Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Drug Sensitivity Test
2.3. Lifespan and Fundamental Physiological Indicators
2.3.1. Lifespan Assay
2.3.2. Fecundity
2.3.3. Motility
2.3.4. Lipofuscin
2.4. Stress Assay
2.4.1. Heat Shock
2.4.2. Oxidative Stress
2.5. ROS Level, Antioxidant Enzyme Activities and MDA Content
2.5.1. ROS Level
2.5.2. Antioxidant Enzyme Activities and MDA Content
2.6. Quantitative Real-Time PCR (RT-qPCR)
2.7. Statistics
3. Results
3.1. SH Has No Antimicrobial Activity against OP50
3.2. Lifespan and Fundamental Physiological Indicators
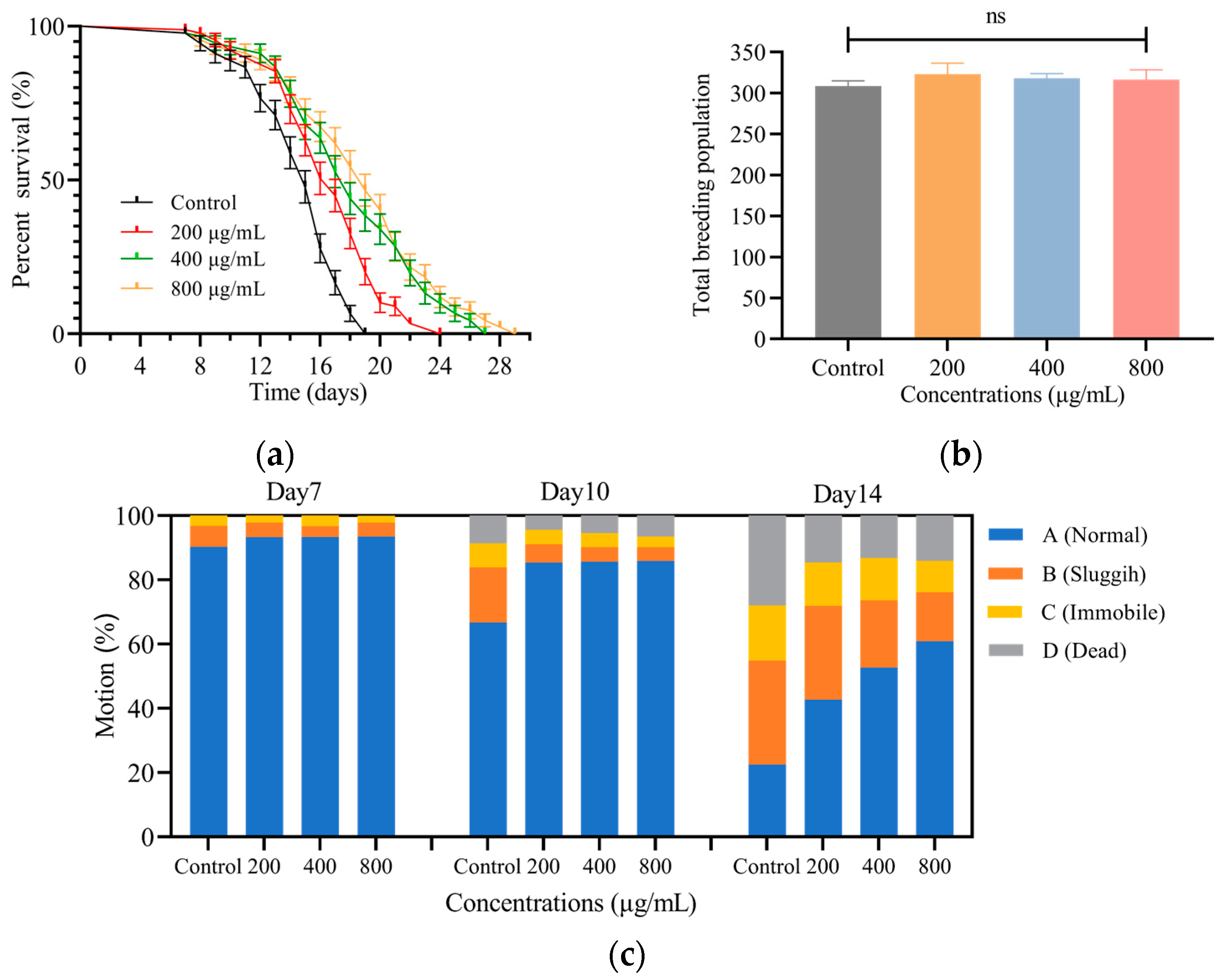
3.2.1. SH Extends the Lifespan of C. elegans
3.2.2. SH Shows No Toxicity on C. elegans Fecundity
3.2.3. SH Slows down the Decline of C. elegans Motility
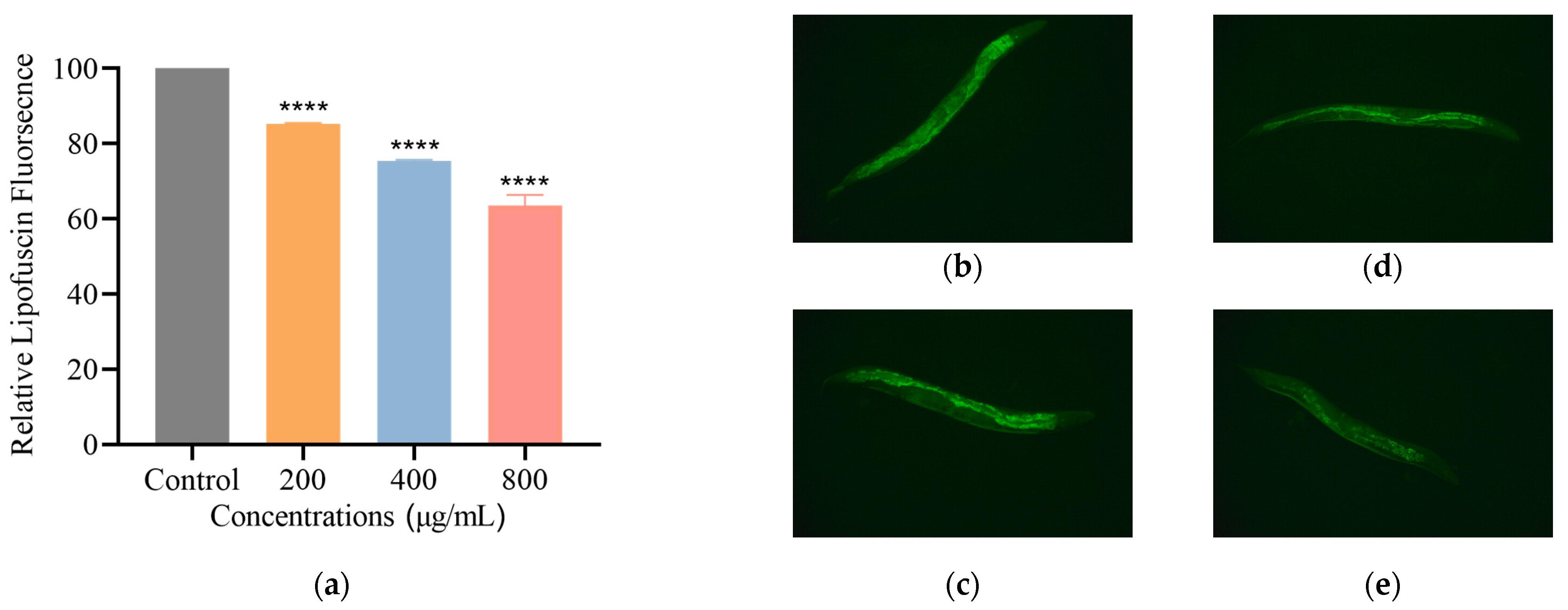
3.2.4. SH Reduces Lipofuscin Level in C. elegans
3.3. SH Enhances the Abilities of C. elegans to Resist Stress
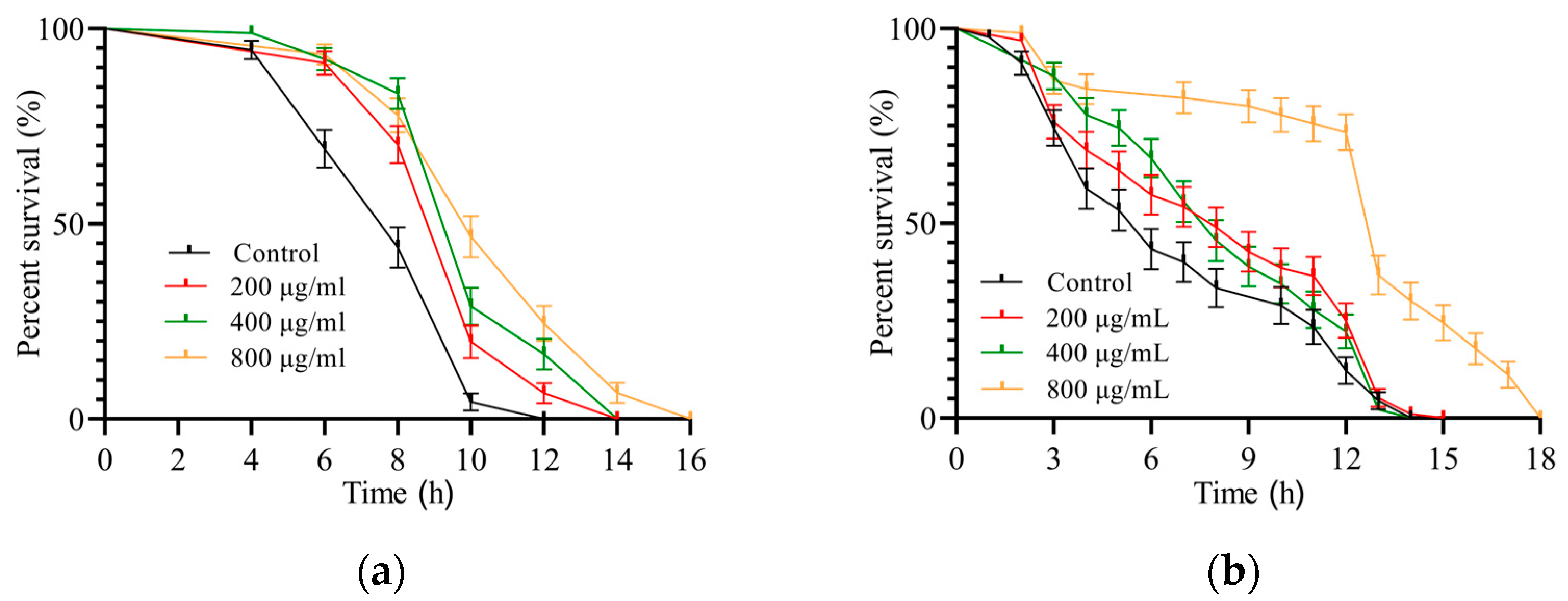
3.3.1. SH Relieves Heat Shock
3.3.2. SH Protects C. elegans during Oxidative Stress
3.4. SH Decreases ROS Level in C. elegans
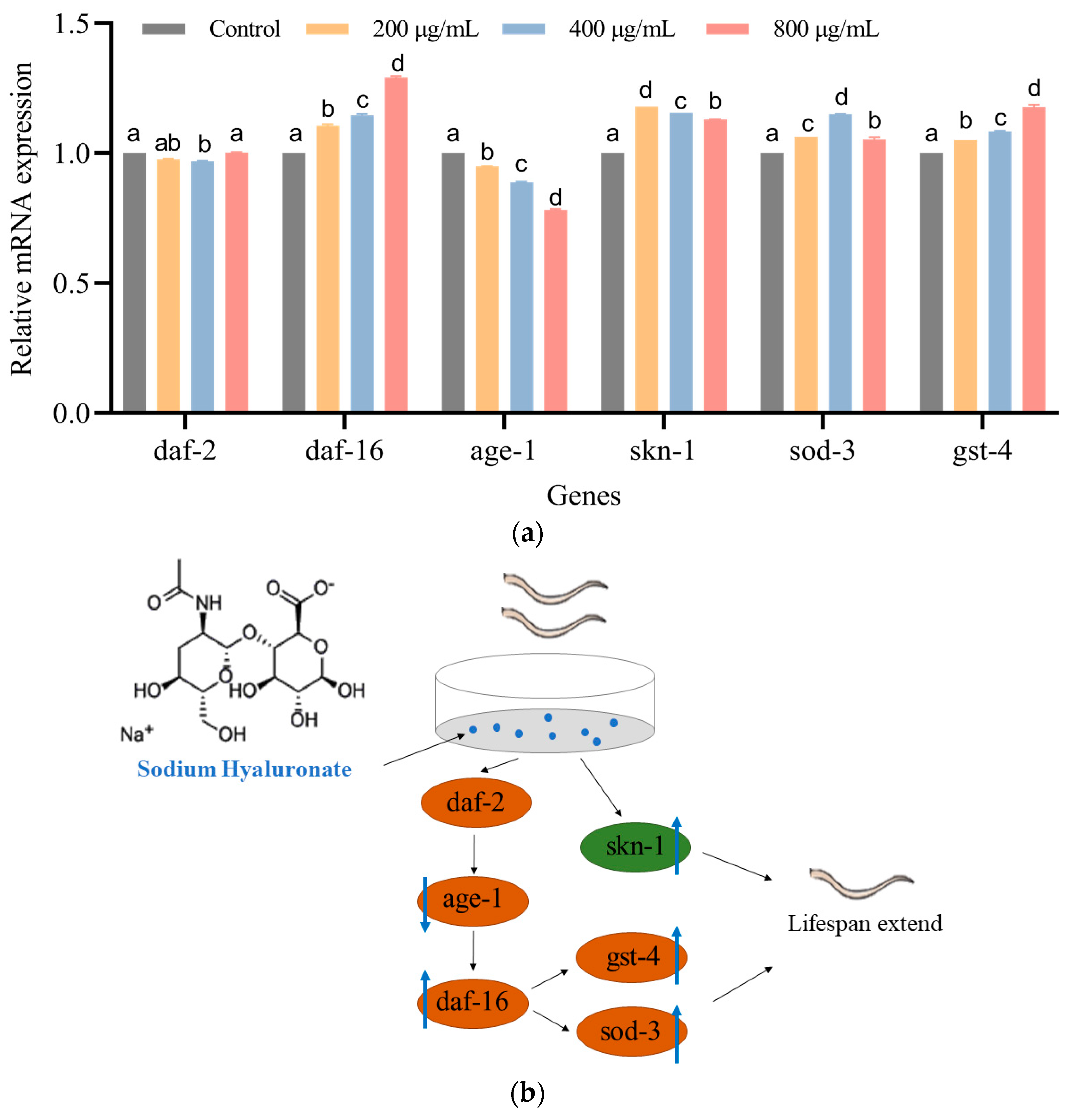
3.5. SH Regulates the mRNA Expression of C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.-W.; Kim, B.; Seo, K.-H.; Bae, D.; Jeong, D.; Song, K.-Y. Physiochemical and Organoleptic Properties of Kefir Containing Different Concentrations of Hyaluronic Acid: A Preliminary Study. J. Dairy Sci. Biotechnol. 2020, 38, 146–153. [Google Scholar] [CrossRef]
- Zając, M.; Kulawik, P.; Tkaczewska, J.; Migdał, W.; Filipczak-Fiutak, M.; Fiutak, G. The effect of hyaluronic acid addition on the properties of smoked homogenised sausages. J. Sci. Food Agric. 2017, 97, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, S.G.; Salunke, P. Effect of Hyaluronic Acid and Kappa-Carrageenan on Milk Properties: Rheology, Protein Stability, Foaming, Water-Holding, and Emulsification Properties. Foods 2023, 12, 913. [Google Scholar] [CrossRef]
