Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health
Abstract
1. Introduction
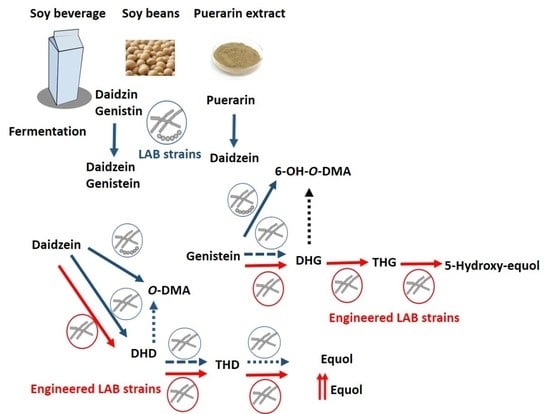
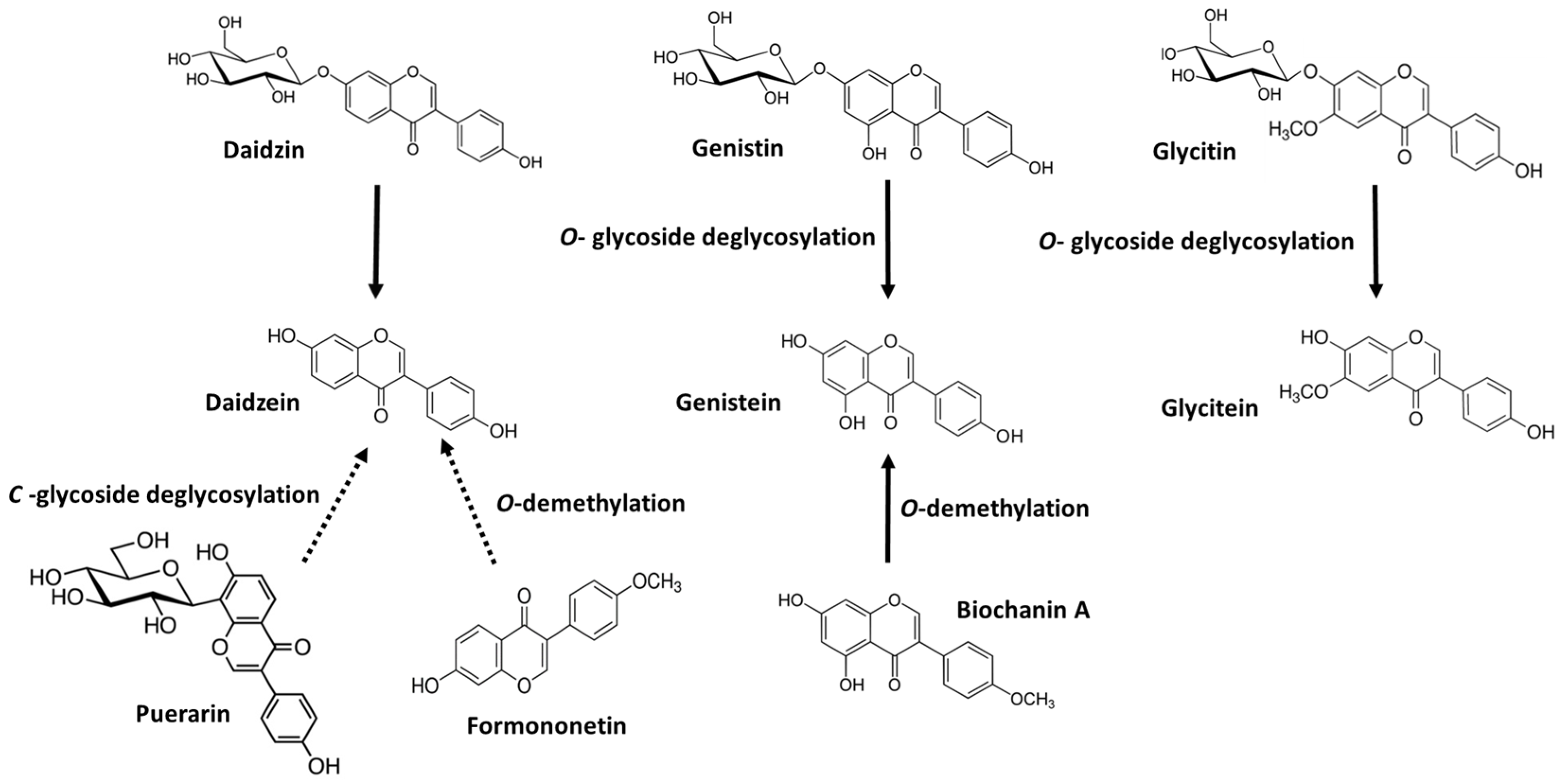
2. Transformation of Glycosylated and Methylated Isoflavones into Their Aglycones by LAB
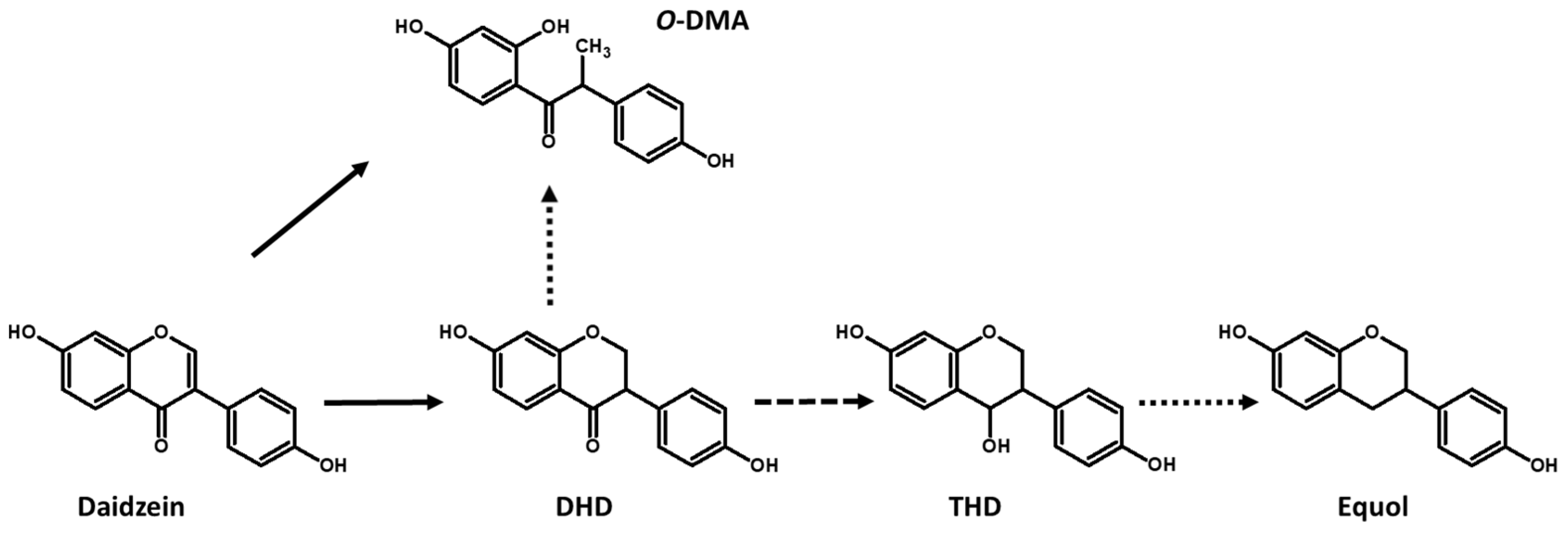
3. Metabolism of Isoflavone Aglycones by LAB
4. Genetic Engineering as a Strategy for the Production of Bioactive Isoflavones by LAB
4.1. Heterologous Expression of Degycosylases and Demethylases from GRAS Bacteria
4.2. Heterologous Expression of a Daidzein Reductase Gene Involved in the Production of DHD and DHG
4.3. Soy Beverage Enriched in Equol and 5-Hydroxy-Equol
5. Effects of the Isoflavones Metabolism by LAB
5.1. Increased Bioavailability of Isoflavones
5.2. Increased Antioxidant Activity of Fermented Foods
5.3. Estrogenic/Anti-Estrogenic Effect
5.4. Other Effects of Isoflavone Metabolism and Fermentation of Soy Foods
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of isoflavones in foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Tutunchi, H.; Yousefi, M.; Naeini, F.; Mobarak, S.; Asadi, M.; Sadeghi, O. Soy isoflavone intake and risk of cardiovascular disease in adults: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs. World Social Report 2023; United Nations: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Langa, S.; Landete, J.M. Strategies to achieve significant physiological concentrations of bioactive phytoestrogens in plasma. Crit. Rev. Food Sci. Nutr. 2021. [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.L. Maximizing the estrogenic potential of soy isoflavones through the gut microbiome: Implication for cardiometabolic health in postmenopausal women. Nutrients 2022, 14, 553. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Zhang, X.; Fujiyoshi, A.; Kadota, A.; Kondo, K.; Torii, S.; Okami, Y.; Hisamatsu, T.; Yano, Y.; Barinas-Mitchell, E.; Magnani, J.; et al. Cross-sectional association of equol producing status with aortic calcification in Japanese men aged 40–79 years. Sci. Rep. 2022, 12, 20114. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Arques, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of phytoestrogens: Intestinal bacteria and health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; Van’t Veer, P.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef]
- Peirotén, A.; Bravo, D.; Landete, J.M. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit. Rev. Food Sci. Nutr. 2020, 60, 1922–1937. [Google Scholar] [CrossRef]
- Cai, J.-S.; Feng, J.-Y.; Ni, Z.-J.; Ma, R.-H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de la Bastida, A.; Peirotén, Á.; Langa, S.; Curiel, J.A.; Arqués, J.L.; Landete, J.M. Effect of storage and heat treatment on the levels of bioactive flavonoids produced in fermented soy beverages. LWT 2022, 154, 112872. [Google Scholar] [CrossRef]
- Wessels, S.; Axelsson, L.; Bech Hansen, E.; De Vuyst, L.; Laulund, S.; Lähteenmäki, L.; Lindgren, S.; Mollet, B.; Salminen, S.; von Wright, A. The lactic acid bacteria, the food chain, and their regulation. Trends Food Sci. Technol. 2004, 15, 498–505. [Google Scholar] [CrossRef]
