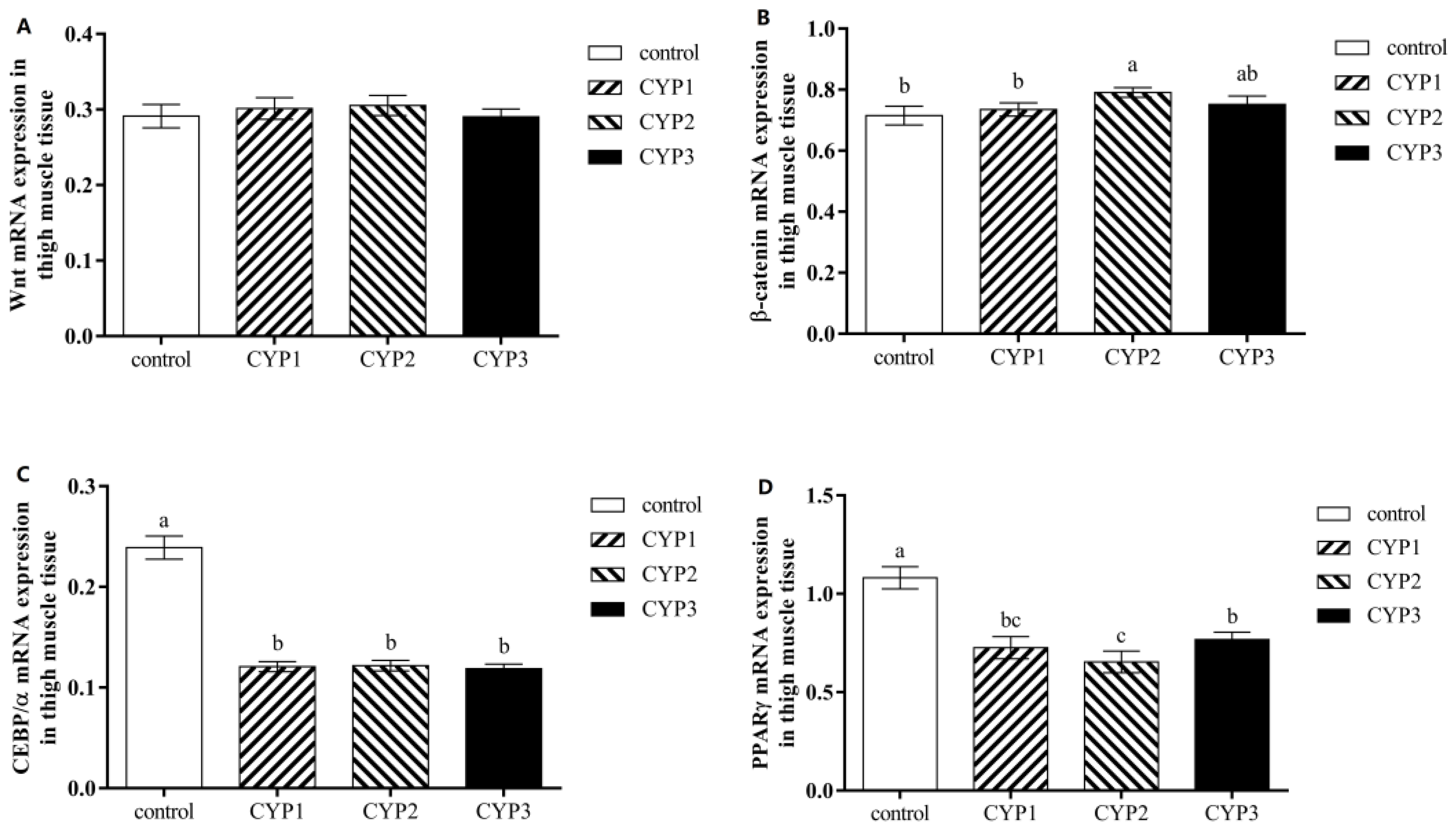
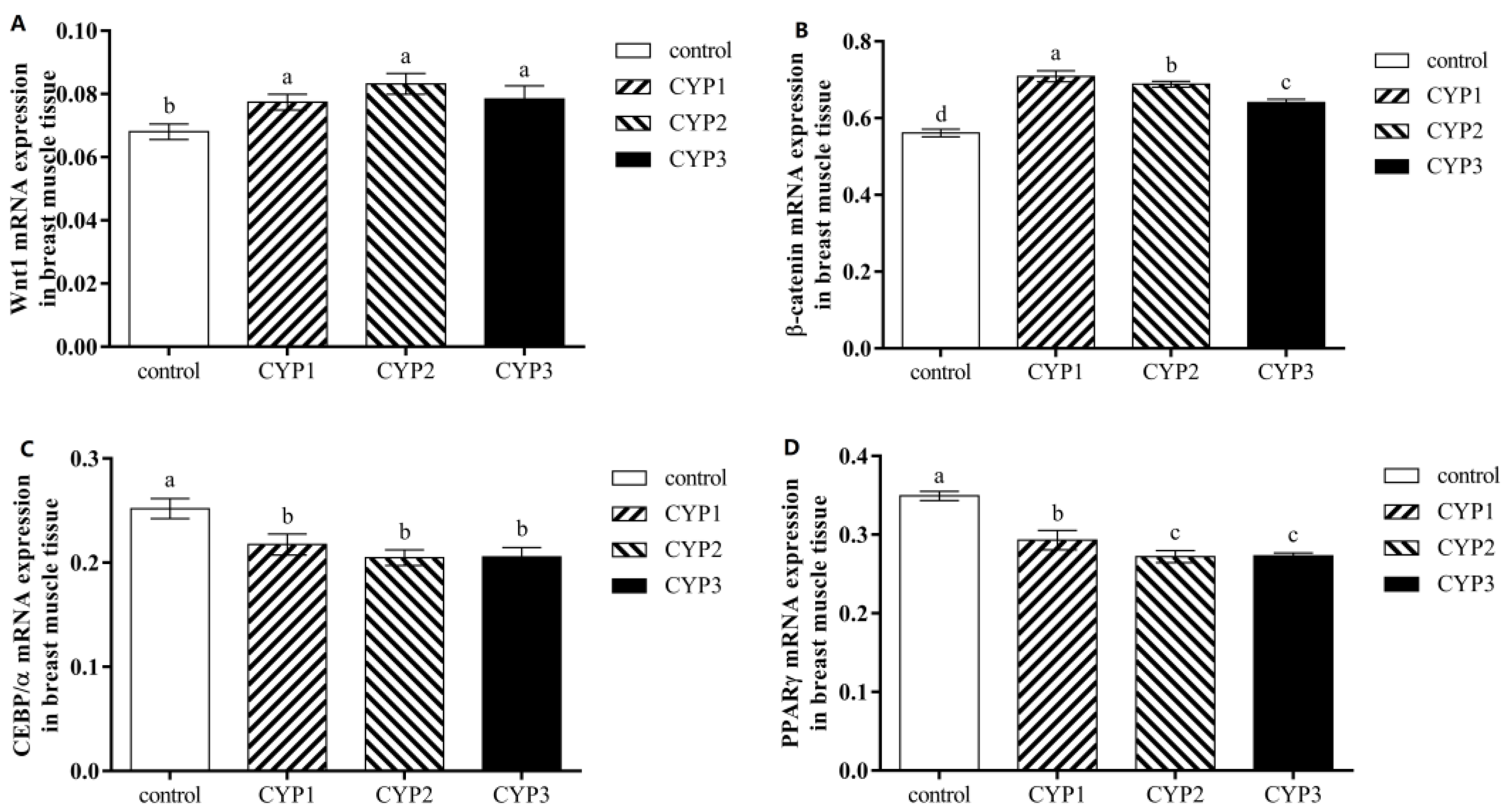
3.2. Intramuscular Fat Related Gene Expression
3.3. Fatty Acid Composition
3.4. Nutritional Indicators of Fatty Acid Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, S.; Kobayashi, M.; Murai, A.; Tsudzuki, M.; Ishikawa, A. Characterization of Growth, Fat Deposition, and Lipid Metabolism-Related Gene Expression in Lean and Obese Meat-Type Chickens. J. Poult. Sci. 2019, 56, 101–111. [Google Scholar] [CrossRef] [PubMed]
- James, W.P.T. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- World Health Organization. Controlling the Global Obesity Epidemic; World Health Organization: Geneva, Switzerland, 2015; Volume 7.