- Kweon, D.-K.; Han, J.-A. Development of hyaluronic acid-based edible film for alleviating dry mouth. Food Sci. Hum. Wellness 2023, 12, 371–377. [Google Scholar] [CrossRef]
- Oe, M.; Mitsugi, K.; Odanaka, W.; Yoshida, H.; Matsuoka, R.; Seino, S.; Kanemitsu, T.; Masuda, Y. Dietary Hyaluronic Acid Migrates into the Skin of Rats. Sci. World J. 2014, 2014, 378024. [Google Scholar] [CrossRef]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef]
- Canut, L.; Zapatero, J.; López, S.; Torrent, A.; Ruhí, R.; Vicente, L. Genotoxicity, acute and subchronic toxicity studies in rats of a rooster comb extract rich in sodium hyaluronate. Regul. Toxicol. Pharmacol. 2012, 62, 532–541. [Google Scholar] [CrossRef]
- Ishihara, M.; Inouye, T.; Ishiyama, Y.; Sakata, T.; Ichikawa, A.; Funahashi, N.; Kato, T.; Takahashi, T.; Horie, K. Toxicity Study on Sodium Hyaluronate (NA-HA) in Rats by Repeated Oral Administration for 90 Days Followed by a 28-Day Recovery Study. Ōyō Yakuri 1996, 51, 97–113. [Google Scholar]
- Juncan, A.M.; Moisa, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Tincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, C.E.P.; Castro, M.A.A.; de Noronha, M.S.; Martins, P.A.; Mendes, R.D.; Caliari, M.V.; Mesquita, R.A.; Ferreira, A.J. Hyaluronic acid accelerates bone repair in human dental sockets: A randomized triple-blind clinical trial. Braz. Oral Res. 2018, 32, 84. [Google Scholar] [CrossRef] [PubMed]
- Tambara, A.L.; de Los Santos Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Gonçalves Soares, A.T.; de Freitas Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Chen, T.; Luo, S.; Wang, X.; Zhou, Y.; Dai, Y.; Zhou, L.; Feng, S.; Yuan, M.; Ding, C. Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants 2021, 10, 1744. [Google Scholar] [CrossRef]
