Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Treatment Solutions
2.3. Preparation of BF
2.4. Use of Treatment Solutions
2.5. Staining of Extracellular Products
2.6. CLSM Observation
2.7. ISA-2 Analysis
2.8. Extraction of Extracellular Substances
2.9. Determination of Fluorescence Intensity
2.10. Degradation Kinetic Model
2.11. Statistical Analysis
3. Results
3.1. Germicidal Rate
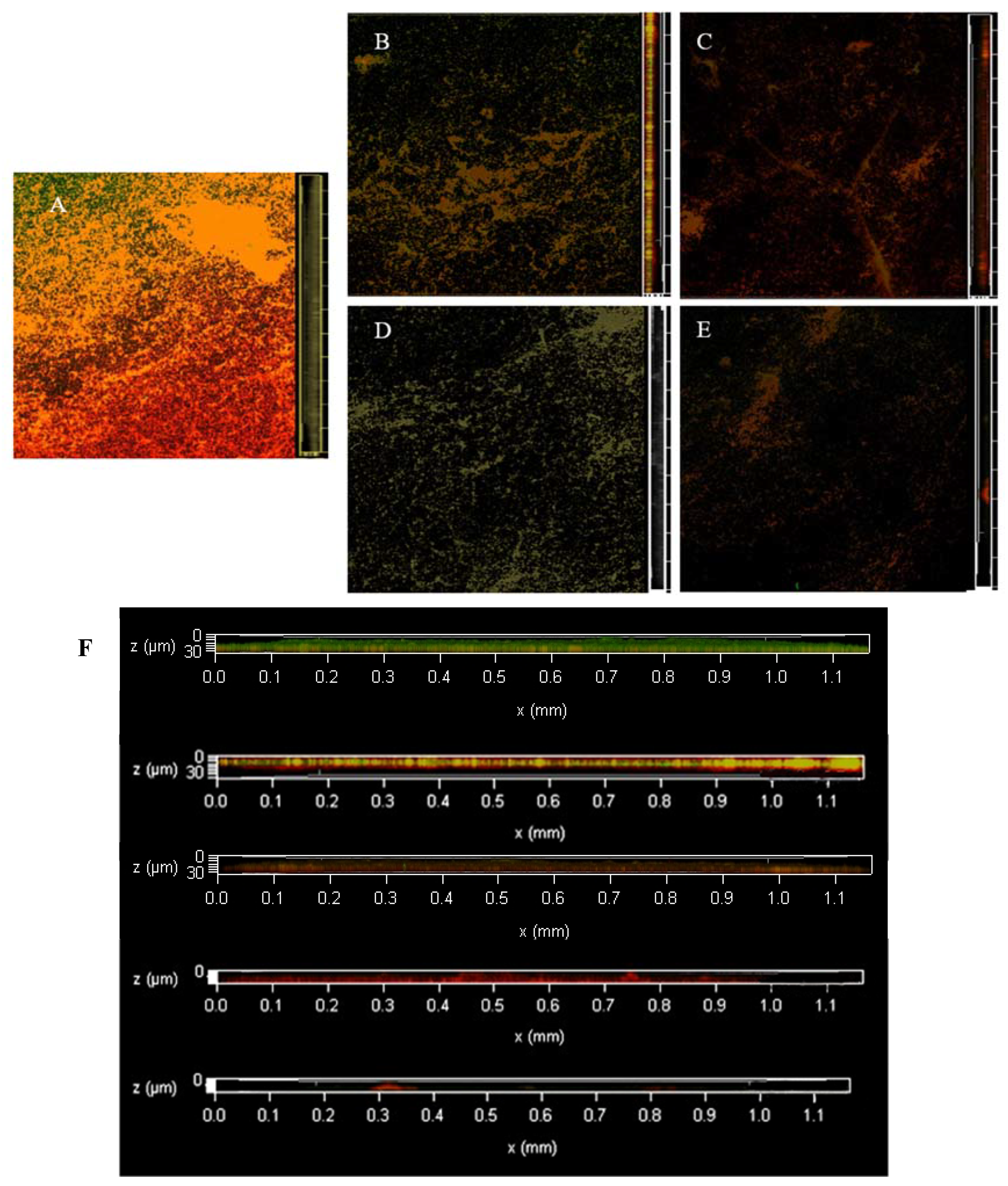
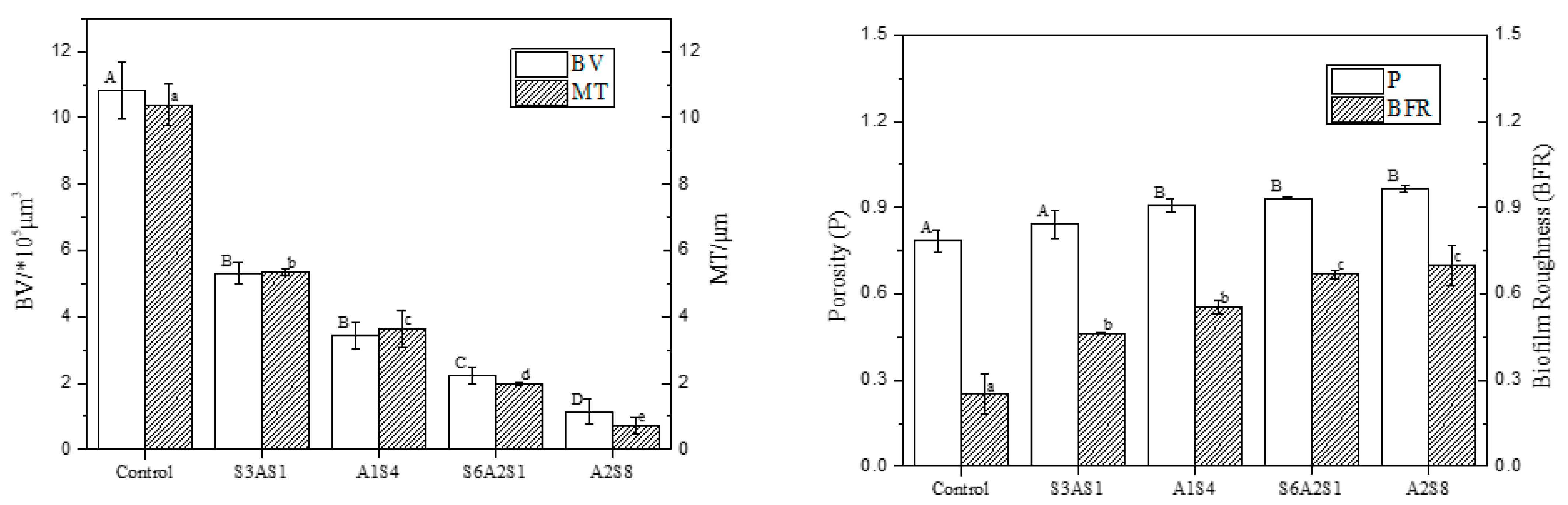
3.2. CLSM Observation of Staining Results with ISA-2 Software Analysis
3.3. Dyeing with PI and Syto®9 after BF Treatment with SAEW
3.4. Dyeing with PI and Syto®9 after BF Treatment with NEHW
3.5. Dyeing with PI and Syto®9 after BF Treatment with AEW and ALEW
3.6. Dyeing with AO after BF Treatment with SAEW
3.7. Dyeing with AO after BF Treatment with NEHW
3.8. Dyeing with AO after BF Treatment with AEW and ALEW
3.9. Multi-Mode Microplate Reader Validation Test
3.10. Degradation Rate and Reaction Order of SAEW and NEHW Treated at Different Times
4. Conclusions
5. Discussion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Décard, B.; Thne, J.; Haghikia, A.; Brnke, C.; Anders, A.; Lukas, C.; Gold, R. Listeria rhombencephalitis mimicking a demyelinating event in an immunocompetent young patient. SAGE Publ. 2017, 23, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ireton, K.; Mortuza, R.; Gyanwali, G.C.; Gianfelice, A.; Hussain, M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes—ScienceDirect. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Milian, A.; Payeras-Cifre, A. What is new in listeriosis? Biomed. Res. Int. 2014, 2014, 358051. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Mah, T.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Guo, L. Study on the Formation of Heterogeneous Biofilms on Abiotic Surfaces of Several Foodborne Pathogens. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar] [CrossRef]
- Oh, E.; Kim, J.C.; Jeon, B. Stimulation of biofilm formation by oxidative stress in Campylobacter jejuni under aerobic conditions. Virulence 2016, 7, 846–851. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Regal, P.; Vazquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Salmonella and Campylobacter biofilm formation: A comparative assessment from farm to fork. J. Sci. Food Agric. 2018, 98, 4014–4032. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Lima, C.A.; Brás, S.; Frana, N.; Cerca, N. Evidence for inter-and intra-species biofilm formation variability among a small group of coagulase-negative staphylococci. FEMS Microbiol. Lett. 2017, 362, fnv175. [Google Scholar] [CrossRef]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Fernández, M.; Sanchez-Vizuete, P.; Briandet, R.; Cabo, M.L. Quantitative image analysis to characterize the dynamics of Listeria monocytogenes biofilms. Int. J. Food Microbiol. 2016, 236, 130–137. [Google Scholar] [CrossRef]
- Díaz-Pascual, F.; Hartmann, R.; Lempp, M.; Vidakovic, L.; Song, B.; Jeckel, H.; Thormann, K.M.; Yildiz, F.H.; Dunkel, J.; Link, H. Breakdown of Vibrio cholerae biofilm architecture induced by antibiotics disrupts community barrier function. Nat. Microbiol. 2019, 4, 2136–2145. [Google Scholar] [CrossRef]
- Yun, M.A.; Yeon, K.M.; Park, J.S.; Lee, C.H.; Chun, J.; Lim, D.J. Characterization of biofilm structure and its effect on membrane permeability in MBR for dye wastewater treatment—ScienceDirect. Water Res. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Beyenal, H.; Donovan, C.; Lewandowski, Z.; Harkin, G. Three-dimensional biofilm structure quantification. J. Microbiol. Methods 2004, 59, 395–413. [Google Scholar] [CrossRef]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Castillo, A. Reduction of Salmonella and Shiga toxin-producing Escherichia coli on alfalfa seeds and sprouts using an ozone generating system. Int. J. Food Microbiol. 2019, 289, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cap, M.; Rojas, D.; Fernandez, M.; Fulco, M.; Mozgovoj, M. Effectiveness of short exposure times to electrolyzed water in reducing Salmonella spp and Imidacloprid in lettuce. LWT-Food Sci. Technol. 2020, 128, 109496. [Google Scholar] [CrossRef]
