Hot Air Convective Drying of Ginger Slices: Drying Behaviour, Quality Characteristics, Optimisation of Parameters, and Volatile Fingerprints Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Drying Experiments
2.3. Drying Curves
2.4. Water Status
2.5. Colour
2.6. Rehydration Coefficient
2.7. Specific Energy Consumption
2.8. Gingerol Contents
2.9. Box–Behnken Design (BBD)
2.10. GC-IMS Analysis Parameters
2.11. Statistical Analysis
3. Results
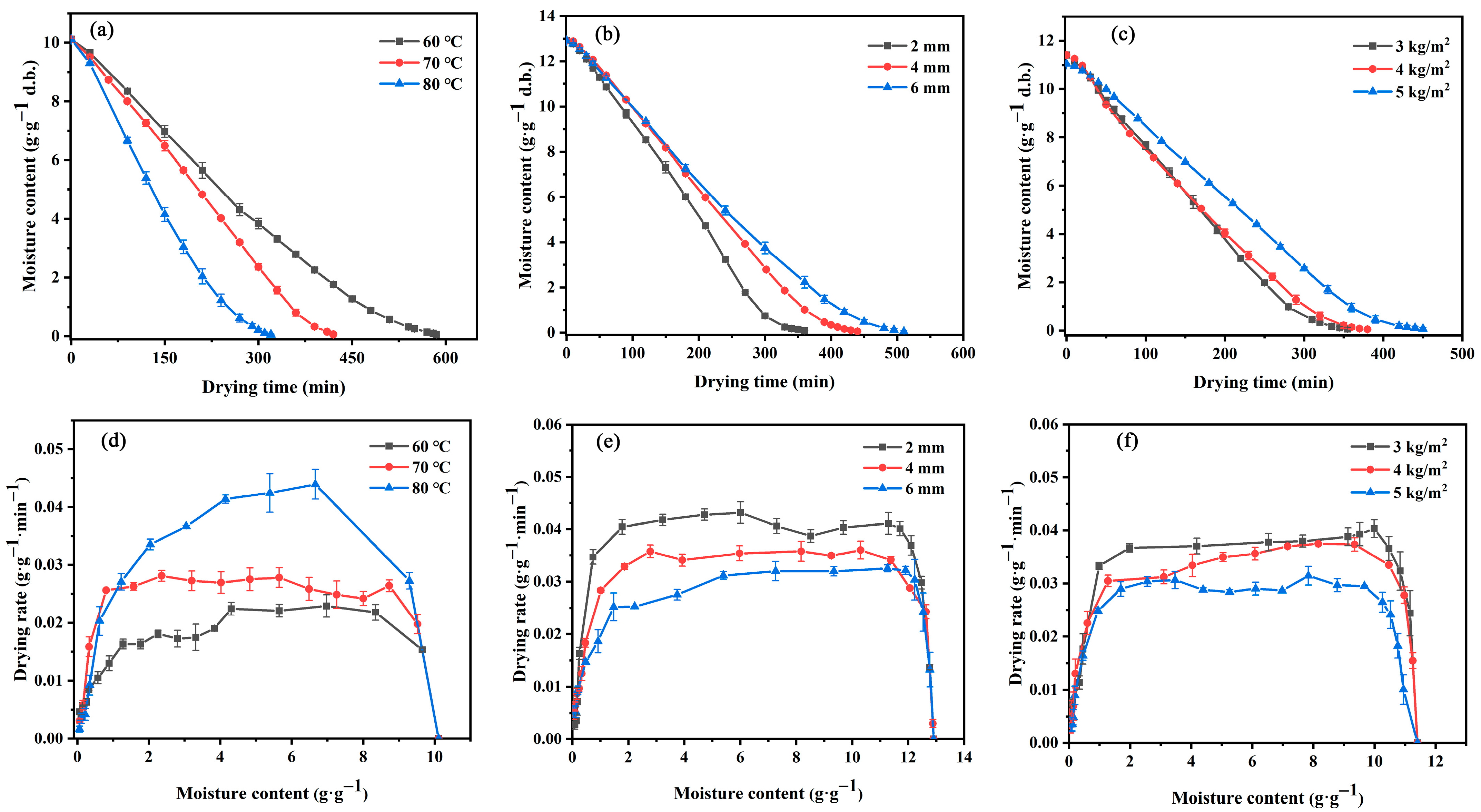
3.1. Drying Characteristics
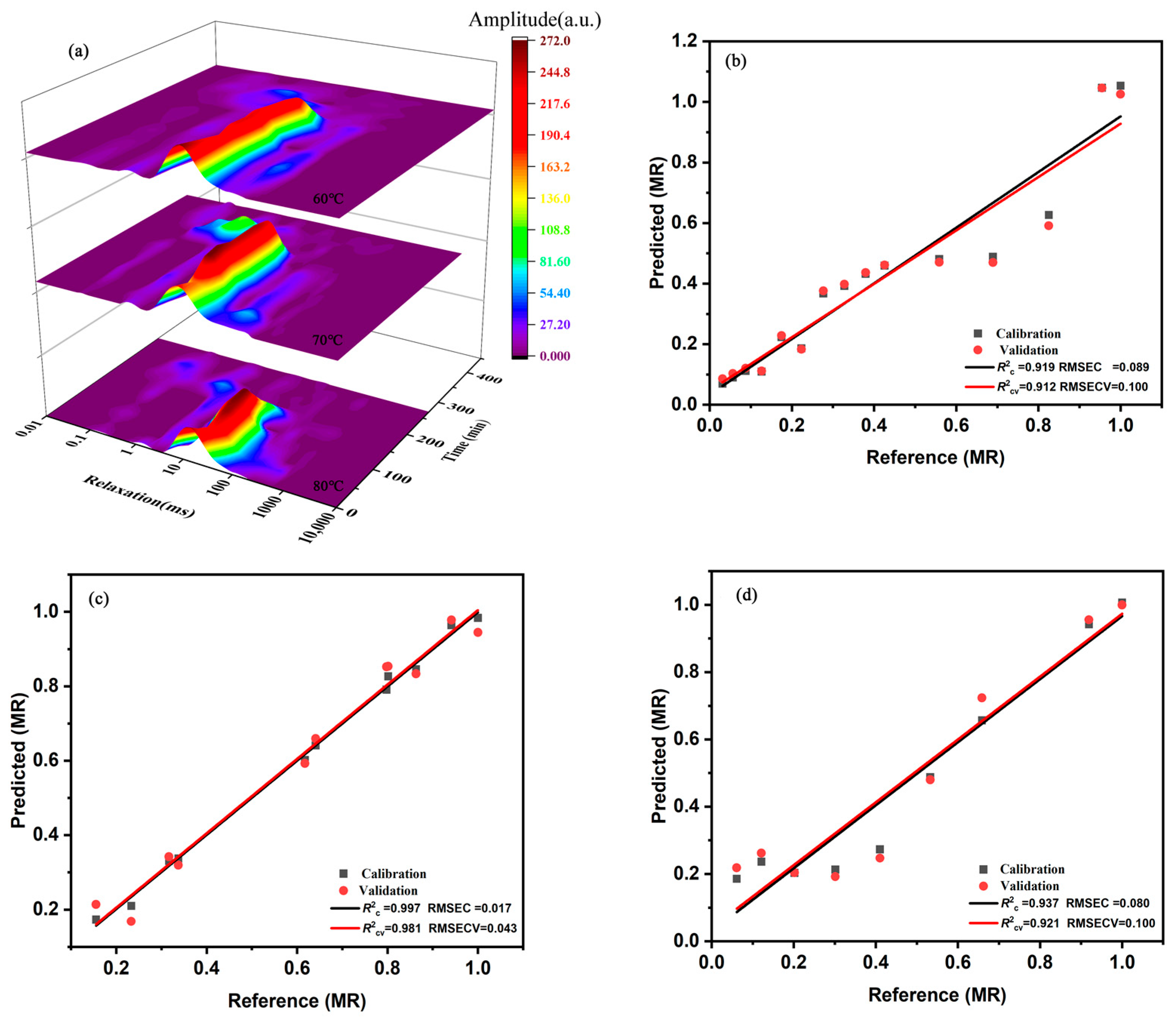
3.2. Evaluation and Prediction of Water State by LF-NMR
3.3. Colour and BI
3.4. Rehydration Coefficients
3.5. Gingerol Content