- Wang, J.; Deng, N.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Effects of orange extracts on longevity, healthspan, and stress resistance in Caenorhabditis elegans. Molecules 2020, 25, 351. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zang, Y.-P.; Cui, Q.; Gai, Q.-Y.; Jiao, J.; Wang, W.; Zu, Y.-G.; Fu, Y.-J. The preservative potential of Amomum tsaoko essential oil against E. coil, its antibacterial property and mode of action. Food Control 2017, 75, 236–245. [Google Scholar] [CrossRef]
- Shahen, M.Z.; Mahmud, S.; Rony, M.; Sohana, S.; Imran, M.; Al Maruf, M.; Azim, M.; Islam, M.; Islam, M.; Uddin, M. Effect of antibiotic susceptibility and inhibitory activity for the control of growth and survival of microorganisms of extracts of Calendula officinalis. Eur. J. Med. Health Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Koch, K.; Weldle, N.; Baier, S.; Büchter, C.; Wätjen, W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef]
- Song, B.B.; Xia, W.; Li, T.; Liu, R.H. Mitochondria are involved in the combination of blueberry and apple peel extracts synergistically ameliorating the lifespan and oxidative stress in Caenorhabditis elegans. Food Funct. 2022, 13, 8204–8213. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.; Yang, S.; Zhang, X.; Cheng, J.; Wang, H. Anti-aging Effects and Underling Mechanism of D-chiro-inosiol on Glucose-inducedOxidative Damage in Caenorhabditis elegans. Sci. Technol. Food Ind. 2019, 40, 282–286. [Google Scholar]
- Cheng, R. The Anti-Aging Effect and Mechanism of Flavonoids in Coreopsis tinctoria Nutt. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2019. [Google Scholar]
- Yang, X.Y.; Wang, H.; Li, T.; Chen, L.; Zheng, B.S.; Liu, R.H. Nobiletin Delays Aging and Enhances Stress Resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2020, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Gerstbrein, B.; Stamatas, G.; Kollias, N.; Driscoll, M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 2005, 4, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, J.; Rao, Y.; Wang, W.; Fu, Y. Gengnianchun Extends the Lifespan of Caenorhabditis elegans via the Insulin/IGF-1 Signalling Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 4740739. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Brown, D.; Choi, H.; Park, S.; Ocampo, B.R.; Chen, S.W.; Le, A.; Sutphin, G.L.; Shamieh, L.S.; Smith, E.D.; Kaeberlein, M. Sorbitol treatment extends lifespan and induces the osmotic stress response in Caenorhabditis elegans. Front. Genet. 2015, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, S.; Zhang, C.; Huang, J.; Wu, J.; Zhou, H.; Jin, L.; Qian, X.; Jin, J.; Lyu, J. Enhanced ROS production leads to excessive fat accumulation through DAF-16 in Caenorhabditis elegans. Exp. Gerontol. 2018, 112, 20–29. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.-L. Fucoidan-Derived Functional Oligosaccharides: Recent Developments, Preparation, and Potential Applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B.L. Understanding the Odd Science of Aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [PubMed]
- El Maradny, E.; Kanayama, N.; Kobayashi, H.; Hossain, B.; Khatun, S.; Liping, S.; Kobayashi, T.; Terao, T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum. Reprod. 1997, 12, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Galimov, E.R.; Gems, D. Shorter life and reduced fecundity can increase colony fitness in virtual Caenorhabditis elegans. Aging Cell 2020, 19, e13141. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, G.J. Does Anti-Aging Equal Anti-Microbial? Sci. Aging Knowl. Environ. 2003, 2003, pe16. [Google Scholar] [CrossRef] [PubMed]
- Kumada, J.I.; Nishiwaki, K.; Iriyama, K.; Hibino, H.; Isowa, K.; Komai, Y.; Takahashi, T.; Miyauchi, S. 50-11 Reproductive and Developmental Toxicity Study of a 1 Per cent Solution of Sodium hyaluronate (SI-4402) 2. Teratogenicity Study in Rats by Subcutaneous Administration. Oyo Yakuri-Pharmacomet. 1995, 50, 105–122. [Google Scholar]