- Fernandez, X.; Monin, G.; Talmant, A. Influence of intramuscular fat content on the quality of pig meat—2. Consumer acceptability of m. longissimus lumborum. Meat Sci. 1999, 53, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.J.; Cui, H.X.; Liu, R.R.; Zhao, G.P.; Wen, J. Transcriptional insights into key genes and pathways controlling muscle lipid metabolism in broiler chickens. BMC Genom. 2019, 20, 863. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, J.; Yin, H.; Duan, C.; Wang, B.; Li, W. Effects of dietary nicotinic acid supplementation on meat quality, carcass characteristics, lipid metabolism, and tibia parameters of Wulong geese. Poult. Sci. 2021, 100, 101430. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Feng, X.; Wang, Z.; Xia, Z. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult. Sci. 2018, 97, 3218–3229. [Google Scholar] [CrossRef]
- Miao, Z.; Guo, L.; Liu, Y.; Zhao, W.; Zhang, J. Effects of dietary supplementation of chitosan on carcass composition and meat quality in growing huoyan geese. Poult. Sci. 2020, 99, 3079–3085. [Google Scholar] [CrossRef]
- Liu, W.C.; Guo, Y.; Zhao, Z.H.; Jha, R.; Balasubramanian, B. Algae-derived polysaccharides promote growth performance by improving anti-oxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020, 7, 601336. [Google Scholar] [CrossRef]
- Price, E.J.; Bhattacharjee, R.; Montes, A.L.; Fraser, P.D. Metabolite profiling of yam (Dioscorea spp.) accessions for use in crop improvement programmes. Metabolomics 2017, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Baah, F.D.; Maziya-Dixon, B.; Asiedu, R.; Oduro, I.; Ellis, W.O. Nutritional and biochemical composition of D. alata (Dioscorea spp.) tubers. J. Food Agr. Environ 2009, 7, 373–378. [Google Scholar]
- Mohan, V.R.; Kalidass, C. Nutritional and antinutritional evaluation of some unconventional wild edible plants. Trop. Subtrop. Agroecosyst. 2010, 12, 495–506. [Google Scholar]
- Kumar, S.; Das, G.; Shin, H.S.; Patra, J.K. Dioscorea spp. (A Wild Edible Tuber): A Study on Its Ethnopharmacological Potential and Traditional Use by the Local People of Similipal Biosphere Reserve. India. Front. Pharmacol. 2017, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Zhang, W.J.; Xiang, C.; Wen-xia, G.E. Effect of supplemental yam on dietary nutrient metabolism and blood physiological-biochemical parameters in broilers. J. Shihezi Univ. 2006, 24, 105–107. [Google Scholar]
- Kusano, Y.; Tsujihara, N.; Masui, H.; Kozai, H.; Takeuchi, W. Consumption of Japanese Yam Improves Lipid Metabolism in High-Cholesterol Diet-Fed Rats. J. Nutr. Sci. Vitaminol. 2016, 62, 350–360. [Google Scholar] [CrossRef]
- McKoy, M.L.; Grant, K.; Asemota, H.; Simon, O.; Omoruyi, F. Renal and Hepatic Function in Hypercholesterolemic Rats Fed Jamaican Bitter Yam (Dioscorea polygonoides). J. Diet. Suppl. 2015, 12, 173–183. [Google Scholar] [CrossRef]
