3.1. Protein Texturisation
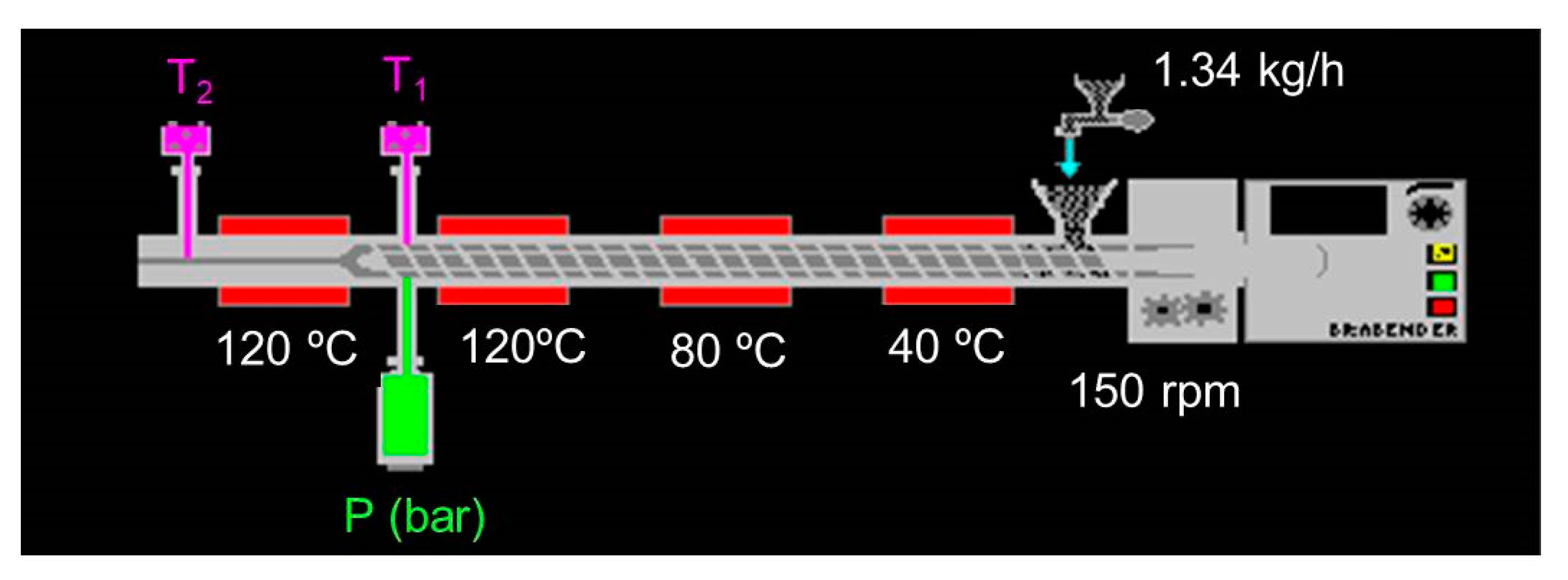
Table 3 shows the process control parameters for texturing protein mixtures and PP. Barrel temperatures (T
1 and T
2) and melt pressure (P) were monitored during extrusion. Specific mechanical energy (SME) can be defined as the energy required to produce 1 g of extrudate [
40]. It was calculated [
41] from torque (C, N m), screw speed (V, rad s
−1) and the mass flow rate (Q, g s
−1) with Equation (5).
The addition of L, S and C caused a significant (
p < 0.05) increase in SME. Other studies have shown that feed composition significantly affects SME. Specifically, they indicate that a higher carbohydrate content in mixtures caused higher SME [
40]. In the formulation, samples containing L, S or C have more carbohydrates than PP (only protein). SME values order the enriched samples from highest to lowest: S > C > L. This is likely due to the composition of the raw material, since, as indicated above, the feed composition exhibits a strong effect on SME [
42].
T1, T2 and P showed no significant (p > 0.05) differences when L, S or C were added into the mixtures.
