Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits
Abstract
1. Introduction
2. Purple Wheat Products
2.1. Bread
2.2. Pasta and Noodle
2.3. Other Cereal Products
2.4. Anthocyanin-Rich Powder
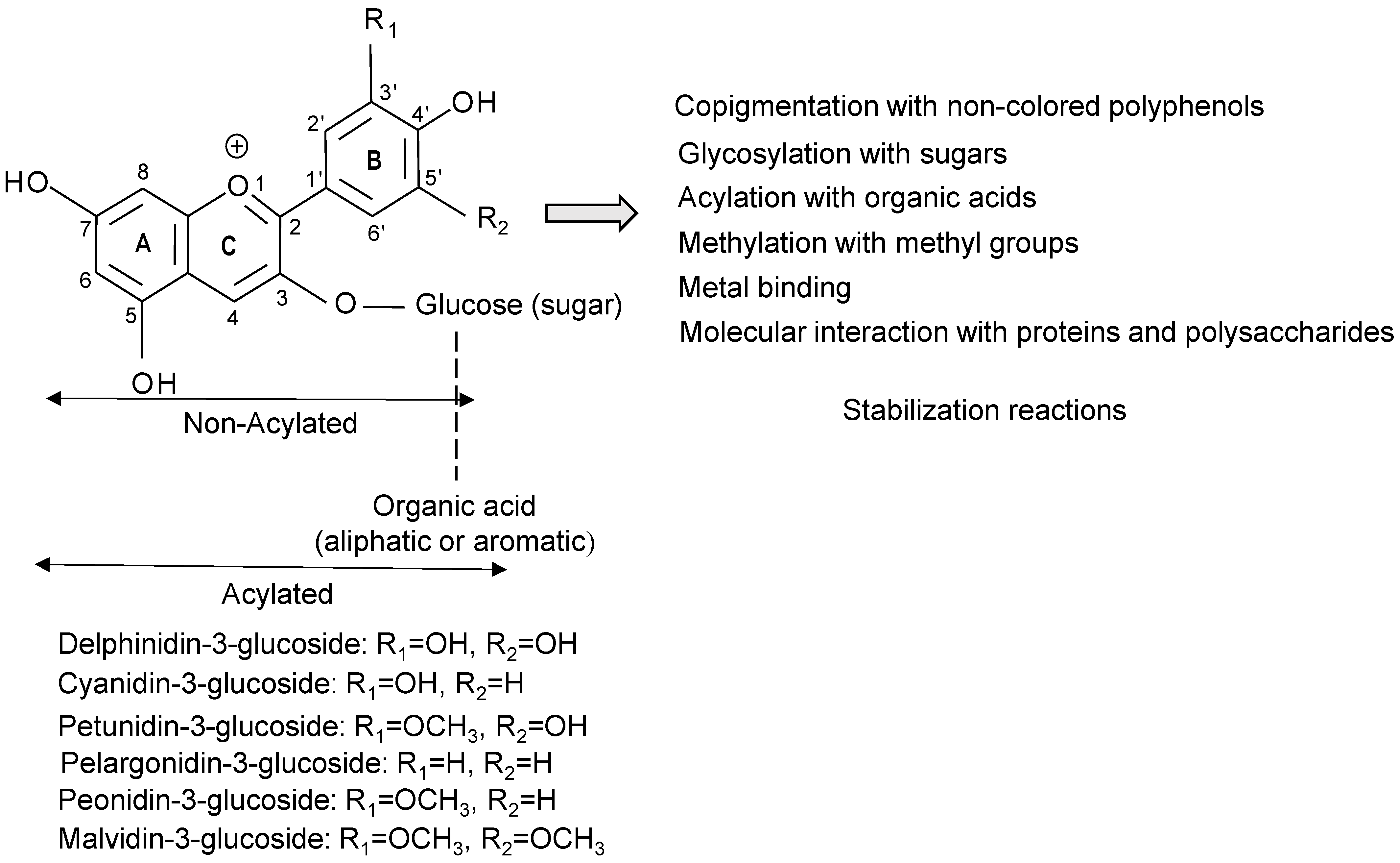
3. Improving Stability of Anthocyanins
4. Health-Enhancing Properties of Purple Wheat Foods
4.1. Antioxidant Properties
4.2. Health Benefits
4.2.1. Animal Studies
4.2.2. Cell Culture Studies
4.2.3. Human Studies
| Wheat Type | Food Type | Model Used | Subjects and Assay | Health Effects | References |
|---|---|---|---|---|---|
| Purple, black, and white wheat | High fat diet supplemented with wheat | Animal study (12 weeks) | Male Swiss albino mice (age 6–7 weeks, 20–22 g) | Both black and purple wheats reduce total cholesterol, triglyceride, and free fatty acid levels in serum, with the restoration of blood glucose and insulin resistance | Sharma et al., 2020 [6] |
| Purple wheat | Bran and anthocyanin-rich powder | In vitro study | ABTS, DPPH, and ORAC assays | Exceptional antioxidant properties | Abdel-Aal et al., 2018 [7] |
| Purple wheat | Bran-enriched crackers and convenience bars | In vitro study | ABTS, DPPH, and ORAC assays | Exceptional antioxidant properties | Gamel et al., 2019 [36] |
| Purple and yellow wheat | Bread | In vitro study | ABTS and DPPH assays | Bread (crust and crumb) made from purple wheat has higher antioxidant activities | Yu and Beta, 2015 [38] |
| Purple wheat and durum semolina | Anthocyanin-rich pasta (25% bran) | Cell culture | Human intestinal epithelial Caco-2 cells | Both types of cooked pasta suppress IL1β-stimulated expression of NF-кB in the cellular model | Parizad et al., 2020 [45] |
| Purple and common wheat | Fresh noodle | In vitro study | ABTS and DPPH assays | Increased antioxidant capacity with the addition of purple wheat bran | Park et al., 2022 [51] |
| Purple wheat | Home- and laboratory-made whole purple wheat infant cereals | In vitro cellular antioxidant activity In vitro cellular proliferation | Primary human fetal small intestine cell line (FHs 74 Int, CCL-241, American Type Culture Collection (ATCC), Manassas, VA) | Higher cellular antioxidant activity than lab made red rice and commercial infant cereals No toxicity against the fetal small intestine cell line | Hirawan et al., 2011 [54] |
| Purple and common wheat | Milled wheat in pelleted form Coarse meal Crushed wheat kernel | Animal study | Wistar Albino male rats (age of 9 weeks, n = 64). Chickens of the hybrid combination COBB 500 (age of 39 days, n = 32) Fingerlings of common carp (Cyprinus carpio L.) (n = 100). | Significant higher antioxidant status in the liver of rats and chickens fed purple wheat No significant differences in hepatopancreas enzymes of fish | Mrkvicová et al., 2016 [81] |
| Purple and regular wheat | Diet containing 60% purple or regular wheat High-fat diet to induce dyslipidaemia | Animal study (6 weeks) | Dyslipidaemic male rats (weight 180–210 g, n = 42) | Reduced triglyceride, total cholesterol and low-density lipoprotein, fatty liver, and mitigation of lipid metabolism disorders and renal injury in groups fed purple wheat diet | Lan et al., 2022 [82] |
| Purple and common wheat | Pelleted feed (purple wheat plus anthocyanin) | Animal study (61 days) | Broiler rabbits (n = 18 HYLA female rabbits, age of 32 days) | No significant effects on plasma biomarkers, oxidative stress enzymes, and antioxidant activity | Stastnik et al., 2019 [83] |