- Meng, X.; Hu, W.; Wu, S.; Zhu, Z.; Lu, R.; Yang, G.; Qin, C.; Yang, L.; Nie, G. Chinese yam peel enhances the immunity of the common carp (Cyprinus carpio L.) by improving the gut defence barrier and modulating the intestinal microflora. Fish Shellfish Immunol. 2019, 95, 528–537. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Wen, Y.; Yao, Y.; Zhu, J.; Liu, X.; Bell, A.; Tikkanen-Kaukanen, C. Emulsification properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 221, 919–925. [Google Scholar] [CrossRef]
- Nishimura, N.; Tanabe, H.; Yamamoto, T.; Fukushima, M. Raw Chinese Yam (Dioscorea opposita) Promotes cecalfermentation and Reduces Plasma Non-HDL Cholesterol Concentration in Rats. J. Nutr. Sci. Vitaminol. 2011, 57, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Shujun, W.; Jinglin, Y.; Hongyan, L.; Weiping, C. Characterisation and preliminary lipid-lowering evaluation of starch from Chinese yam. Food Chem. 2008, 108, 176–181. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Xia, Y.; Wang, H.; Wu, S.; Huang, L.; Liu, C.; Xiao, P. Characterizations of starches isolated from five different Dioscorea, L. species. Food Hydrocoll. 2012, 29, 35–41. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Mao, X.; Huang, H.; Wang, T.; Qu, Z.; Miao, J.; Gao, W. Effects of drying processes on starch-related physicochemical properties, bioactive components and antioxidant properties of yam flours. Food Chem. 2017, 224, 224–232. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Jiao, J.; Su, J.; Sun, W.T.; Guo, Y.; Ma, S.X.; Zhang, T.; Meng, D.X. Effect of nano yam polysaccharide on the blood glucose and blood lipid in rats. Pak. J. Pharm. Sci. 2020, 33, 481–487. [Google Scholar]
- Zhao, W.; Cao, M.; Jin, Y.; Zhang, J.; Miao, Z. Effect of Chinese yam polysaccharide on immune performance and intestinal morphology of weaned piglets. Feed Sci. China 2021, 667, 37–41. (In Chinese) [Google Scholar]
- Deng, J.; Zhang, J.; Chang, Y.; Wang, S.; Shi, M.; Miao, G. Effects of Chinese yam polysaccharides on the immune function and serum biochemical indexes of broilers. Front. Vet. Sci. 2022, 9, 1013888. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, J.; Jin, Y.; Deng, J.; Shi, M.; Miao, Z. Effects of Dietary Supplementation of Chinese Yam Polysaccharide on Carcass Composition, Meat Quality, and Antioxidant Capacity in Broilers. Animals 2023, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; The National Academy Press: Washington, DC, USA, 1994.
- Cui, H.X.; Zheng, M.Q.; Liu, R.R.; Zhao, G.P.; Chen, J.L.; Wen, J. Liver dominant expression of fatty acid synthase (FAS) gene in two chicken breeds during intramuscular-fat development. Mol. Biol. Rep. 2012, 39, 3479–3484. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Logue, J.A.; De Vries, A.L.; Fodor, E.; Cossins, A.R. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J. Exp. Biol. 2000, 203, 2105–2115. [Google Scholar] [CrossRef]