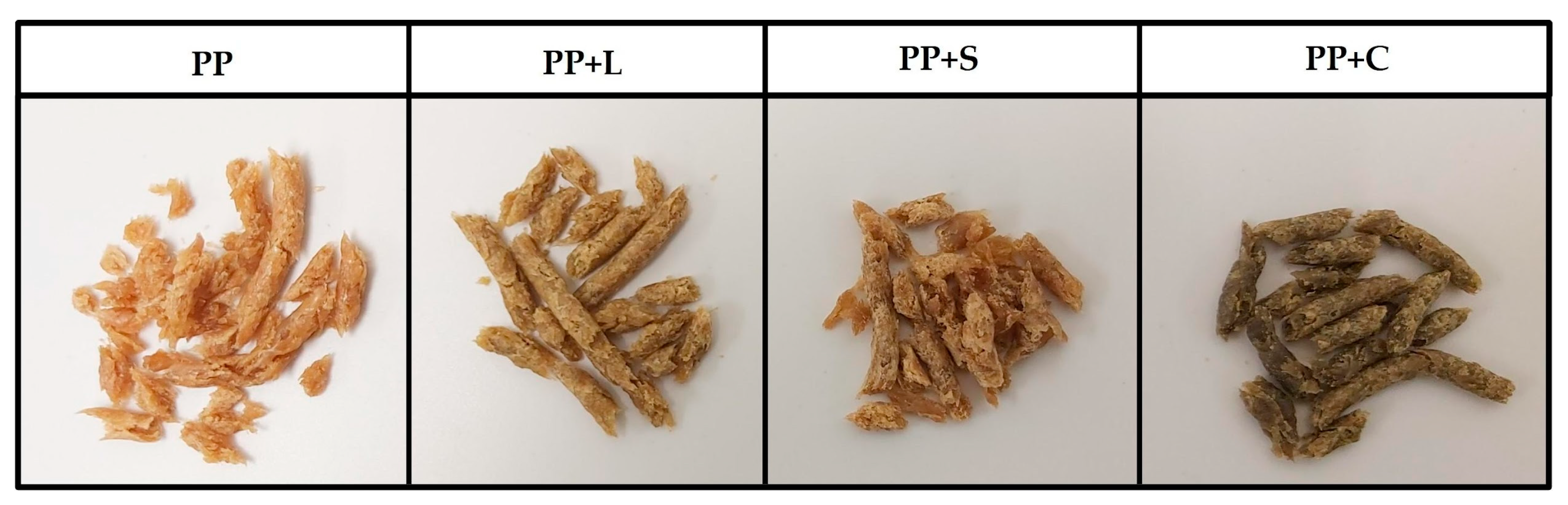
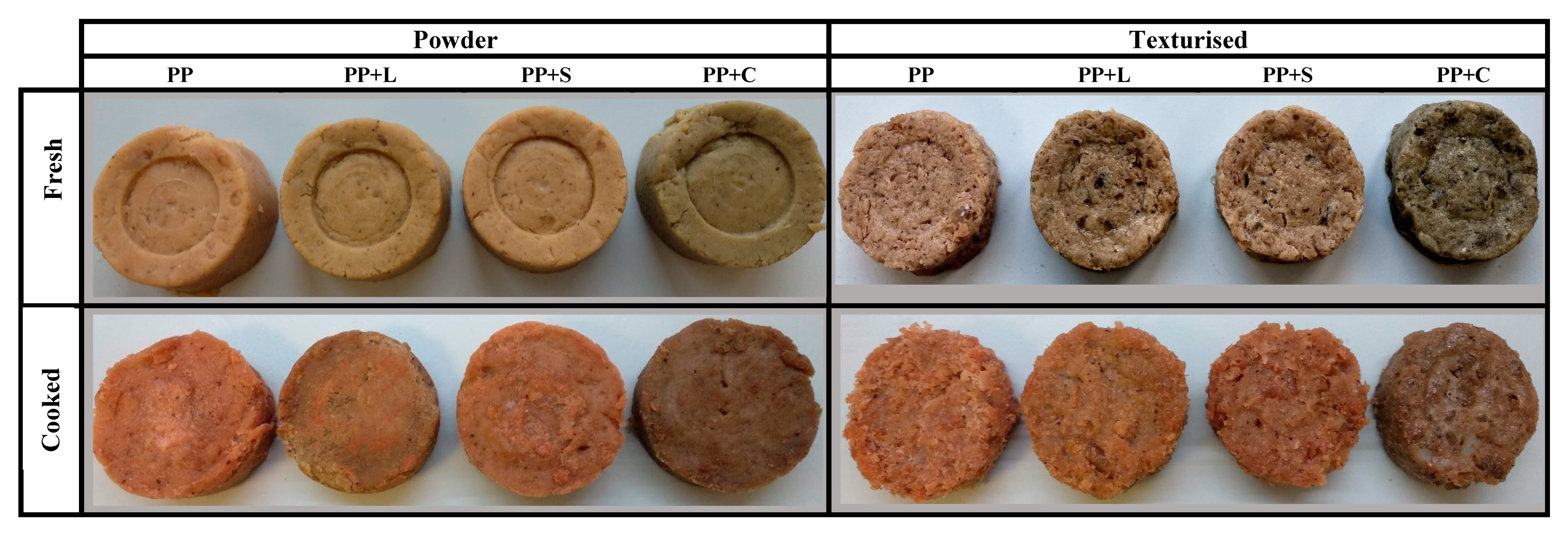
The appearance of the samples obtained after the texturisation process can be seen in
Figure 2. They all show fibrous structures typical of texturised protein [
5]. The wetted proteins were plasticised in the extruder barrel during extrusion by applying heat, pressure and mechanical shear. The plasticised dough was then pushed through the die openings, during which the water in the dough partially evaporated, and protein molecules rapidly aligned to generate fibrous textures [
43]. Native protein structures were altered in response to extrusion energy, resulting in denaturation, conformational changes and alterations in technological properties [
5,
10].