- Hur, H.G.; Lay, J.O., Jr.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimazu, T.; Tanimoto, H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J. Sci. Food Agric. 2011, 91, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Nagino, T.; Kano, M.; Masuoka, N.; Kaga, C.; Anbe, M.; Miyazaki, K.; Kamachi, K.; Isozaki, M.; Suzuki, C.; Kasuga, C.; et al. Intake of a fermented soymilk beverage containing moderate levels of isoflavone aglycones enhances bioavailability of isoflavones in healthy premenopausal Japanese women: A double-blind, placebo-controlled, single-dose, crossover trial. Biosci. Microbiota Food Health 2016, 35, 9–17. [Google Scholar] [CrossRef]
- Richelle, M.; Pridmore-Merten, S.; Bodenstab, S.; Enslen, M.; Offord, E.A. Hydrolysis of isoflavone glycosides to aglycones by β-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J. Nutr. 2002, 132, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kim, G.M.; Lee, K.W.; Choi, I.D.; Kwon, G.-H.; Park, J.-Y.; Jeong, S.-J.; Kim, J.-S.; Kim, J.H. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J. Food Sci. 2007, 72, M39–M44. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2017, 39, 198–205. [Google Scholar] [CrossRef]
- Baú, T.R.; Garcia, S.; Ida, E.I. Changes in soymilk during fermentation with kefir culture: Oligosaccharides hydrolysis and isoflavone aglycone production. Int. J. Food Sci. Nutr. 2015, 66, 845–850. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Garro, M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Raimondi, S.; Roncaglia, L.; De Lucia, M.; Amaretti, A.; Leonardi, A.; Pagnoni, U.M.; Rossi, M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2009, 81, 943–950. [Google Scholar] [CrossRef]
- Peirotén, A.; Gaya, P.; Álvarez, I.; Landete, J.M. Production of O-desmethylangolensin, tetrahydrodaidzein, 6′-hydroxy-O-desmethylangolensin and 2-(4-hydroxyphenyl)-propionic acid in fermented soy beverage by lactic acid bacteria and Bifidobacterium strains. Food Chem. 2020, 318, 126521. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-S.; Nishihata, T.; Kakiuchi, N.; Hattori, M. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol. Pharm. Bull. 2008, 31, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.; Han, J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J. Sci. Food Agric. 2015, 95, 1925–1931. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environ. Microbiol. 2011, 13, 482–494. [Google Scholar] [CrossRef]
- Wu, Q.L.; Wang, M.F.; Simon, J.E. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J. Chromatogr. A 2003, 1016, 195–209. [Google Scholar] [CrossRef]
- Hur, H.G.; Rafii, F. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol. Lett. 2000, 192, 21–25. [Google Scholar] [CrossRef]
- Curiel, J.A.; Landete, J.M. Identification and cloning of the first O-demethylase gene of isoflavones from Bifidobacterium breve INIA P734. LWT 2022, 162, 113510. [Google Scholar] [CrossRef]