- Witting, L.A.; Horwitt, M.K. Effect of degree of fatty acid unsaturation in tocopherol deficiency-induced creatinuria. J. Nutr. 1964, 82, 19. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Kong, Y.; Li, F.; Yue, X. Effects of intramuscular fat on meat quality and its regulation mechanism in Tan sheep. Front. Nutr. 2022, 9, 908355. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, Q.; Wang, Z.; Pan, Y. Association study between gene polymorphisms in PPAR signaling pathway and porcine meat quality traits. Mamm. Genome 2013, 24, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Tunim, S.; Phasuk, Y.; Aggrey, S.E.; Duangjinda, M. Increasing Fat Deposition Via Upregulates the Transcription of Peroxisome Proliferator-Activated Receptor Gamma in Native Crossbred Chickens. Animals 2021, 11, 90. [Google Scholar] [CrossRef]
- Darlington, G.J.; Rossi, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Y.; Lin, S.; Wang, Y.; Zhu, J.; Lin, Y. KLF4 Inhibits the Differentiation of Goat Intramuscular Preadipocytes through Targeting C/EBPβ Directly. Front. Genet. 2021, 12, 663759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Hou, L.; Wu, W.; Zhao, S.; Xiong, Y. Fibroblast Growth Factor 21 Suppresses Adipogenesis in Pig Intramuscular Fat Cells. Int. J. Mol. Sci. 2015, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Moldes, M.; Zuo, Y.; Morrison, R.F.; Silva, D.; Park, B.H.; Liu, J.; Farmer, S.R. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem. J. 2003, 376, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Qimuge, N.; Ma, M.; Wang, Y.; Tang, G.; Zhang, Q.; Sun, Y.; Chen, X.; Yu, T.; Dong, W.; et al. MicroRNA-664-5p promotes myoblast proliferation and inhibits myoblast differentiation by targeting serum response factor and Wnt1. J. Biol. Chem. 2018, 293, 19177–19190. [Google Scholar] [CrossRef] [PubMed]
- Torró-Montell, L.; Cortés-Castell, E.; Sirvent-Segura, E.; Veciana-Galindo, C.; Gil-Guillén, V.; Rizo-Baeza, M. Influence of Olive Extracts on the Expression of Genes Involved in Lipid Metabolism in Medaka Fish. Molecules 2019, 24, 3068. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lee, J.I.; Ryu, Y.B.; Choi, Y.J.; Tang, Y.; Oh, M.; Moon, S.H.; Lee, B.; Kim, E.K. Comparative Analysis of Metabolite Profiling of Momordica charantia Leaf and the Anti-Obesity Effect through Regulating Lipid Metabolism. Int. J. Environ. Res. Public Health 2021, 18, 5584. [Google Scholar] [CrossRef]
- Xu, D.; Li, J.; Lin, L.; Ma, H.; Xie, Y. Effects of Phytosterols on performance, immune indices and Wnt1 gene expression in different tissues of pigs. Feed Res. 2021, 17, 14–17. [Google Scholar]
- WEBB, E.C.; O’NEILL, H.A. The animal fat paradox and meat quality. Meat Sci. 2008, 80, 28–36. [Google Scholar] [CrossRef]