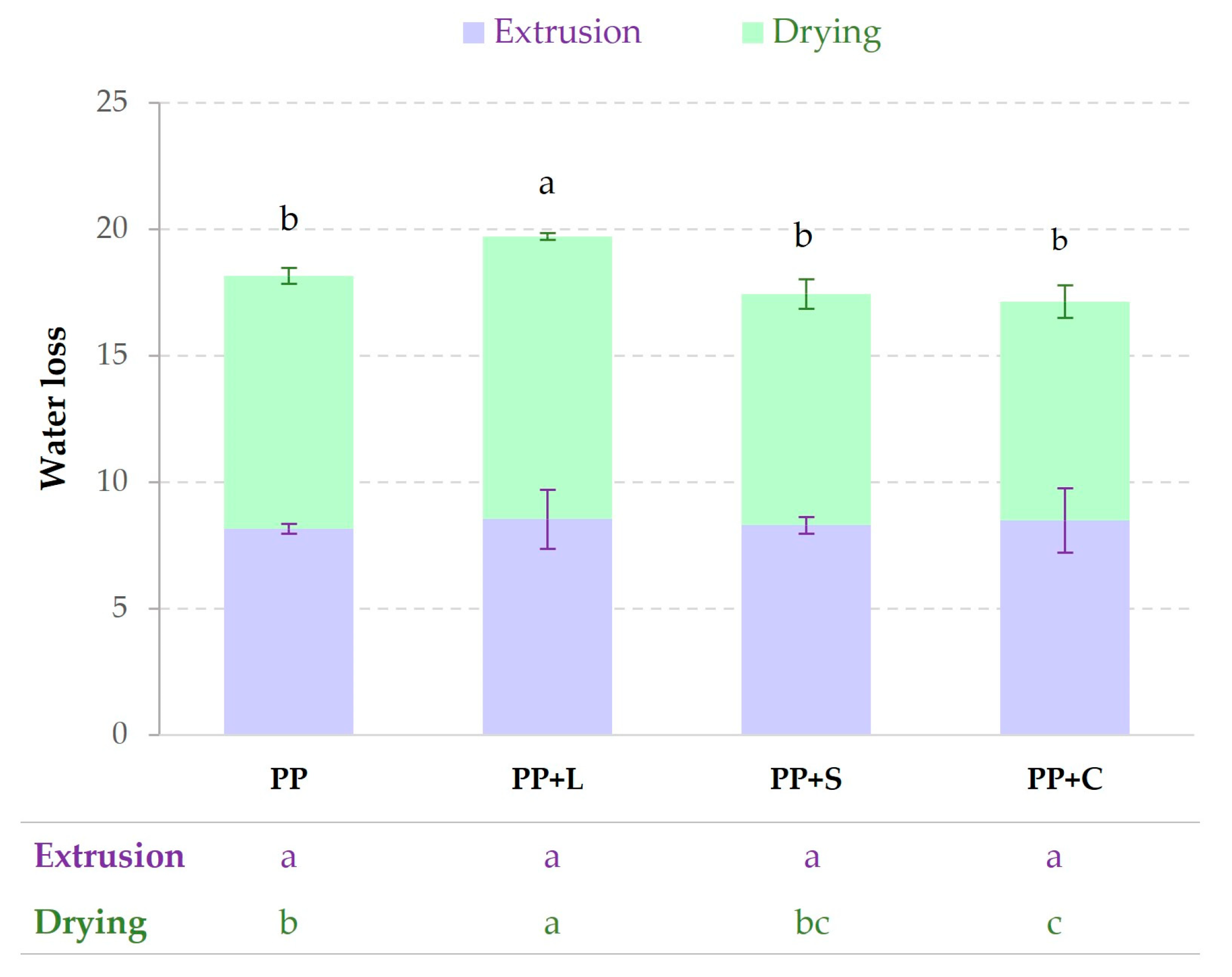
The wet protein was extruded and dried to obtain the samples, as described in
Section 2.3. During extrusion, water was evaporated from the sample, and during the process of drying, the sample suffered water losses.
Figure 3 shows the water losses in both stages (extrusion and drying) before the final product was obtained. No significant (
p > 0.05) differences in water losses during extrusion were observed. The total water losses of PP+L showed significant (
p < 0.05) differences due to drying, probably because the water was less bound to the matrix than in the other samples.
The moisture and hygroscopic character of a sample are indicators of the stability of the samples.