- Wang, X.L.; Shin, K.H.; Hur, H.G.; Kim, S.I. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. J. Biotechnol. 2005, 115, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zang, X.; Dou, S.-J.; Wang, D.-Y.; Wang, X.-L. Fermentation of soymilk by Lactobacillus acidipiscis isolated from Chinese stinky tofu capable of efficiently biotransforming isoflavone glucosides to dihydrodaidzein and dihydrogenistein. J. Sci. Food Agric. 2022, 102, 7221–7230. [Google Scholar] [CrossRef]
- Yu, F.; Wang, S.; Li, J.; Zhang, Q.; Li, C.; Wang, X. C-ring cleavage of isoflavone daidzein by a newly-isolated facultative Enterococcus hirae AUH-HM195 from Crossoptilon mantchuricum feces. Wei Sheng Wu Xue Bao 2009, 49, 479–484. [Google Scholar] [PubMed]
- Gaya, P.; Peirotén, Á.; Álvarez, I.; Medina, M.; Landete, J.M. Production of the bioactive isoflavone O-desmethylangolensin by Enterococcus faecium INIA P553 with high efficiency. J. Funct. Foods 2018, 40, 180–186. [Google Scholar] [CrossRef]
- Landete, J.M. Development of soy beverages enriched in O-desmethylangolesin and 6-hydroxy-O-desmethylangolesin by engineered lactic acid bacteria. LWT 2022, 163, 113526. [Google Scholar] [CrossRef]
- Peirotén, Á.; Gaya, P.; Landete, J.M. Application of recombinant lactic acid bacteria and bifidobacteria able to enrich soy beverage in dihydrodaidzein and dihydrogenistein. Food Res. Int. 2020, 134, 109257. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ueno, T.; Suzuki, T. Identification of a newly isolated equol-producing lactic acid bacterium from the human feces. J. Intest. Microbiol. 2007, 21, 217–220. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lim, J.; Kim, I.; Kim, D.; Kang, S.C. Isolation and identification of new bacterial stains producing equol from Pueraria lobata extract fermentation. PLoS ONE 2018, 13, e0192490. [Google Scholar] [CrossRef]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; Vincentini, O.; Silano, M.; Giuliani, G.; De Angelis, M.; Gobbetti, M. Synthesis of isoflavone aglycones and equol in soy milks fermented by food-related lactic acid bacteria and their effect on human intestinal Caco-2 cells. J. Agric. Food Chem. 2010, 58, 10338–10346. [Google Scholar] [CrossRef]
- Langa, S.; Ruiz de la Bastida, A.; Peirotén, Á.; Curiel, J.A.; Landete, J.M. Development of the first fermented soy beverages enriched in equol and 5-hydroxy-equol. LWT 2022, 168, 113899. [Google Scholar] [CrossRef]
- Larkin, T.; Price, W.E.; Astheimer, L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit. Rev. Food Sci. Nutr. 2008, 48, 538–552. [Google Scholar] [CrossRef]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of a novel dihydrodaidzein racemase essential for biosynthesis of equol from daidzein in Lactococcus spp. strain 20-92. Appl. Environ. Microbiol. 2012, 78, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Kim, H.-J.; Kang, S.-I.; Kim, S.-I.; Hur, H.-G. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 2007, 187, 155–160. [Google Scholar] [CrossRef]