| Purple wheat | Anthocyanin-rich methanol extract | Animal model (Study duration = the whole life span of the Nematodes) | Wild type strain N2 worms of nematode Caenorhabditis elegans and mev-1(hn1) mutants | 10% extension of life span | Chen et al., 2013 [84] |
| Inhibition of insulin/IGF-1-like signaling pathway Increased stress response and reduced oxidative stress | |||||
| Purple and common wheat | Wheat diet | Animal study (5–6 months) | Male mice of C57Bl/6J strain (age 2.5 months, Neurodegenerative disorder induced by central injection of an amyloid beta | Prolong memory extinction and improve neurodegenerative disorder | Tikhonova et al., 2020 [85] |
| Purple, blue, black, and white wheat | Acidified methanol extract | Cell culture | Murine macrophage cell line RAW 264.7 | Reduced nitrite oxide production in lipopolysaccharide-induced pro-inflammatory stress. | Sharma et al., 2018 [86] |
| Inhibition of pro-inflammatory cytokines (TNF-α and IL-1β) | |||||
| Purple wheat | Bran-enriched crackers and convenience bars | Human study— A randomized, semi-blinded crossover acute Study | Healthy participants, 4 servings (6.7 mg anthocyanins and 176–213 mg phenolic acids, plasma antioxidant status and short-term markers of inflammation markers | Few anthocyanin metabolites in urine and none in plasma No short-term impact on plasma antioxidant activity or inflammatory biomarkers, IL-6 and TNF-α | Gamel et al., 2019 [88] |
| Purple wheat and regular wheat | Bran-enriched convenience bars | Human study A randomised, single-blind parallel-arm study for 8 weeks. | Overweight and obese adults (n = 29) with chronic inflammation (high-sensitivity CRP > 1 mg/L) | Significant reduction in IL-6 and increase in adiponectin within the purple wheat group and lower TNF-α in both groups comparing to the starting point | Gamel et al., 2020 [89] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Aal, E.-S.M.; Hucl, P.; Shipp, J.; Rabalski, I. Compositional differences in anthocyanins from blue- and purple-grained spring wheat grown in four environments in Central Saskatchewan. Cereal Chem. 2016, 93, 32–38. [Google Scholar] [CrossRef]
- Knievel, D.C.; Abdel-Aal, E.-S.M.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Lachman, J.; Martinek, P.; Kotíková, Z.; Orsák, M.; Šulc, M. Genetics and chemistry of pigments in wheat grain–A review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Morgounov, A.; Karaduman, Y.; Akin, B.; Aydogan, S.; Baenziger, P.S.; Bhatta, M.; Chudinov, V.; Dreisigacker, S.; Govindan, V.; Güler, S.; et al. Yield and quality in purple-grained wheat isogenic lines. Agronomy 2020, 10, 86. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; Finocchiaro, F. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. spp. turgidum var. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-biofortified colored wheat prevents high fat diet-induced alterations in mice: Nutrigenomics studies. Mol. Nutr. Food Res. 2020, 64, 1900999. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–29. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Garg, M.; Kaur, S.; Sharma, A.; Kumari, A.; Tiwari, V.; Sharma, S.; Kapoor, P.; Sheoran, B.; Goyal, A.; Krishania, M. Rising demand for healthy foods-anthocyanin biofortified colored wheat is a new research trend. Front. Nutr. 2022, 9, 878221. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic digestion, bioavailability, therapeutic effects, current pharmaceutical/industrial use, and innovation potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Joye, I.J. Anthocyanins in whole grain cereals and their potential effect on health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Franco-San Sebastián, D.; Alaniz-Monreal, S.; Rabadán-Chávez, G.; Vázquez-Manjarrez, N.; Hernández-Ortega, M.; Gutiérrez-Salmeán, G. Anthocyanins: Potential therapeutic approaches towards obesity and diabetes mellitus type 2. Molecules 2023, 28, 1237. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, J.M.; Lodge., J.K.; Howatson, G. Dietary intake of anthocyanin and risk of cardiovascular diseases: A systemic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 21, 3809. [Google Scholar] [CrossRef]
- Shipp, J.; Abdel-Aal, E.-S.M. Food applications and physiological effects of anthocyanins as functional food ingredients. The Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. J. Hum. Nutr. Diet. 2019, 32, 578–590. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food system-An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: A review of plant sources, extraction, stability, content determination and modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J.; et al. An updated review on the stability of anthocyanins regarding the interaction with food proteins and polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Belwal, T.; Xu, Y.; Ma, Q.; Li, D.; Li, L.; Xiao, H.; Luo, Z. Updated insights into anthocyanin stability behavior from bases to cases: Why and why not anthocyanins lose during food processing. Crit. Rev. Food Sci. Nutr. 2022, 62, 2063250. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailon, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M. Anthocyanin-Pigmented Grain Products. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; Oxford University Press, Inc.: Washington, DC, USA, 2011; pp. 76–109. [Google Scholar]
- Dhua, S.; Kumar, K.; Kumar, Y.; Singh, L.; Sharanagat, V.S. Composition, characteristics and health promising prospects of black wheat: A review. Trends Food Sci. Technol. 2021, 112, 780–794. [Google Scholar] [CrossRef]
- Gupta, R.; Meghwal, M.; Prabhakar, P.K. Bioactive compounds of pigmented wheat (Triticum aestivum): Potential benefits in human health. Trends Food Sci. Technol. 2021, 110, 240–252. [Google Scholar] [CrossRef]
- Saini, P.; Kumar, N.; Kumar, S.; Mwaurah, P.W.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.k.; Singh, V. Bioactive compounds, nutritional benefits and food applications of colored wheat: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Beta, T. Flour and bread from black-, purple-, and blue-colored wheats. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 59–67. [Google Scholar]
- Li, W.; Beta, T.; Sun, S.; Corke, H. Protein characteristics of Chinese black-grained wheat. Food Chem. 2006, 98, 463–472. [Google Scholar] [CrossRef]
- Sharma, N.; Kumari, A.; Chunduri, V.; Kaur, S.; Banda, J.; Goyal, A.; Garg, M. Anthocyanin biofortified black, blue and purple wheat exhibited lower amino acid cooking losses than white wheat. LWT-Food Sci. Technol. 2022, 154, 112802. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of biofortified colored wheats (purple, blue, black) on physicochemical, antioxidant and sensory characteristics of chapatti (Indian flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef] [PubMed]