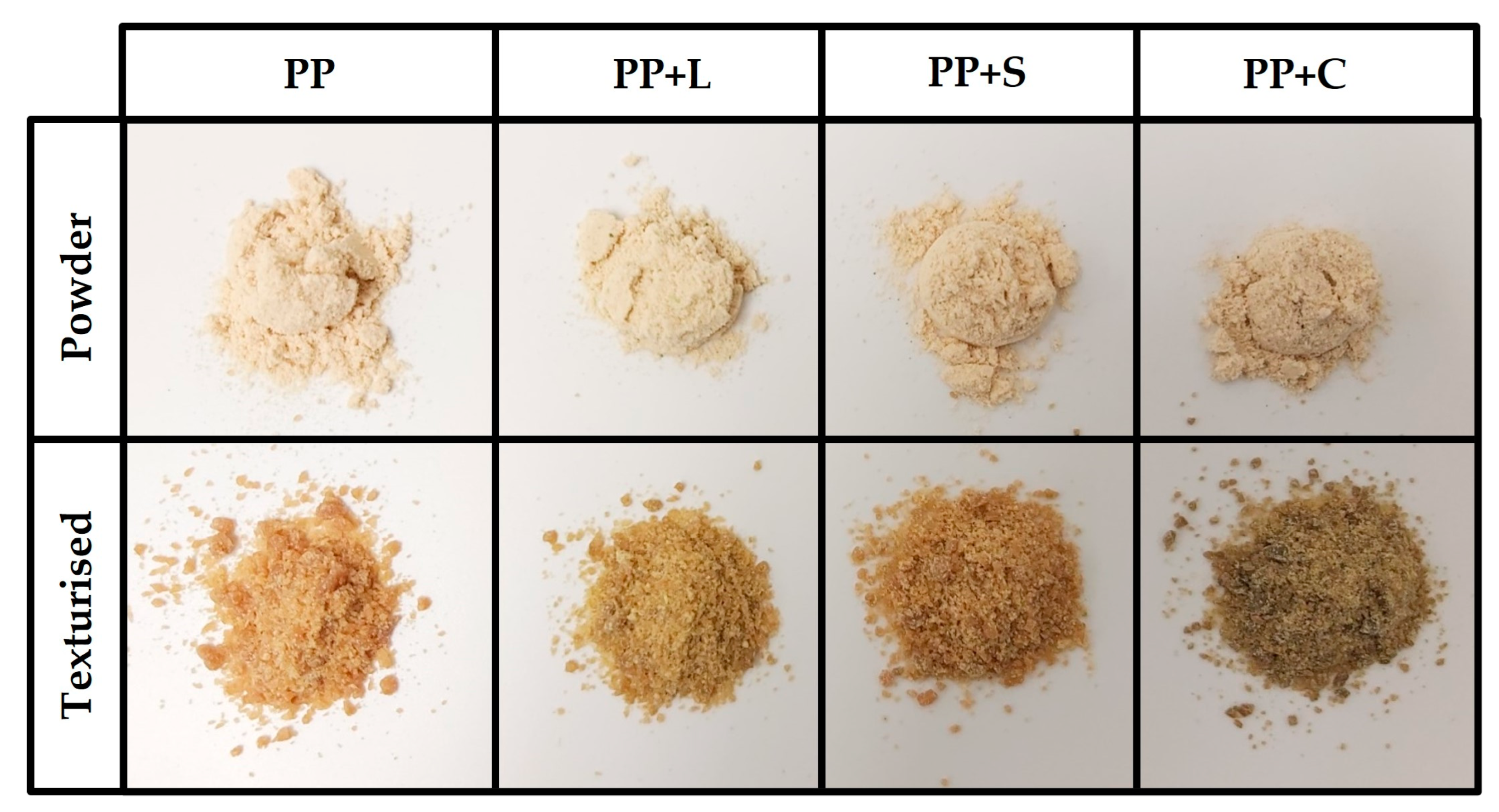
Table 4 shows the mean values and deviations of the moisture and hygroscopicity of the samples. In the protein texturisation process, water is added before the texturisation process, which is then lost in the stages mentioned above. However, the texturised samples had significantly (
p < 0.05) higher water contents than untextured powder samples. Comparing the powdered samples, PP+C was the least moist. In the case of the texturised samples, the least moist was PP+L, as it lost more water than the others in the texturisation process (
Figure 3). When the samples were stored for 1d in an environment with 81% relative humidity, the textured samples took up approximately half of the water content from the environment that the untextured powder samples did. They are, therefore, more stable, as less water uptake makes them less favourable to degradation reactions. The hygroscopicity values of the textured samples were significantly (
p < 0.05) lower than the non-textured samples for the three studied times.
Figure 4 shows the behaviour of the samples in contact with water (WAI, WSI and SWE) and oil (FAI). The WAI values were similar for textured and non-textured samples. PP+L showed significant differences as a function of texturisation, with a higher WAI in the texturised sample. PP+C showed the opposite trend, with the texturised sample showing the lowest WAI. In terms of formulation, the non-texturised powder sample with the highest WAI was PP+C; however, it was PP+L for the texturised samples. WSI refers to water-solubilised components released during texturisation [
44]. Texturisation reduced the number of soluble components released, as indicated by WSI values in
Figure 4, thus reducing the molecular degradation in samples because there are fewer soluble particles to react with water and degrade the matrix. Protein solubility is often a vital indicator of the degree of protein texturisation [
45]. After extrusion cooking, the protein is thermally denatured with a series of cleavages and aggregations, decreasing the soluble protein content. Thus, texturised proteins with lower solubility than their native counterparts are often observed [
10,
44]. This effect was observed in maize samples fortified with cowpea as a protein source [
46].
Figure 4 also shows the SWE values, which decreased significantly (
p < 0.05) when the samples were textured. This decrease in SWE was observed in all samples equally.
Texturisation of the samples led to a significant (
p < 0.05) decrease in FAI in all samples (
Figure 4), which is in agreement with the results obtained by Sotelo-Díaz et al. [
46] that FAI was reduced with the extrusion of corn samples with cowpea. Extrusion processing caused a decrease in the number of available hydrophobic sites in the samples. This may be due to the higher extrusion temperature. When protein denaturation takes place together with aggregation, interactions among hydrophobic groups occur during the formation of aggregates, leading to an overall decrease in the hydrophobicity of the product. Owing to this interaction, blocking of the possible hydrophobic sites that can react with oil may occur [
47]. On the other hand, adding L, S and C to untextured PP powder increased FAI, as its composition includes fibres and carbohydrates that facilitate oil absorption.