- Ruiz de la Bastida, A.; Peirotén, Á.; Langa, S.; Arqués, J.L.; Landete, J.M. Heterologous production of equol by lactic acid bacteria strains in culture medium and food. Int. J. Food Microbiol. 2021, 360, 109328. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Expression of a β-glucosidase in bacteria with biotechnological interest confers them the ability to deglycosylate lignans and flavonoids in vegetal foods. Appl. Microbiol. Biotechnol. 2020, 104, 4903–4913. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Muñoz, R. Aryl glycosidases from Lactobacillus plantarum increase antioxidant activity of phenolic compounds. J. Funct. Foods 2014, 7, 322–329. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chu, L.H.; Lee, C.L.; Pan, T.M. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soymilk supplemented with Momordica charantia. J. Agric. Food Chem. 2009, 57, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Yang, C.; Xu, X.; Meng, Y. Antioxidant and hypolipidemic effects of soymilk fermented via Lactococcus acidophilus MF204. Food Funct. 2017, 8, 4414–4420. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Lee, Y.-C. Effect of lactic acid fermentation on enrichment of antioxidant properties and bioactive isoflavones in soybean. J. Food Sci. 2005, 70, S215–S220. [Google Scholar] [CrossRef]
- Rimbach, G.; De Pascual-Teresa, S.; Ewins, B.A.; Matsugo, S.; Uchida, Y.; Minihane, A.M.; Turner, R.; VafeiAdou, K.; Weinberg, P.D. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 2003, 33, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Madjirebaye, P.; Xiao, M.; Mahamat, B.; Xiong, S.; Mueed, A.; Wei, B.; Huang, T.; Peng, F.; Xiong, T.; Peng, Z. In vitro characteristics of lactic acid bacteria probiotics performance and antioxidant effect of fermented soymilk. Food Biosci. 2022, 49, 101952. [Google Scholar] [CrossRef]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.H.; Habib, H.M.; Chow, C.K.; Bruckner, G.G. Isoflavone-rich soy isolate reduces lipid peroxidation in mouse liver. Int. J. Vitam. Nutr. Res. 2008, 78, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef]
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S. Soy intake and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef]
- Markiewicz, L.; Garey, J.; Adlercreutz, H.; Gurpide, E. In vitro bioassays of non-steroidal phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993, 45, 399–405. [Google Scholar] [CrossRef]
- Sathyamoorthy, N.; Wang, T.T. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur. J. Cancer 1997, 33, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, G.; Dalla Via, A.; Licata, A.; Duncan, R.; Gardana, C.; Gargari, G.; Alamprese, C.; Arioli, S.; Taverniti, V.; Karp, M.; et al. Use of kefir-derived lactic acid bacteria for the preparation of a fermented soy drink with increased estrogenic activity. Food Res. Int. 2023, 164, 112322. [Google Scholar] [CrossRef]