- Wada, K.; Hashimato, Y.; Mizutani, M.; Chiaki, T. Reproductive and divelopmental toxicity studies of sodium hyaluronate (SL-1010)(III)-Teratogenicity study in rabbits. Jpn. Pharmacol. Ther. 1991, 19, 111–119. [Google Scholar]
- Furuhashi, T.; Nakazawa, M. Reproduction studies of sodium hyaluronate (SPH) (3). Teratological study in rabbits. Pharmacometrics 1985, 29, 131–138. [Google Scholar]
- Ono, C.; Iwama, A.; Nakajima, Y.; Kitsuya, A.; Nakamura, T. Reproductive and developmental toxicity study on sodium hyaluronate (SH). I: Study on subcuataneous administration to rats during period of organogenesis. Yakuri Chiryo 1992, 20, 11–26. [Google Scholar]
- Wang, H.N.; Webster, P.; Chen, L.Z.; Fisher, A.L. Cell-autonomous and non-autonomous roles of daf-16 in muscle function and mitochondrial capacity in aging C. elegans. Aging-Us 2019, 11, 2295–2311. [Google Scholar] [CrossRef]
- Solyga, M.; Solari, F. DAF-2 receptor signalling pathway (Insulin/IGF-1), a key role in muscular aging. M S-Med. Sci. 2020, 36, 938-841. [Google Scholar]
- Crombie, T.A.; Tang, L.; Choe, K.P.; Julian, D. Inhibition of the oxidative stress response by heat stress in Caenorhabditis elegans. J. Exp. Biol. 2016, 219, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Elkhedir, A.; Iqbal, A.; Albahi, A.; Tao, M.; Rong, L.; Xu, X. Capsaicinoid-Glucosides of Fresh Hot Pepper Promotes Stress Resistance and Longevity in Caenorhabditis elegans. Plant Foods Hum. Nutr. 2022, 77, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan Extending and Oxidative Stress Resistance Properties of a Leaf Extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2019, 2019, 9012396. [Google Scholar] [CrossRef]