Table 5 shows the colour coordinates and the differences between samples due to the formulation effect or texturisation effect. Texturisation decreased significantly (
p < 0.05) Texturisation decreased significantly (
p < 0.05) L* coordinate and increased significantly (
p < 0.05) b* and C*. The samples darkened and lost their yellowish tones after texturisation. In terms of the colour differences induced by texturisation (ΔE
1), the most significant differences were observed in PP, followed by PP+S and PP+C, and, finally, PP+L. All of these differences were perceptible to the human eye, as they consisted of more than 3 units of change [
48]. The colour perception of the samples can be seen in
Figure 5, and the effect of texturisation on the colour appearance of the samples is evident. The addition of L, S or C to the protein powder decreased L* and a*. Concretely, the addition of L or S to the protein powder showed no changes perceptible to the human eye in the samples (ΔE
2 > 3 units, [
48]). However, using C increased the perceptibility of the colour differences (ΔE
2 > 3 units). The effect of enrichment with L, S or C in the textured samples caused perceptible changes compared to PP (ΔE
2 > 3 units).
3.2. Application as a Substitute for Meat in Hamburgers
Table 6 shows the results of the instrumental colour- and water-holding capacities (WHC) of the different hamburger formulations. The CIELab colour showed significant differences (
p < 0.05) between formulations (PP, PP+L, PP+S and PP+C) in both formats (powder and texturised), with the PP+C sample having the lowest values for all colour coordinates except for the hue angle, which was higher (
p < 0.05). This formulation produced darker final products, with lower lightness (L*) and C* and higher h* values, with less reddish and yellowish and more greenish colourations. This decrease in the L* value and these colourations was mainly due to the different colours of the chlorophyll pigments (green and blue-green) of the microalgae species used to enrich the pea protein extract compared to the colour of the legume [
19]. The formulation with pea only (PP) had the highest score for brightness and reddish colour (a*), and the lowest for hue (h*), followed by the PP+S formulation. For the b* and C* coordinates, both formulations showed similar values (
p ≥ 0.05). In general, the colour changes in the formulations were closely related to the chlorophyll content of the ingredients used in the fortification (lucerne, spinach and Chlorella) of each formulation. The main source of chlorophyll was algae, followed by cereals such as lucerne, and, to a lesser extent, green leafy vegetables such as spinach [
49]. Similar results were obtained by Žugčić et al. [
7], who observed how adding Chlorella or spirulina into the extrudate mixtures affected the colour parameters. The higher the concentration of chlorophyll pigments, the lesser the brightness and reddish colouring of meat hamburgers.
Regarding the extrusion process, significant changes (
p < 0.05) were observed in all colour coordinates, except h* for the enriched formulations and L* for the PP+ L and PP+C formulations (
p ≥ 0.05). There were significantly more differences in L*, a*, b* and C* in the vegetal hamburgers produced with powder than with texturised protein, and fewer differences in h* in the PP formulation. Therefore, extrusion affected the heat-sensitive pigments of the samples. Colour is highly dependent on raw materials and extrusion process parameters, as the high temperature used during the extrusion process involves pigment degradation [
50]. Consequently, at a high extrusion temperature of 170 °C, Igual et al. [
51] found a decrease in the L*, a*, b* and C* values and an increase in the tone of corn extrudates added with lucerne, as was the case in our study. In addition, the colour of extruded products is also affected by food moisture. In the work of Selani et al. [
52], the researchers observed a decrease in lightness and redness with higher moisture content in extrudates with pomace.
Water-holding capacity (WHC) is a fundamental property of meat products. It has a significant influence on the performance and sensory acceptability of the product, as it measures the ability of the protein to retain water by capillary and tensile force and to form the protein gel network [
8], so it is of great importance in meat analogues. Among the formulations (PP, PP+L, PP+S and PP+C), significant differences (
p < 0.05) were observed for both formats. PP+S and PP+C showed higher water-holding capacities in the powder samples, as PP+L and PP+S did in the texturised ones; these results coincide with those obtained for the water absorption index (WAI). The WHC depends on the amino acid composition and protein–polysaccharide interactions, which are based on electrostatic forces, hydrogen bonds and the microstructure of the binding agent, but are also related to the presence of fibres [
53]. The addition of fibre has been shown to improve the water-holding capacity and stability of emulsions during storage [
9,
18]. Thus, our results indicate this, since the ingredients used for the fortification of the formulations were rich sources of fibre, especially lucerne and spinach, with fibre contents of 3.5 (forage) and 27.3 (leaf meal) g/100 g for lucerne [
54] and 6.3 g/100 g for spinach [
55].
Although differences between formulations were observed, these were not very pronounced, with all formulations obtaining high WHC values around 91.99–93.76% in the powder and 82.23–86.30% in the texturised samples. Similar results were observed by Bakhsh et al. [
30], who studied the technological properties of different concentrations of methylcellulose in soybean patties. They found that the use of high concentrations of methylcellulose (3–4%) reduced water release and cooking losses, which increased the water-holding capacity of the samples. Methylcellulose has high binding and moisture retention capacities, as it is a potent gelling and thickening agent that increases volume and improves the texture of processed meat and meat analogues [
56].
Regarding the source of protein application in vegetal hamburgers (powder and texturised), the results showed that burgers made with powder had a higher water retention capacity in all formulations (
p < 0.05). Protein isolate powder can absorb more water in its native globular structure than during protein denaturation after extrusion [
57]; similar results were observed by Kaleda et al. [
22] in oat and pea protein meat analogues.