- Berghofer, E.; Kreilmayr, I.; Rogenhofer, M.; Mar, A. Functional properties of food products from purple wheat. In Using Cereal Science and Technology for the Benefit of Consumers, Proceedings of the 12th International ICC Cereal and Bread Congress, Harrogate, UK, 24–26 May 2004; Woodhead Publishing: Cambridge, UK, 2005; pp. 344–348. [Google Scholar]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlova, B.; Ostadlova, M.; Smejkal, K.; Vovk, I.; Ulrih, N.P.N.P.; Tremlová, B.; Ošťádalová, M. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Gamel, T.H.; Wright, A.J.; Pickard, M.; Abdel-Aal, E.-S.M. Characterization of anthocyanin-containing purple wheat prototype products as functional foods with potential health benefits. Cereal Chem. 2019, 97, 34–38. [Google Scholar] [CrossRef]
- Eliasova, M.; Kotikova, Z.; Lachman, J.; Orsak, M.; Martinek, P. Influence of baking on anthocyanin content in coloured-grain wheat bread. Plant Soil Environ. 2020, 66, 381–386. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and antioxidant properties of phenolic compounds during production of bread from purple wheat grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanin content of crackers and bread made with purple and blue wheat varieties. Molecules 2022, 27, 7180. [Google Scholar] [CrossRef]
- Seo, Y.; Moon, Y.; Kweon, M. Effect of purple-colored wheat bran addition on quality and antioxidant property of bread and optimization of bread-making conditions. Appl. Sci. 2021, 11, 4034. [Google Scholar] [CrossRef]
- Klupsaite, D.; Kaminskaite, A.; Rimsa, A.; Gerybaite, A.; Stankaityte, A.; Sileikaite, A.; Svetlauskaite, E.; Cesonyte, E.; Urbone, G.; Pilipavicius, K.; et al. The contribution of new breed purple wheat (8526-2 and 8529-1) varieties wholemeal flour and sourdough to quality parameters and acrylamide formation in wheat bread. Fermentation 2022, 8, 724. [Google Scholar] [CrossRef]
- Szoke-Trenyik, E.; Mihalkó, J.; Sipos, P.; Szabó, B.P. Development of high-fibre, ready-to-bake flour mixtures from purple wheat. Processes 2023, 11, 389. [Google Scholar] [CrossRef]
- Král, M.; Pokorná, J.; Tremlová, B.; Ošťádalova, M.; Trojan, V.; Vyhnánek, T.; Walczycka, M. Colored wheat: Anthocyanin content, grain firmness, dough properties, bun texture profile. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018, 66, 685–690. [Google Scholar] [CrossRef]
- Björck, I.; Liljeberg, H.; Östman, E. Low glycaemic-index foods. Br. J. Nutr. 2000, 83, 149S–155S. [Google Scholar] [CrossRef] [PubMed]
- Parizad, P.A.; Marengo, M.; Bonomi, F.; Scarafoni, A.; Cecchini, C.; Pagani, M.A.; Marti, A.; Iametti, S. Bio-functional and structural properties of pasta enriched with a debranning fraction from purple wheat. Foods 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, M.; Parizad, P.A.; Lavelli, V.; Cecchini, C.; Menesatti, P.; Marti, A.; Pagani, M.A. Debranning of purple wheat: Recovery of anthocyanin-rich fractions and their use in pasta production. LWT-Food Sci. Tech. 2017, 75, 663–669. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; Leonardis, A.M.D.; Giovanniello, V.; Nobile, M.A.D.; Padalino, L.; Lecce, L.; Borrelli, G.M.; Vita, P.D. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Pompei, F.; Bonfini, M.; Mustafa, A.M.; Sagratini, G.; Wang, Z.; Vittadini, E. Quality of wholemeal pasta made with pigmented and ancient wheats. Int. J. Gastron. Food Sci. 2023, 31, 100665. [Google Scholar] [CrossRef]