- Aso, T. Equol improves menopausal symptoms in Japanese women. J. Nutr. 2010, 140, S1386–S1389. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Liao, J.-W.; Pan, T.-M. Effect of bioactive compounds in lactobacilli-fermented soy skim milk on femoral bone microstructure of aging mice. J. Sci. Food Agric. 2012, 92, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Desfita, S.; Sari, W.; Yusmarini, Y.; Pato, U.; Zakłos-Szyda, M.; Budryn, G. Effect of fermented soymilk-honey from different probiotics on osteocalcin level in menopausal women. Nutrients 2021, 13, 3581. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Nakatsugi, S.; Watanabe, K.; Kawamori, T.; Ishikawa, F.; Morotomi, M.; Sugie, S.; Toda, T.; Sugimura, T.; Wakabayashi, K. Inhibitory effects of Bifidobacterium-fermented soy milk on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones. Carcinogenesis 2000, 21, 937–941. [Google Scholar] [CrossRef]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef]
- Hwang, S.T.; Yang, M.H.; Baek, S.H.; Um, J.-Y.; Ahn, K.S. Genistin attenuates cellular growth and promotes apoptotic cell death breast cancer cells through modulation of ERalpha signaling pathway. Life Sci. 2020, 263, 118594. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.N.; Zakaria, S.; Nawaya, A.R.; Eldomany, A.R.; El-Shishtawy, M.M. Daidzein and chicory extract arrest the cell cycle via inhibition of cyclin D/CDK4 and cyclin A/CDK2 gene expression in hepatocellular carcinoma. Recent Pat. Anticancer Drug Discov. 2023, 18, 187–199. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Gercel-Taylor, C.; Feitelson, A.K.; Taylor, D.D. Inhibitory effect of genistein and daidzein on ovarian cancer cell growth. Anticancer Res. 2004, 24, 795–800. [Google Scholar] [PubMed]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-rich isoflavones aglycone inhibits lung cancer growth through inhibition of NF-κB signaling pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. O-desmethylangolensin inhibits the proliferation of human breast cancer MCF-7 cells by inducing apoptosis and promoting cell cycle arrest. Oncol. Lett. 2013, 6, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Hod, R.; Maniam, S.; Mohd Nor, N.H. A systematic review of the effects of equol (soy metabolite) on breast cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Yu, M.; Liu, X.; Ma, W.; Huang, L.; Li, X.; Ye, X. S-equol inhibits proliferation and promotes apoptosis of human breast cancer MCF-7 cells via regulating miR-10a-5p and PI3K/AKT pathway. Arch. Biochem. Biophys. 2019, 672, 108064. [Google Scholar] [CrossRef]
- Go, J.; Kim, J.E.; Kwak, M.H.; Koh, E.K.; Song, S.H.; Sung, J.E.; Kim, D.S.; Hong, J.T.; Hwang, D.Y. Neuroprotective effects of fermented soybean products (Cheonggukjang) manufactured by mixed culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on trimethyltin-induced cognitive defects mice. Nutr. Neurosci. 2016, 19, 247–259. [Google Scholar] [CrossRef]
- Wang, X.; Yin, Z.; Meng, X.; Yang, D.; Meng, H.; Liao, C.; Wei, L.; Chen, Y.; Yang, X.; Han, J.; et al. Daidzein alleviates neuronal damage and oxidative stress via GSK3β/Nrf2 pathway in mice. J. Funct. Foods 2022, 92, 105060. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, J.; Li, S.; Li, Z. Neuroprotective effects of genistein in a SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2019, 14, 688–696. [Google Scholar] [CrossRef]