Table 6.
Mean values (and standard deviations) of colour coordinates and WHC of fresh vegetal hamburgers manufactured using enriched pea protein (PP, PP+L, PP+S and PP+C). PP, pea protein; PP+L, pea protein with lucerne; PP+S, pea protein with spinach; PP+C, pea protein with chlorella.
Table 6.
Mean values (and standard deviations) of colour coordinates and WHC of fresh vegetal hamburgers manufactured using enriched pea protein (PP, PP+L, PP+S and PP+C). PP, pea protein; PP+L, pea protein with lucerne; PP+S, pea protein with spinach; PP+C, pea protein with chlorella.
| | Sample | L* | a* | b* | C* | h* | WHC |
|---|
| Powder | pp | 58.14 (1.15) aA | 8.22 (0.26) aA | 13.19 (0.83) aA | 15.54 (0.80) aA | 58.06 (1.27) dB | 92.33 (1.12) bA |
| PP+L | 55.10 (1.54) bA | 4.55 (0.07) cA | 12.29 (1.37) aA | 13.11 (1.29) bA | 69.57 (1.91) bA | 91.99 (0.61) bA |
| PP+S | 57.41 (0.83) aA | 6.43 (0.19) bA | 13.27 (1.05) aA | 14.75 (0.99) aA | 64.10 (1.47) cA | 93.76 (0.99) aA |
| PP+C | 51.78 (0.99) cA | 2.13 (0.16) dA | 9.13 (0.90) bA | 9.38 (0.88) cA | 76.81 (1.51) aA | 92.91 (0.67) abA |
| Texturised | pp | 56.67 (0.57) aB | 6.85 (0.48) aB | 11.95 (0.75) aB | 13.78 (0.78) aB | 60.17 (1.81) dA | 83.39 (0.43) bB |
| PP+L | 55.29 (1.42) bA | 4.33 (0.23) cB | 11.39 (0.64) abB | 12.19 (0.56) bB | 69.17 (1.82) cA | 86.30 (1.82) aB |
| PP+S | 54.69 (0.44) bB | 5.04 (0.27) bB | 10.28 (1.27) bB | 11.45 (1.23) bB | 63.75 (2.02) bA | 85.04 (0.43) abB |
| PP+C | 51.97 (1.09) cA | 1.85 (0.12) dB | 7.89 (1.20) cB | 8.10 (1.19) aB | 76.62 (1.68) aA | 83.23 (1.91) bB |
The physical and mechanical properties of the cooked patties are presented in
Table 7. Regarding the CIELab colour of the cooked hamburger formulations, all colour coordinates showed similar trends to those described for the fresh vegetal hamburgers (
p < 0.05), with the formulation enriched with Chlorella showing the lowest values for L*, a*, b* and C* and the highest values for h* (
p < 0.05). During the extrusion process, changes were also observed in the colour coordinates, with the burgers made with powder showing the highest scores for these coordinates (
p < 0.05). Chroma being the only colour parameter that showed significant differences between the two protein formats in all the hamburger formulations (PP, PP+L, PP+S and PP+C).
Figure 6 shows digital photographs of the same vegetal hamburger formulations before and after cooking. Although the cooked and fresh samples showed similar trends, the cooking process was evident in the colour coordinates, with the cooked vegetal hamburgers showing lower lightness (L*), yellow (b*) and hue (h*) in their colouring and higher reddish (a*) colouring, while the chroma scores were very similar to those of the fresh vegetal hamburgers. During cooking, the high temperatures can degrade the pigments in the samples, especially chlorophyll, which is very sensitive to high temperatures. Heat disintegrates the cellular structure of the chlorophyll and leaves the pigment exposed to various enzymatic and non-enzymatic reactions that brown the product by conversion to pheophytins (devoid of their magnesium ion in the porphyrin ring) [
58]. Hence, cooked patties have lower brightness values and less green colour than fresh vegetal hamburgers. In addition, other reactions occur during cooking, such as protein denaturation and aggregation, water evaporation and Maillard reactions, which could contribute to the changes in the samples [
59]. Maillard reactions occur during heating between amino acids and reducing sugars, causing caramelisation of the samples and giving an orange and brownish colouring [
60].
In meat products, the reactions of water evaporation, denaturation and protein aggregation also affect the ability of the emulsion to bind water and fat, and are, therefore, responsible for cooking losses (CL). Regarding CL, significant differences were found between the formulations for both protein formats (
p < 0.05), with the PP+S and PP+C formulations showing fewer losses in powder and PP+L and PP+S in texturised samples. These results coincide with those obtained for WHC, as it is associated with the weight losses of meat products during cooking [
8]. Therefore, the formulations that presented greater WHCs were the ones that showed the lowest cooking losses. Angiolillo et al. [
13] established that adding fibres to non-meat protein ingredients reduces weight loss, as some dietary fibres can strengthen the connections between the different components of the matrix by preventing water diffusion after cooking [
61].