- Reiser, R. Fatty acid changes in egg yolk of hens on a fat-free and a cottonseed oil ration. J. Nutr. 1949, 40, 429–440. [Google Scholar] [CrossRef]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, Cd011094. [Google Scholar] [PubMed]
- de Roos, B.; Mavrommatis, Y.; Brouwer, I.A. Long-chain n-3 polyunsaturated fatty acids: New insights into mechanisms relating to inflammation and coronary heart disease. Br. J. Pharmacol. 2009, 158, 413–428. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Fereidoon, S.; Ying, Z. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar]
- Banskalieva, V.; Sahlu, T.; Goetsch, A. Fatty acid composition of goat muscles and fat depots: A review. Small Rumin. Res. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, nutritional quality and oxidative stability of cold-pressed Camelina (Camelina sativa L.) oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Bobe, G.; Zimmerman, S.; Hammond, E.G.; Freeman, A.E.; Porter, P.A.; Luhman, C.M.; Beitz, D.C. Butter composition and texture from cows with different milk fatty acid compositions fed fish oil or roasted soybeans. J. Dairy Sci. 2007, 90, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef]
- Mir, N.A.; Tyagi, P.K.; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Kumar, F.; Sharma, D.; Biswas, A.; Verma, A.K. Inclusion of flaxseed, broken rice, and distillers dried grains with solubles (DDGS) in broiler chicken ration alters the fatty acid profile, oxidative stability, and other functional properties of meat. Eur. J. Lipid Sci. Technol. 2018, 120, 1700470. [Google Scholar] [CrossRef]
| Ingredients | Content | |
|---|---|---|
| 1–28 Days | 29–48 Days | |
| Corn (%) | 60.00 | 63.50 |
| Soybean meal (%) | 32.00 | 29.00 |
| Wheat bran (%) | 1.00 | |
| Soybean oil (%) | 1.00 | 2.00 |
| Fish meal (%) | 2.00 | 1.60 |
| CaHPO4 (%) | 1.30 | 1.30 |
| Lime stone (%) | 1.40 | 1.30 |
| NaCl (%) | 0.30 | 0.30 |
| Premix 1 (%) | 1.00 | 1.00 |
| Total (%) | 100.00 | 100.00 |
| Nutrient levels | ||
| ME/(MJ·kg−1) 2 | 12.13 | 12.55 |
| CP (%) | 21.00 | 20.00 |
| Ca (%) | 1.00 | 0.90 |
| TP (%) | 0.65 | 0.60 |
| AP (%) | 0.45 | 0.35 |
| Lys (%) | 0.50 | 0.38 |
| Met (%) | 1.10 | 1.00 |
| Genes (Accession) | Primer Sequence | Length (bp) |
|---|---|---|
| PPARγ (NM_001001460) | F:5′-CGAATGCCACAAGCGGAGAAGG-3′ R:5′-CACTGCCTCCACAGAGCGAAAC-3′ | 330 |
| C/EBPα (NM_001031459) | F:5′-GCCAACTTCTACGAGGTCGATTCC-3′ R:5′-TTGTGCTTCTCCTGCTGCTTGC-3′ | 260 |
| Wnt1 (NM_001396681) | F:5′-GGCTCTTCGGGAGGGAATTTGTG-3′ R:5′-TGCCTTTGTTGCCGTAGATGACC-3′ | 255 |
| β-catenin (U82964) | F:5′-GCTATTGTTGAGGCTGGTGGGATG-3′ R:5′-GCTTCCTGATGTCTGCTGGTGAG-3′ | 368 |
| β-actin (L08165) | F:5′-CATTGAACACGGTATTGTCACCAACTG-3′ R:5′-GTAACACCATCACCAGAGTCCATCAC-3′ | 270 |
| Item | CYP Level (mg/kg) 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | CYP1 | CYP2 | CYP3 | |||
| Breast muscle fat ratio (%) | 6.25 a | 6.09 a | 5.45 b | 5.96 ab | 0.22 | 0.032 |