- Song, X.; Zhu, W.; Pei, Y.; Ai, Z.; Chen, J. Effects of wheat bran with different color on the qualities of dry noodles. J. Cereal Sci. 2013, 58, 400–407. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Park, G.; Cho, H.; Kim, K.; Kweon, M. Quality characteristics and antioxidant activity of fresh noodles formulated with flour-bran blends varied by particle size and blend ratio of purple-colored wheat bran. Processes 2022, 10, 584. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Hirawan, R.; Diehl-Jones, W.; Beta, T. Comparative evaluation of the antioxidant potential of infant cereals produced from purple wheat and red rice grain and LC-MS analysis of their anthocyanins. J. Agric. Food Chem. 2011, 59, 12330–12341. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.M.; Abou-Arab, A.A.; Gamel, T.H.; Hucl, P.; Young, J.C.; Rabalski, I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J. Agric. Food Chem. 2008, 56, 11171–11177. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sanch-Garcia, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Chen, L.; Chen, N.; He, Q.; Sun, Q.; Zeng, W.-C. Effect of casein on the stability, antioxidant activity and bioavailability of lotus anthocyanins. J. Food Biochem. 2022, 46, e14288. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT-Food Sci. Tech. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Gauche, C.; Malagoli, E.S.; Luiz, M.T.B. Effect of pH on the copigmentation of anthocyanins from Cabernet Sauvignon grape extracts with organic acids. Sci. Agric. 2010, 67, 41–46. [Google Scholar] [CrossRef]
- Tian, X.-Z.; Wang, X.; Ban, C.; Luo, Q.-Y.; Li, J.-X.; Lu, Q. Effect of purple corn anthocyanin on antioxidant activity, volatile compound and sensory property in milk during storage and light prevention. Front. Nutr. 2022, 9, 862689. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Provenzano, S.; Spelt, C.; Hosokawa, S.; Nakamura, N.; Brugliera, F.; Demelis, L.; Geerke, D.P.; Schubert, A.; Tanaka, Y.; Quattrocchio, F.; et al. Genetic control and evolution of anthocyanin methylation. Plant Physiol. 2014, 164, 962–977. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chem. 2016, 208, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.; Steiner, B.; Schantz, M.; Oidtmann, J.; Mader, K.; Richling, E.; Kulozik, U. Antioxidant capacity of bilberry extract microencapsulated in whey protein hydrogels. Food Res. Int. 2012, 47, 51–57. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Liu, J.N.; Tan, Y.B.; Zhou, H.L.; Mundo, J.L.M.; McClements, D.J. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J. Food Eng. 2019, 254, 1–9. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Marostica Junior, M.R. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Enaru, B.; Dretcanu, G.; Daria Pop, T.; Stanila, A.; Diaconneasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compar. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; Pacheco-Hernandez, M.D.L.; Paez-Hernandez, M.E.; Rodriguez, J.A.; Galan-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanin in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Bartłomiej, S.; Justyna, R.K.; Ewa, N. Bioactive compounds in cereal grains–occurrence, structure, technological significance and nutritional benefits—A review. Food Sci. Technol. Inter. 2012, 18, 559–568. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; Garcia-Viguers, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1730. [Google Scholar] [CrossRef] [PubMed]