- Schreihofer, D.A.; Oppong-Gyebi, A. Genistein: Mechanisms of action for a pleiotropic neuroprotective agent in stroke. Nutr. Neurosci. 2019, 22, 375–391. [Google Scholar] [CrossRef]
- Saadoun, J.H.; Calani, L.; Cirlini, M.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. Effect of fermentation with single and co-culture of lactic acid bacteria on okara: Evaluation of bioactive compounds and volatile profiles. Food Funct. 2021, 12, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef] [PubMed]


| Strain | Phytoestrogen Precursor | Product of Metabolism | Reference |
|---|---|---|---|
| L. mucosae INIA P508 | Daidzin, genistin and glycitin | Daidzein, genistein and glycitein | [18] |
| Enterococcus sp. MRG-IFC-2 | Puerarin | Daidzein | [34] |
| Lactococcus sp. MRG-IFC-1 | Puerarin | Daidzein | [34] |
| E. faecalis INIA P90 | Puerarin | Daidzein | [27] |
| E. faecium INIA P1 | Puerarin | Daidzein | [27] |
| L. rhamnosus INIA P540 | Biochanin A | Genistein | [38] |
| L. plantarum ESI144 | Biochanin A | Genistein | [38] |
| L. paracasei INIA P272 | Biochanin A | Genistein | [38] |
| L. fermentum INIA 584L | Biochanin A | Genistein | [38] |
| L. acidipiscis HAU-FR7 | Daidzein and genistein | DHD, DHG | [40] |
| E. hirae AUH-HM195 | Daidzein and genistein | O-DMA; 6-hydroxy-O-DMA | [41] |
| E. faecium INIA P553 | Daidzein and genistein | O-DMA; 6-hydroxy-O-DMA | [42] |
| L. plantarum ESI144 | Daidzein and genistein | O-DMA; 6-hydroxy-O-DMA | [44] |
| L. rhamnosus INIA P540 | Daidzein and genistein | O-DMA; 6-hydroxy-O-DMA | [44] |
| L. paracasei INIA P461 | Daidzein | THD | [44] |
| L. garvieae 20-92 | Daidzein | Equol | [45] |
| P. pentosaceus CS1 | Pueraria extract | Equol | [46] |
| L. intestinalis JCM 7548 | Pueraria extract | Equol | [46] |
| L. paracasei CS2 | Pueraria extract | Equol | [46] |
| L. sakei CS3 | Pueraria extract | Equol | [46] |
| L. intestinalis KTCT13676BP | Daidzein/chungkookjang | Equol | [47] |
| L. fermentum DPPMA114, L. plantarum DPPMA24W and DPPMASL33, and L. rhamnosus DPPMAAZ1 | Soy beverage | Equol | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langa, S.; Peirotén, Á.; Curiel, J.A.; de la Bastida, A.R.; Landete, J.M. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods 2023, 12, 1293. https://doi.org/10.3390/foods12061293
Langa S, Peirotén Á, Curiel JA, de la Bastida AR, Landete JM. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods. 2023; 12(6):1293. https://doi.org/10.3390/foods12061293
Chicago/Turabian StyleLanga, Susana, Ángela Peirotén, José Antonio Curiel, Ana Ruiz de la Bastida, and José María Landete. 2023. "Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health" Foods 12, no. 6: 1293. https://doi.org/10.3390/foods12061293
APA StyleLanga, S., Peirotén, Á., Curiel, J. A., de la Bastida, A. R., & Landete, J. M. (2023). Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods, 12(6), 1293. https://doi.org/10.3390/foods12061293