- Schultze, D.M.; Couto, R.; Temelli, F.; Mcmullen, L.M.; Gnzle, M. Lethality of high-pressure carbon dioxide on Shiga toxin-producing Escherichia coli, Salmonella and surrogate organisms on beef jerky. Int. J. Food Microbiol. 2020, 321, 108550. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Honjoh, K.I.; Miyamoto, T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT 2018, 91, 353–360. [Google Scholar] [CrossRef]
- Peng, L.H.; Liang, X.; Chang, R.H.; Mu, J.Y.; Yang, J.L. A bacterial polysaccharide biosynthesis-related gene inversely regulates larval settlement and metamorphosis of Mytilus coruscus. Biofouling 2020, 36, 753–765. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.C.; Russell, S.M. Efficacy of electrolyzed water in the prevention and removal of fecal material attachment and its microbicidal effectiveness during simulated industrial poultry processing. Poult. Sci. 2005, 84, 1778–1784. [Google Scholar] [CrossRef]
- Hussain, M.S.; Tango, C.N.; Oh, D.H. Inactivation kinetics of slightly acidic electrolyzed water combined with benzalkonium chloride and mild heat treatment on vegetative cells, spores, and biofilms of Bacillus cereus. Food Res. Int. 2019, 116, 157–167. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Wang, H.; Cai, L.; Li, Y.; Xu, X.; Zhou, G. Biofilm formation by meat-borne Pseudomonas fluorescens on stainless steel and its resistance to disinfectants. Food Control 2018, 91, 397–403. [Google Scholar] [CrossRef]