- Grausgruber, H.; Atzgersdorfer, K.; Bohmdorefr, S. Purple and blue wheat-Health promoting grains with increased antioxidant activity. Cereal Foods World 2018, 63, 217–220. [Google Scholar]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Júnior, M.R.M. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef] [PubMed]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant capacity and profiles of phenolic acids in various genotypes of purple wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Mrkvicova, E.; Pavlata, L.; Karasek, F.; Šťastník, O.; Doležalová, E.; Trojan, V.; Vyhnánek, T.; Hřivna, L.; Holeksová, L.; Mareš, J.; et al. The influence of feeding purple wheat with higher content of anthocyanins on antioxidant status and selected enzyme activity of animals. Acta Vet. Brno 2016, 85, 371–376. [Google Scholar] [CrossRef]
- Lan, S.; Meng, Y.; Wang, M.; Yang, J.; Li, G.; Mou, R.; Zhang, Y.; Li, X.; Chen, F.; Bi, R.; et al. Purple wheat alleviates dyslipidaemia in rat model. Food Sci. Technol. 2022, 42, e01021. [Google Scholar] [CrossRef]
- Stastnik, O.; Mrkvicova, E.; Pavlata, L.; Anzenbacherova, E.; Prokop, J.; Roztocilova, A.; Umlaskova, B.; Novotny, J.; Metnarova, E.; Vyhnanek, T.; et al. Purple wheat as a source of anthocyanins and its effect on the metabolism of rabbits. Vet. Med. 2019, 64, 539–546. [Google Scholar] [CrossRef]
- Chen, W.; Müller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the effects of grain of isogenic wheat lines differing in the content of anthocyanins in mouse models of neurodegenerative disorders. Nutrients 2020, 12, 3877. [Google Scholar] [CrossRef]
- Sharma, S.; Chunduri, V.; Kumar, A.; Kumar, R.; Khare, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin bio-fortified colored wheat: Nutritional and functional characterization. PLoS ONE 2018, 13, e0194367. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Gamel, T.H.; Wright, A.J.; Tucker, A.J.; Pickard, M.; Rabalski, I.; Podgorski, M.; Di Ilio, N.; O’Brien, C.; Abdel-Aal, E.-S.M. Absorption and metabolites of anthocyanins and phenolic acids after consumption of purple wheat crackers and bars by healthy adults. J. Cereal Sci. 2019, 86, 60–68. [Google Scholar] [CrossRef]
- Gamel, T.H.; Abdel-Aal, E.-S.M.; Tucker, A.J.; Pare, S.M.; Faughnan, K.; O’Brien, C.D.; Dykun, A.; Rabalski, I.; Pickard, M.; Wright, A.J. Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein: A randomised, single-blind parallel-arm study. Br. J Nutr. 2020, 124, 1179–1189. [Google Scholar] [CrossRef]
- Chen, K.; Kortesniemi, K.M.; Linderborg, K.M.; Yang, B. Anthocyanins as promising molecules affecting energy homeostasis, inflammation, and gut microbiota in type 2 diabetes with special reference to impact of acylation. J. Agric. Food Chem. 2023, 71, 1002–1017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamel, T.H.; Saeed, S.M.G.; Ali, R.; Abdel-Aal, E.-S.M. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods 2023, 12, 1358. https://doi.org/10.3390/foods12071358
Gamel TH, Saeed SMG, Ali R, Abdel-Aal E-SM. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods. 2023; 12(7):1358. https://doi.org/10.3390/foods12071358
Chicago/Turabian StyleGamel, Tamer H., Syed Muhammad Ghufran Saeed, Rashida Ali, and El-Sayed M. Abdel-Aal. 2023. "Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits" Foods 12, no. 7: 1358. https://doi.org/10.3390/foods12071358
APA StyleGamel, T. H., Saeed, S. M. G., Ali, R., & Abdel-Aal, E.-S. M. (2023). Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods, 12(7), 1358. https://doi.org/10.3390/foods12071358