Among the protein formats, it was observed that powders showed the lowest cooking losses compared to texturised proteins (
p < 0.05) at around 10–14% and 25–28%, respectively. These differences may have to do with the greater ability of protein isolate powders to absorb water into their structures than proteins denatured during extrusion [
57]. In general, with texturised vegetable proteins, the cooking losses obtained were higher than those reported by other authors for commercial and non-commercial soy or pea vegetable burgers, which were around 10–15% CL [
10,
30,
59]. These lower losses could be attributed to high concentrations or a mixture of binding agents during the production of vegetal hamburgers, such as gums, wheat gluten and corn starch, and not only methyl cellulose as used in this study; these contribute to emulsion stability [
59].
In terms of the textural properties (TPA) of the cooked vegetal hamburgers, there were no significant differences (
p > 0.05) for the parameters of hardness, adhesiveness, resilience, cohesiveness, elasticity or gumminess between any of the formulations made with protein powder. For those made with texturised protein, no significant differences (
p > 0.05) were observed for the parameters of hardness, adhesiveness, elasticity or gumminess, but significant differences were observed for resilience, cohesiveness and chewiness (
p < 0.05), with the PP formulation showing the highest values for resilience and PP and PP+S for cohesiveness. The chewiness was the only parameter affected by formulation (
p < 0.05) in both protein formats, being higher in PP+S and PP+L formulations in the powder and in PP, PP+S and PP+C in the texturised samples. Resilience measures how fast and strong the recovery is, and cohesiveness indicates the strength of the internal bonds to hold together; in meat analogues, it is the proteins that mainly contribute to the three-dimensional internal structure through hydrophobic interactions, and the structure is stabilised by hydrogen and disulphide bonds [
62]. The moisture and fibre content of vegetal hamburgers are factors responsible for their mechanical properties; decreasing the moisture content and increasing the fibre content of the food would increase its toughness and chewiness [
8]. López-López et al. [
63] showed that the addition of seaweed extracts (
H. elongata) rich in fibre leads to an increase in the hardness and chewiness values, while it reduces the elasticity and cohesion of sausages. In the work of Zhou et al. [
59], they obtained similar results to ours in terms of hardness, cohesiveness, gumminess and adhesiveness in commercial vegetable burgers, with a hardness of 440 g for vegetal hamburgers made with textured pea protein and 1300 g for those made with soybean flour. However, resilience, elasticity and chewiness had higher scores than in our study [
59]; elasticity has been found to correlate with higher protein concentrations and, thus, greater chewiness [
17].
Among protein formats, the powder had the highest values for hardness, resilience, cohesiveness, elasticity, gumminess and chewiness (
p < 0.05). The toughness of the vegetal hamburgers appears to be related to their cooking losses and water-holding capacities, as toughness tends to decrease as the moisture content of the samples increases [
8]. In particular, the cooking losses of the protein powder samples were lower than those of the texturised samples. This may be due to their denser structure; as they had higher oil absorption capacities, they could better capture the oil incorporated in the emulsion and form a network more resistant to deformation during cooking. Protein–lipid and carbohydrate–lipid interactions stabilised the structure more effectively [
64].
The results of the sensory analysis are presented in
Table 8. Statistically significant differences (
p < 0.05) were observed in all the sensory attributes analysed, both for the effects of the protein format (PF: powder and texturised) and hamburger formulations (FM: PP, PP+L, PP+S and PP+C) and for the interaction between them (PF × FM). However, significant differences were not found for the fibrousness attributes of the two formulations, nor for the interactions of FP and FM (
p ≥ 0.05) or for the hardness of FP x FM (
p ≥ 0.05). In terms of the appearance of the burgers, it could be seen that as the chlorophyll content increased, the colouring of the formulations darkened. PP+C showed the darkest colour, followed by PP+L and PP+S, with the formulation without enrichment (PP) showing the colouring most characteristic of a pea-based hamburger. As seen in the instrumental colour coordinates, this is mainly due to the higher concentration of chlorophyll pigments in Chlorella [
7]. Regarding brightness, the PP+C sample had the highest values for this attribute (
p < 0.05) compared to the other formulations, which did not differ from each other (
p ≥ 0.05). Brightness is determined by the amount of water or fat on the surface of the food that is capable of reflecting light [
65]; the formulation enriched with Chlorella had the lowest water retention capacity and the highest cooking losses, so when cooked, it released more water to the surface of the hamburger, providing a gloss effect. No significant differences were observed in the fibrousness between formulations (
p ≥ 0.05), with the panellists giving the same fibrousness score to all formulations. Regarding the effect of protein format, the texturised samples showed higher colour tones due to Maillard reactions during extrusion [
58], as well as higher brightness and fibrousness values, than burgers formulated with protein powder (
p < 0.05), which did not have fibrous structures, as can be seen in
Figure 7. Extrusion cooking of vegetable proteins allows us to obtain a fibrous matrix that mimics the fibrillar structure of meat muscle [
17].