- Bridier, A.; Dubois-Brissonnet, F.; Boubetra, A.; Thomas, V.; Briandet, R. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 2010, 82, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chaochao, G.; Guo, Y.; Sun, X.; Liu, Z.; Xue, G.; Jia, H.; Gao, P. Comparative Study on Extracellular Polymeric Substance Extraction Method for Biofilms in Biological Aerated Filters. J. Donghua Univ. (Nat. Sci.) 2017, 43, 720–726. [Google Scholar]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341. [Google Scholar] [CrossRef]
- Rogers, S.; Honma, K.; Mang, T.S. Confocal Fluorescence Imaging To Evaluate The Effect Of Antimicrobial Photodynamic Therapy Depth On P. Gingivalis And T. Denticola Biofilms. Photodiagnosis Photodyn. Ther. 2018, 23, 18–24. [Google Scholar] [CrossRef]
- Alhede, M.; Qvortrup, K.; Liebrechts, R.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Combination of microscopic techniques reveals a comprehensive visual impression of biofilm structure and composition. FEMS Immunol. Med. Microbiol. 2012, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Dominguez, I.J.; Manzo-Merino, J.; Taja-Chayeb, L.; Duenas-Gonzalez, A.; Perez-Cardenas, E.; Trejo-Becerril, C. The role of extracellular DNA (exDNA) in cellular processes. Cancer Biol. Ther. 2021, 22, 267–278. [Google Scholar] [CrossRef]
- Das, T.; Sharma, P.K.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J. Role of eDNA on the Adhesion Forces betweenStreptococcus mutansand Substratum Surfaces: Influence of Ionic Strength and Substratum Hydrophobicity. Langmuir 2011, 27, 10113–10118. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, C.; Zhao, H.; Chen, G.; Chen, X. Interaction between copper and extracellular nucleic acids in the EPS of unsaturated Pseudomonas putida CZ1 biofilm. Environ. Sci. Pollut. Res. 2018, 25, 24172–24180. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications. Pure Appl. Chem. 2012, 2, 377–410. [Google Scholar] [CrossRef]
- Cherny, K.E.; Sauer, K. Untethering and degradation of the polysaccharide matrix are essential steps in the dispersion response of Pseudomonas aeruginosa biofilms. J. Bacteriol. 2020, 202, e00575-19. [Google Scholar] [CrossRef] [PubMed]
- Khosravian, N.; Bogaerts, A.; Huygh, S.; Yusupov, M.; Neyts, E.C. How do plasma-generated OH radicals react with biofilm components? Insights from atomic scale simulations. Biointerphases 2015, 10, 029501. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit Rev Microbiol 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Bossù, M.; Selan, L.; Artini, M.; Relucenti, M.; Familiari, G.; Papa, R.; Vrenna, G.; Spigaglia, P.; Barbanti, F.; Salucci, A.; et al. Characterization of Scardovia wiggsiae Biofilm by Original Scanning Electron Microscopy Protocol. Microorganisms 2020, 8, 807. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhao, F.J. SEM Analysis of Surface Impact on Biofilm Antibiotic Treatment. Scanning 2017, 2017, 2960194. [Google Scholar] [CrossRef]