- Jayarathne, S.; Ramalingam, L.; Edwards, H.; Vanapalli, S.A.; Moustaid-Moussa, N. Tart Cherry Increases Lifespan inCaenorhabditis elegansby Altering Metabolic Signaling Pathways. Nutrients 2020, 12, 1482. [Google Scholar] [CrossRef]
- Song, B.; Wang, H.; Xia, W.; Zheng, B.; Li, T.; Liu, R.H. Combination of apple peel and blueberry extracts synergistically induced lifespan extension via DAF-16 in Caenorhabditis elegans. Food Funct. 2020, 11, 6170–6185. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Dong, J.; Yang, W.; Liu, T.; Wang, Y.; Wang, X.; Wang, M.; Zhi, D. Rose essential oil delayed Alzheimer’s disease-like symptoms by SKN-1 pathway in C. elegans. J. Agric. Food Chem. 2017, 65, 8855–8865. [Google Scholar] [CrossRef]
- Wang, E.; Wang, N.; Zou, Y.; Fahim, M.; Zhou, Y.; Yang, H.; Liu, Y.; Li, H. Black mulberry (Morus nigra) fruit extract alleviated AD-Like symptoms induced by toxic Abeta protein in transgenic Caenorhabditis elegans via insulin DAF-16 signaling pathway. Food Res. Int. 2022, 160, 111696. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Song, B.; Zhong, Y.; Yin, H.; Li, Z.; Wang, Z.; Cheong, K.-L.; Huang, R.; Zhong, S. Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans. Foods 2023, 12, 1400. https://doi.org/10.3390/foods12071400
Lin Q, Song B, Zhong Y, Yin H, Li Z, Wang Z, Cheong K-L, Huang R, Zhong S. Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans. Foods. 2023; 12(7):1400. https://doi.org/10.3390/foods12071400
Chicago/Turabian StyleLin, Qianmin, Bingbing Song, Yingxiong Zhong, Huan Yin, Ziyu Li, Zhuo Wang, Kit-Leong Cheong, Riming Huang, and Saiyi Zhong. 2023. "Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans" Foods 12, no. 7: 1400. https://doi.org/10.3390/foods12071400
APA StyleLin, Q., Song, B., Zhong, Y., Yin, H., Li, Z., Wang, Z., Cheong, K.-L., Huang, R., & Zhong, S. (2023). Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans. Foods, 12(7), 1400. https://doi.org/10.3390/foods12071400