For the odour attributes, it was observed that the formulation with microalgae (PP+C) had general, vegetable, and meat odours of higher intensity and legume and spiced aromas of lower intensity (
p < 0.05). In contrast, the PP+L formulation had the lowest scores for overall and meat odour, followed by the PP formulation, which also had the lowest meat odour and vegetable odour scores. The highest scores for legume and spiced aroma were shown by PP and PP+S (
p < 0.05). In general, an inverse relationship between the increase in legume and spiced aromas and the decrease in odour intensity can be observed. The addition of 1% Chlorella could have contributed to softening the intensity of the volatile compounds responsible for the legume odour [
17,
21], which is usually not very appreciated by consumers [
66] because of its stronger vegetable odour. Spices and herbs with intense, fresh scents are typically used to mask these products’ unpleasant connotations [
67]. One of the most outstanding properties of Chlorella is its fresh green fruity odour and flavour, which exert an odour-neutralising effect [
68]. Regarding the effect of protein format, it could be seen that the vegetal hamburgers made with texturised protein presented higher overall, spiced, vegetable and meat odour intensities, but lower legume odour intensity (
p < 0.05), compared to protein powders. This could be related to the occurrence of Maillard reactions of amino acids/peptides during the extrusion process resulting in volatile aromatic substances, which could decrease the perception of legume odour and be responsible for the meat odour and flavour [
22,
69].
In terms of flavour, the PP+C formulation was perceived as the least salty and with the least umami taste (
p < 0.05). The main umami agent is monosodium glutamate, which, like salt, is a flavour enhancer [
70]. Therefore, the less salty samples were those with less of an umami taste. In contrast, the PP formulation was tastier, sweeter and, thus, the least bitter (
p < 0.05), as sweet and umami taste receptors share a subunit, namely, the T1R receptor family (T1R1/T1R3 responding to umami and T1R2/T1R3 responding to sweet), hence the similar results for these attributes [
71]. In this study, brewer’s yeast was added to enhance the umami taste [
72]. Umami taste has been shown to modulate sweetness and suppress bitterness [
70]. Vegetable proteins are often associated with bitterness, which may be caused by the presence of anti-nutritional factors such as saponins or phenolic compounds (tannins and catechins) [
73]. De Angelis et al. [
17] obtained similar results for both odour and flavour in texturised pea protein isolate analogues. As was the case for odour, the PP+C formulation had higher overall vegetable and meat odour intensity and lower legume and spiced flavour (
p < 0.05), and it was observed that overall odour scores were higher than flavour scores. The flavour of the vegetal hamburgers made with protein powder followed the same trend as the odour, being sweeter, more bitter, more leguminous and, therefore, less salty, with less overall, umami, spiced, vegetable and meaty flavour intensity (
p < 0.05) compared to the texturised protein. As discussed above, a more intense Maillard reaction during the extrusion process could have led to a better sensory profile of the texturised protein. To achieve the meat flavour during the thermal degradation of amino acids/peptides, starting raw materials such as free amino acids, yeast extracts and hydrolysed vegetable protein, in our case, are important [
69].
For the texture attributes, the enriched formulations had the highest scores for toughness, juiciness, and chewiness and the lowest scores for adhesiveness (
p < 0.05). As determined for instrumental texture, increasing fibre content increased toughness and chewiness [
8]. As for juiciness, it is related to the moisture content of the patties (WHC and CL); formulations with a higher capacity to retain water by capillarity were juicier [
60]. For the gumminess attribute, the panellists gave the highest scores to the PP and PP+S formulations (
p < 0.05), with almost negligible values ranging between 0.17 and 0.16, respectively. For graininess, the least granular formulation was PP+C (
p < 0.05), probably due to the lipid content of Chlorella [
74], which likely helped to improve the cohesiveness of the vegetal hamburgers [
7].
Regarding the format, in contrast to the values obtained for the instrumental texture, the panellists gave the textured protein the highest values for hardness, juiciness, chewiness and gumminess, and the lowest values for graininess and adhesiveness, the latter not being adhesive at all (
p < 0.05). This is probably due to the fibrous texture obtained after the extrusion process, which was more similar to the texture of meat and less grainy than powder [
17]. In general, producing vegetal hamburgers with texturised protein yielded an excellent sensory profile.
Pearson correlations were carried out to relate the properties of the textured and non-textured proteins with the properties of the vegetal hamburgers obtained from them (
Table 9). WAI was not significantly (
p > 0.05) correlated with any property measured in the vegetal hamburgers. AD also showed no significant (
p > 0.05) correlations with the studied properties of the L-, S- and C-enriched proteins. All other parameters were significantly correlated with high coefficients. FAI, SWE and WSI showed a negative correlation with CL. CL was higher when the enriched protein showed lower values of FAI, SWE and WSI, as it has been shown that the lower capacity for swelling and absorption of fat and water by the enriched protein structure leads to lower stability of the vegetal hamburgers in the emulsion to bind water and fat, and, therefore, to higher losses during cooking [
7,
57]. The x
w of proteins was negatively related to the mechanical properties of the hamburgers and their WHC values, probably due to tensile and capillary forces of the protein gel network, as increasing moisture content hampers the protein’s ability to retain water, decreasing, in particular, its hardness, cohesiveness and chewiness [
8,
59]. However, x
w was positively correlated with CL; moister proteins resulted in vegetal hamburgers that lost more water during cooking. In general, SWE showed the highest correlation coefficients with mechanical properties. A higher protein swelling capacity produces firmer and more cohesive vegetal hamburgers, although more gummy at the same time, as these proteins have a greater capacity for swelling and oil absorption. Thus, they were able to better capture the oil incorporated during the preparation of the vegetal hamburgers and form a network that was denser and more resistant to deformation during cooking due to protein–lipid and carbohydrate–lipid interactions [
64].