- Suzuki, I.; Shimizu, T.; Senpuku, H. Short chain fatty acids induced the type 1 and type 2 fimbrillin-dependent and fimbrillin-independent initial attachment and colonization of Actinomyces oris monoculture but not coculture with streptococci. BMC Microbiol. 2020, 20, 329. [Google Scholar] [CrossRef]
- Niederdorfer, R.; Besemer, K.; Battin, T.J.; Peter, H. Ecological strategies and metabolic trade-offs of complex environmental biofilms. npj Biofilms Microbiomes 2017, 3, 21. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef]
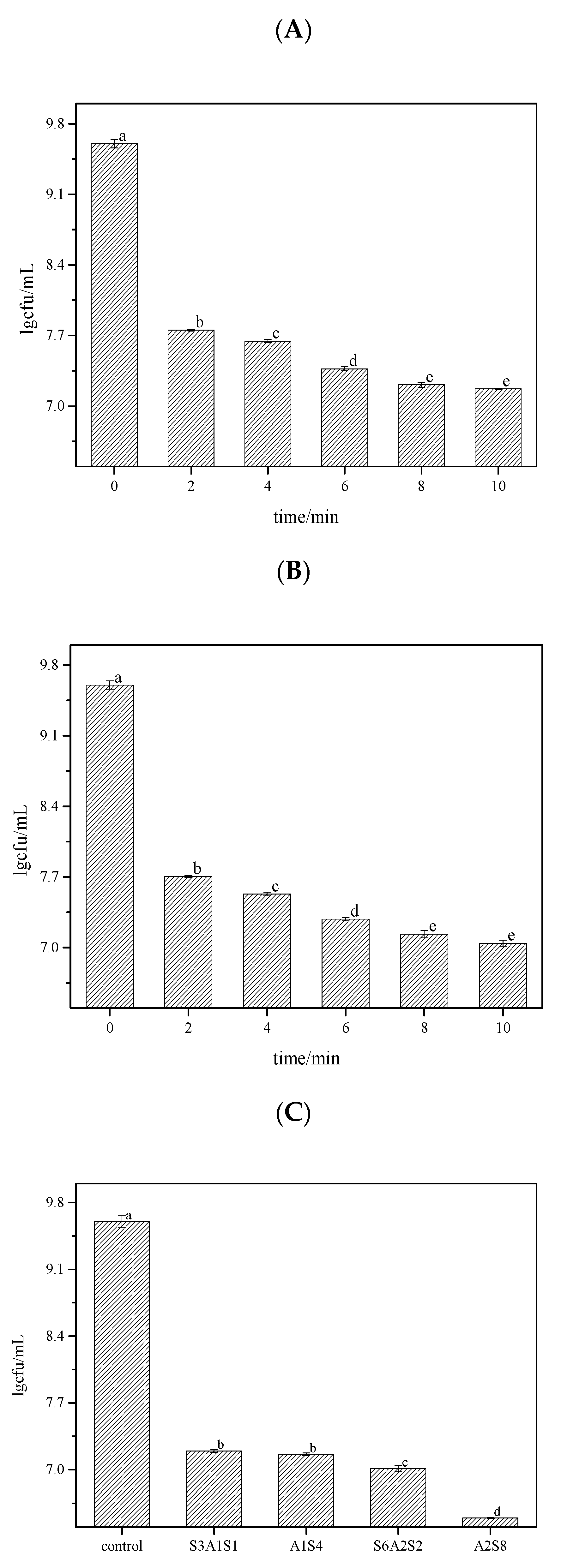

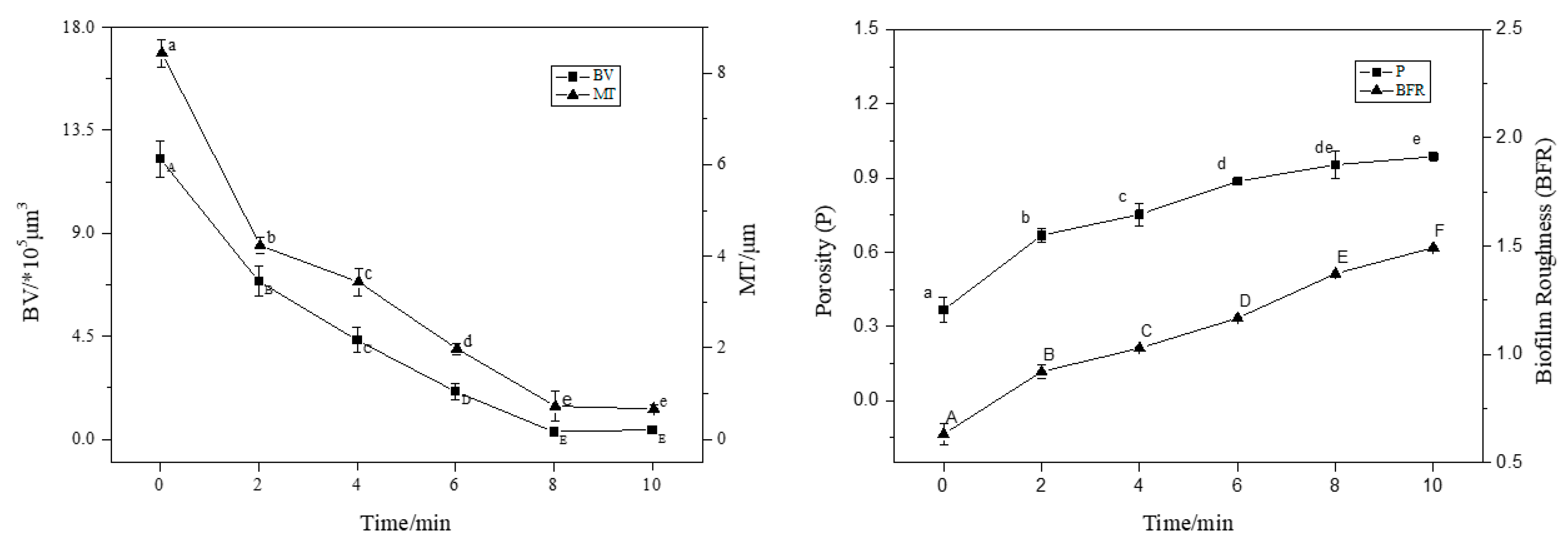




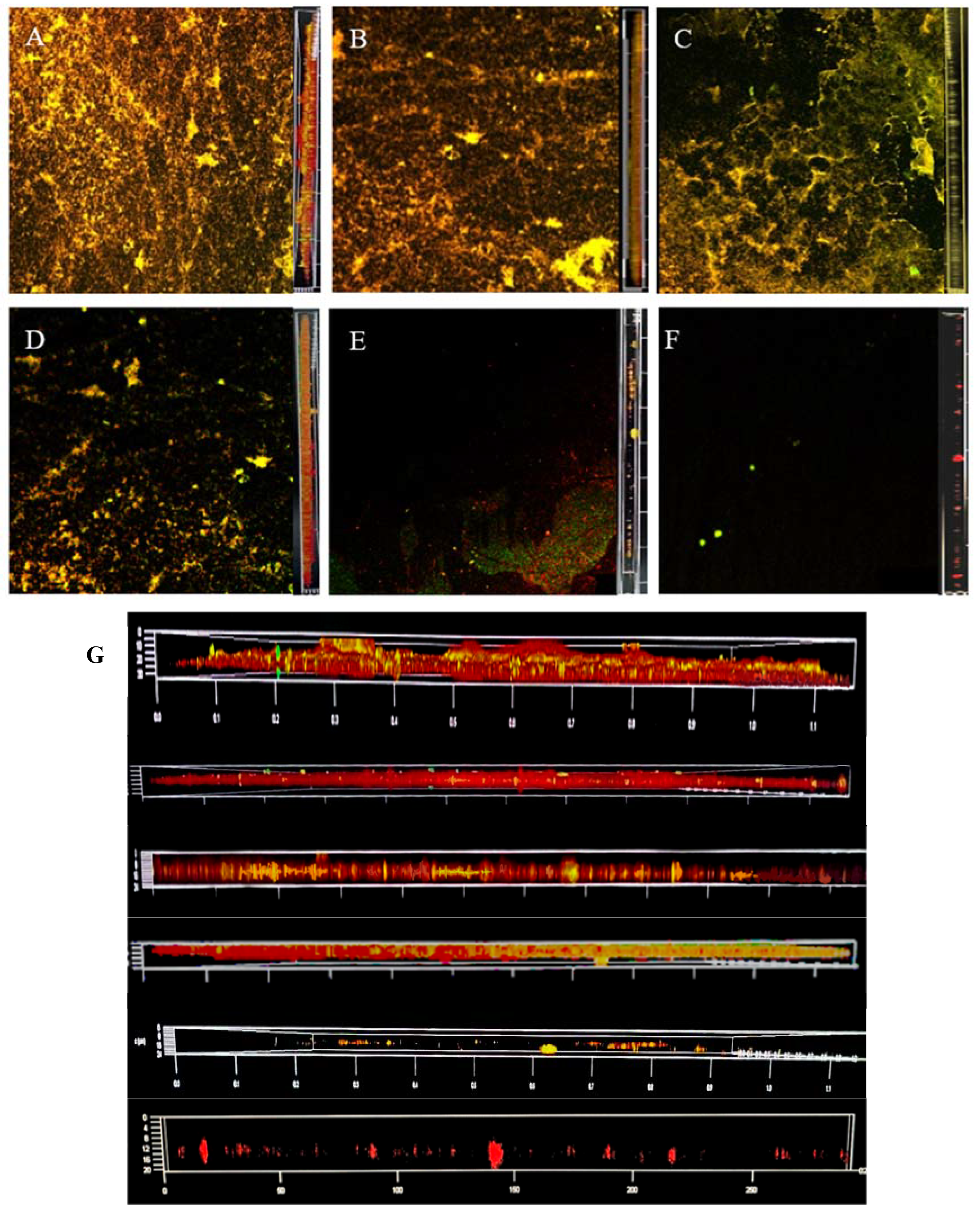


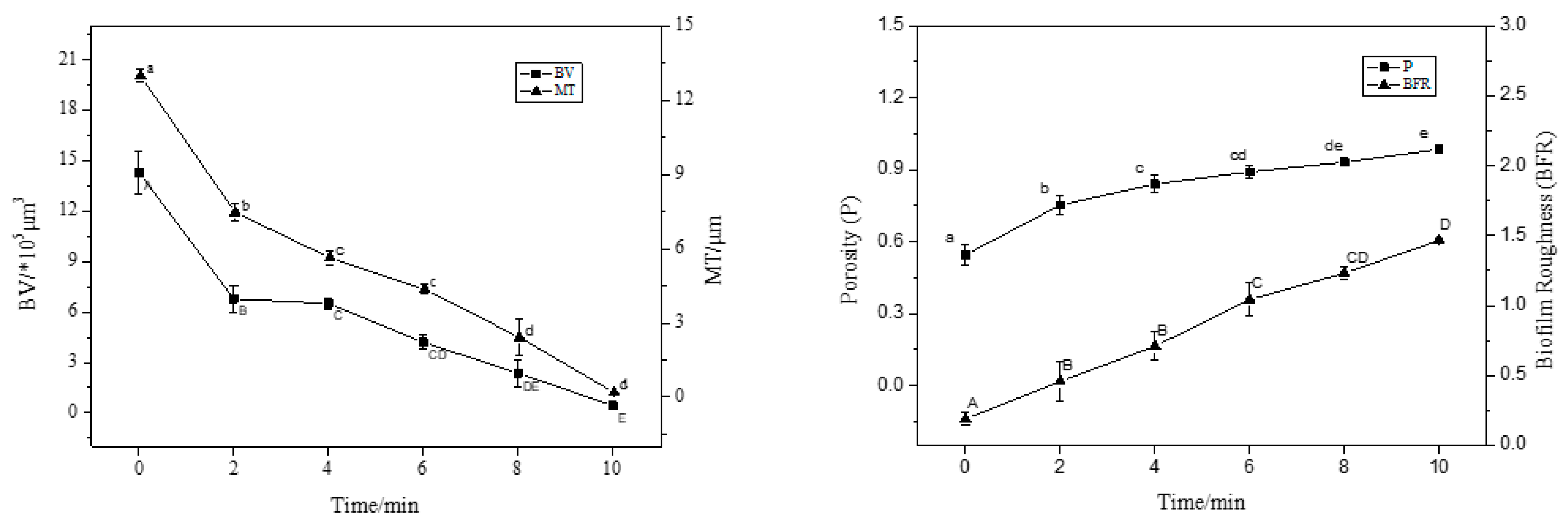


| Fluorescent Dye | Binding Substances | Excitation Wavelength/nm | Emission Wavelength/nm |
|---|---|---|---|
| PI | Dead cells | 485 | 630 |
| Syto®9 | live cells | 485 | 530 |
| AO | DNA | 490 | 528 |
| AO | RNA | 555 | 620 |
| SAEW | NEHW | A1S4 | A2S8 | S3A1S1 | S6A2S2 | |
|---|---|---|---|---|---|---|
| Control | 967.76 ± 3.25 | 967.76 ± 3.25 | 967.76 ± 3.25 | 967.76 ± 3.25 | 967.76 ± 3.25 | 967.76 ± 3.25 |
| 2 min | 102.64 ± 2.01 | 45.18 ± 1.33 | ||||
| 4 min | 32.51 ± 0.51 | 10.45 ± 0.12 | ||||
| 5 min | 0.085 ± 0.013 | 0.077 ± 0.011 | ||||
| 6 min | 9.31 ± 0.12 | 2.26 ± 0.07 | ||||
| 8 min | 3.40 ± 0.0044 | 0.22 ± 0.0021 | ||||
| 10 min | 2.24 ± 0.0084 | 0.16 ± 0.0023 | 0.039 ± 0.0093 | 0.036 ± 0.0032 |