| Thigh muscle fat ratio (%) | 24.41 a | 22.07 b | 21.45 b | 23.55 a | 0.59 | 0.004 |
| Item | CYP Level (mg/kg) 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | CYP1 | CYP2 | CYP3 | |||
| SFA | ||||||
| C14:0 (%) | 0.46 a | 0.40 b | 0.35 c | 0.47 a | 0.021 | 0.001 |
| C16:0 (%) | 25.71 | 24.85 | 24.87 | 25.44 | 0.43 | 0.098 |
| C17:0 (%) | 4.49 | 4.32 | 4.46 | 4.54 | 0.24 | 0.798 |
| C18:0 (%) | 10.46 a | 10.40 a | 9.37 b | 9.80 ab | 0.24 | 0.017 |
| C20:0 (%) | 0.041 a | 0.029 b | 0.025 b | 0.038 a | 0.0021 | 0.001 |
| MUFA | ||||||
| C14:1(%) | 0.052 a | 0.047 a | 0.035 b | 0.034 b | 0.0035 | 0.002 |
| C16:1 (%) | 1.36 a | 1.19 b | 1.41 a | 1.39 a | 0.072 | 0.05 |
| C18:1 (%) | 30.03 | 30.32 | 30.79 | 28.88 | 0.92 | 0.273 |
| C20:1 (%) | 0.41 a | 0.30 b | 0.25 b | 0.27 b | 0.03 | 0.003 |
| C22:1 (%) | 0.10 a | 0.10 a | 0.059 b | 0.10 a | 0.07 | 0.001 |
| C24:1 (%) | 0.11 a | 0.59 b | 0.45 b | 0.50 b | 0.012 | 0.003 |
| PUFA | ||||||
| C18:2 n-6 (%) | 17.67 b | 17.64 b | 18.52 a | 17.74 b | 0.34 | 0.05 |
| C18:3 n-6 (%) | 0.23 | 0.21 | 0.22 | 0.22 | 0.016 | 0.184 |
| C18:3 n-3 (%) | 0.47 b | 0.47 b | 0.53 a | 0.49 b | 0.031 | 0.001 |
| C20:2 n-6 (%) | 0.53 | 0.59 | 0.47 | 0.64 | 0.07 | 0.189 |
| C20:3 n-6 (%) | 0.66 | 0.63 | 0.54 | 0.60 | 0.04 | 0.091 |
| C20:4 n-6 (%) | 0.028 a | 0.024 b | 0.024 b | 0.026 ab | 0.0017 | 0.012 |
| C20:5 n-3 (%) | 0.21 b | 0.25 ab | 0.23 b | 0.27 a | 0.018 | 0.041 |
| C22:6 n-3(%) | 0.91 b | 1.19 a | 1.30 a | 1.22 a | 0.047 | 0.001 |
| Total SFA (%) | 41.16 a | 40.00 b | 39.07 c | 40.29 b | 0.25 | 0.001 |
| Total MUFA (%) | 32.07 | 32.02 | 32.59 | 30.73 | 0.92 | 0.291 |
| Total PUFA (%) | 20.72 b | 21.03 ab | 21.83 a | 21.18 ab | 0.38 | 0.047 |
| Item | CYP Level (mg/kg) 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | CYP1 | CYP2 | CYP3 | |||
| SFA | ||||||
| C14:0 (%) | 0.67 a | 0.60 a | 0.45 b | 0.63 a | 0.040 | 0.003 |
| C16:0 (%) | 22.02 a | 21.05 b | 21.30 b | 22.00 a | 0.28 | 0.018 |
| C17:0 (%) | 2.64 | 2.28 | 2.25 | 2.59 | 0.16 | 0.094 |
| C18:0 (%) | 5.19 a | 4.32 b | 3.84 b | 4.34 b | 0.31 | 0.015 |
| C20:0 (%) | 0.074 a | 0.046 b | 0.037 b | 0.044 a | 0.0060 | 0.001 |
| MUFA | ||||||
| C14:1(%) | 0.050 a | 0.046 ab | 0.042 b | 0.049 a | 0.0030 | 0.05 |
| C16:1 (%) | 4.25 a | 4.28 b | 3.06 b | 3.11 a | 0.26 | 0.002 |
| C18:1 (%) | 39.50 b | 41.75 a | 42.30 a | 39.39 b | 0.97 | 0.033 |
| C20:1 (%) | 0.41 a | 0.41 a | 0.32 b | 0.35 b | 0.02 | 0.006 |
| C22:1 (%) | 0.10 | 0.0.091 | 0.083 | 0.085 | 0.030 | 0.916 |
| C24:1 (%) | 0.073 a | 0.079 a | 0.052 b | 0.055 b | 0.0040 | 0.001 |
| PUFA | ||||||
| C18:2 n-6 (%) | 20.42 b | 20.53 b | 21.43 a | 20.91 ab | 0.26 | 0.017 |
| C18:3 n-6 (%) | 0.19 | 0.19 | 0.23 | 0.18 | 0.024 | 0.23 |
| C18:3 n-3 (%) | 0.77 a | 0.61 b | 0.88 a | 0.77 a | 0.052 | 0.001 |
| C20:2 n-6 (%) | 0.33 | 0.26 | 0.28 | 0.32 | 0.06 | 0.647 |