| 0 min | 2 min | 4 min | 6 min | 8 min | 10 min | |
|---|---|---|---|---|---|---|
| SAEW | 60.694 ± 0.23 | 4.842 ± 0.11 | 3.228 ± 0.024 | 2.201 ± 0.022 | 1.198 ± 0.017 | 1.079 ± 0.013 |
| NEHW | 60.694 ± 0.23 | 4.078 ± 0.12 | 2.559 ± 0.013 | 1.789 ± 0.067 | 1.132 ± 0.015 | 0.967 ± 0.032 |
| Zero-Order Reaction | First-Order Reaction | Second-Order Reaction | |||||
|---|---|---|---|---|---|---|---|
| K0/min−1 | R2 | K1/min−1 | R2 | K2/min−1 | R2 | ||
| eDNA | SAEW | 11.3955 | 0.6374 | 0.4954 | 0.9661 | 0.0431 | 0.8778 |
| NEHW | 5.0135 | 0.5746 | 0.7574 | 0.9567 | 0.8453 | 0.7829 | |
| eRNA | SAEW | 0.4778 | 0.9068 | 0.1997 | 0.9619 | 0.0983 | 0.9297 |
| NEHW | 0.3825 | 0.8779 | 0.1847 | 0.9731 | 0.1035 | 0.9729 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Gao, X.; Huang, H.; Hao, J. Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes. Foods 2023, 12, 1361. https://doi.org/10.3390/foods12071361
He J, Gao X, Huang H, Hao J. Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes. Foods. 2023; 12(7):1361. https://doi.org/10.3390/foods12071361
Chicago/Turabian StyleHe, Jialin, Xiangyu Gao, Hanbing Huang, and Jianxiong Hao. 2023. "Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes" Foods 12, no. 7: 1361. https://doi.org/10.3390/foods12071361
APA StyleHe, J., Gao, X., Huang, H., & Hao, J. (2023). Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes. Foods, 12(7), 1361. https://doi.org/10.3390/foods12071361