| C20:3 n-6 (%) | 0.32 | 0.21 | 0.39 | 0.41 | 0.09 | 0.183 |
| C20:4 n-6 (%) | 0.034 b | 0.038 b | 0.038 b | 0.048 a | 0.0038 | 0.037 |
| C20:5 n-3 (%) | 0.059 b | 0.10 a | 0.11 a | 0.11 a | 0.0066 | 0.001 |
| C22:6 n-3(%) | 0.49 b | 0.71 a | 0.73 a | 0.58 b | 0.043 | 0.001 |
| Total SFA (%) | 30.59 a | 28.30 b | 27.89 b | 29.60 a | 0.55 | 0.001 |
| Total MUFA (%) | 44.40 bc | 46.66 a | 45.87 ab | 43.04 c | 0.87 | 0.014 |
| Total PUFA (%) | 22.59 c | 22.59 c | 24.07 a | 23.36 b | 0.30 | 0.003 |
| Item 2 | CYP Level (mg/kg) 1 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | CYP1 | CYP2 | CYP3 | |||
| PUFA: SFA | 0.50 b | 0.53 b | 0.56 a | 0.53 b | 0.01 | 0.006 |
| n-6: n-3 | 12.00 a | 9.84 b | 9.63 b | 9.86 b | 0.22 | 0.001 |
| DHA + EPA (%) | 1.13 b | 1.46 a | 1.52 a | 1.47 a | 0.04 | 0.001 |
| UI | 4.83 | 4.69 | 4.67 | 4.70 | 0.16 | 0.766 |
| PI | 0.30 b | 0.33 a | 0.34 a | 0.33 a | 0.006 | 0.002 |
| NVI | 1.62 | 1.59 | 1.61 | 1.52 | 0.05 | 0.304 |
| IA | 0.52 | 0.50 | 0.48 | 0.53 | 0.015 | 0.063 |
| IT | 1.20 a | 1.13 b | 1.07 c | 1.15 ab | 0.02 | 0.002 |
| HHR | 1.94 | 2.03 | 2.09 | 1.93 | 0.058 | 0.078 |
| HPI | 1.92 | 2.01 | 2.07 | 1.90 | 0.056 | 0.052 |
| Item 2 | CYP Level (mg/kg) 1 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | CYP1 | CYP2 | CYP3 | |||
| PUFA: SFA | 0.74 c | 0.80 b | 0.86 a | 0.79 b | 0.016 | 0.001 |
| n-6: n-3 | 16.98 a | 14.81 a | 12.90 b | 15.10 a | 0.636 | 0.007 |
| DHA + EPA (%) | 0.55 c | 0.82 a | 0.85 a | 0.69 b | 0.046 | 0.001 |
| UI | 4.69 a | 3.76 b | 5.36 a | 5.05 a | 0.304 | 0.004 |
| PI | 0.29 c | 0.30 b | 0.33 a | 0.31 b | 0.005 | 0.001 |
| NVI | 2.03 b | 2.19 a | 2.17 a | 1.99 b | 0.041 | 0.003 |
| IA | 0.37 a | 0.34 b | 0.33 b | 0.37 a | 0.006 | 0.001 |
| IT | 0.76 a | 0.68 b | 0.65 b | 0.73 a | 0.016 | 0.001 |
| HHR | 2.73 b | 2.97 a | 3.05 a | 2.77 b | 0.047 | 0.001 |
| HPI | 2.71 b | 2.96 a | 3.02 a | 2.71 b | 0.047 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Chang, Y.; Sun, Z.; Deng, J.; Jin, Y.; Shi, M.; Zhang, J.; Miao, Z. Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers. Foods 2023, 12, 1479. https://doi.org/10.3390/foods12071479
Guo L, Chang Y, Sun Z, Deng J, Jin Y, Shi M, Zhang J, Miao Z. Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers. Foods. 2023; 12(7):1479. https://doi.org/10.3390/foods12071479
Chicago/Turabian StyleGuo, Liping, Yadi Chang, Zhe Sun, Jiahua Deng, Yan Jin, Mingyan Shi, Jinzhou Zhang, and Zhiguo Miao. 2023. "Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers" Foods 12, no. 7: 1479. https://doi.org/10.3390/foods12071479
APA StyleGuo, L., Chang, Y., Sun, Z., Deng, J., Jin, Y., Shi, M., Zhang, J., & Miao, Z. (2023). Effects of Chinese Yam Polysaccharide on Intramuscular Fat and Fatty Acid Composition in Breast and Thigh Muscles of Broilers. Foods, 12(7), 1479. https://doi.org/10.3390/foods12071479