3.6. Optimisation of Drying Conditions by RSM
3.6.1. Specific Energy Consumption
3.6.2. Total Gingerol Content
3.6.3. Determination and Experimental Validation of Optimal Conditions
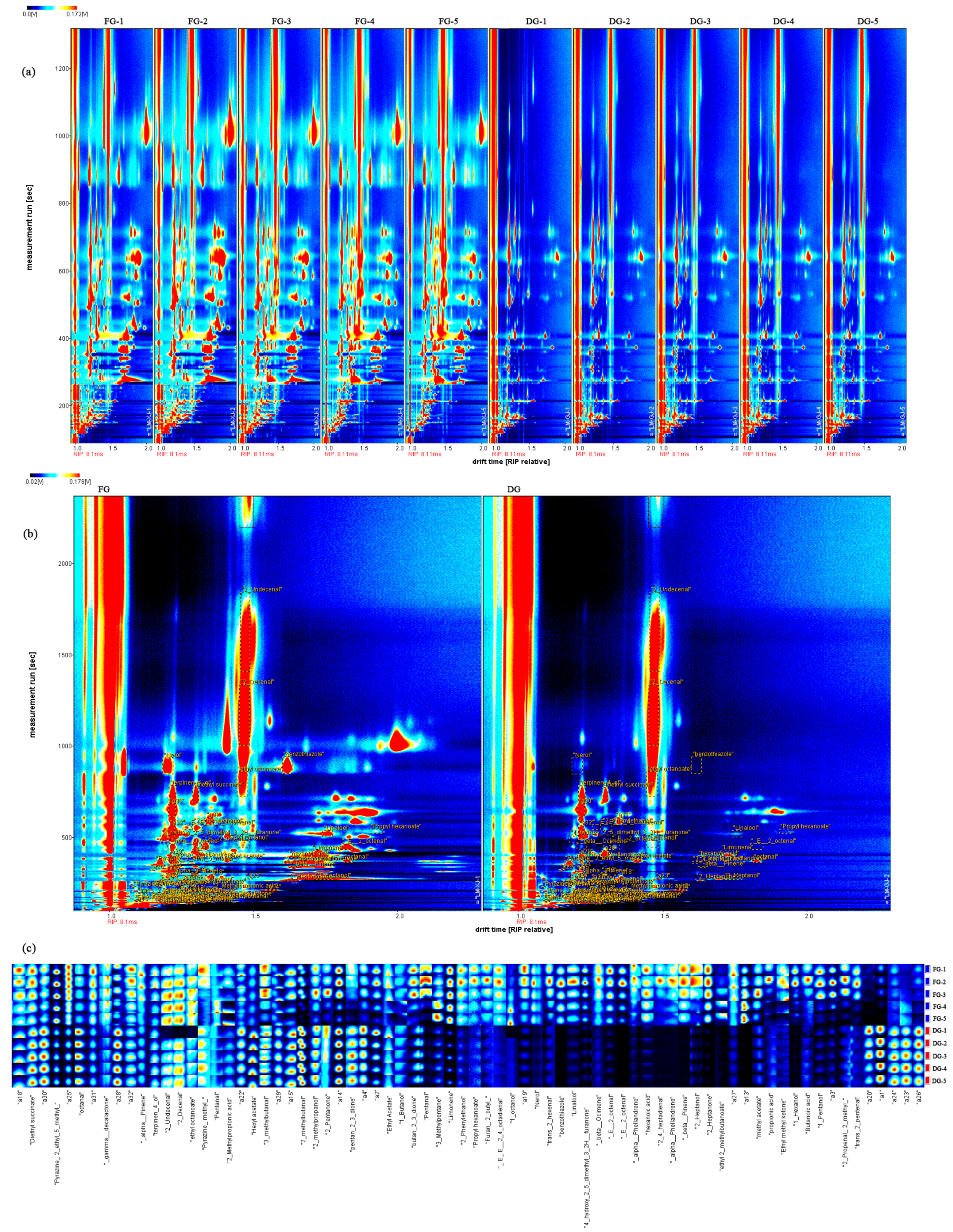
3.7. The Volatile Compounds Identified and the Volatile Fingerprints under Fresh and Dried Ginger by HS-GC–MS
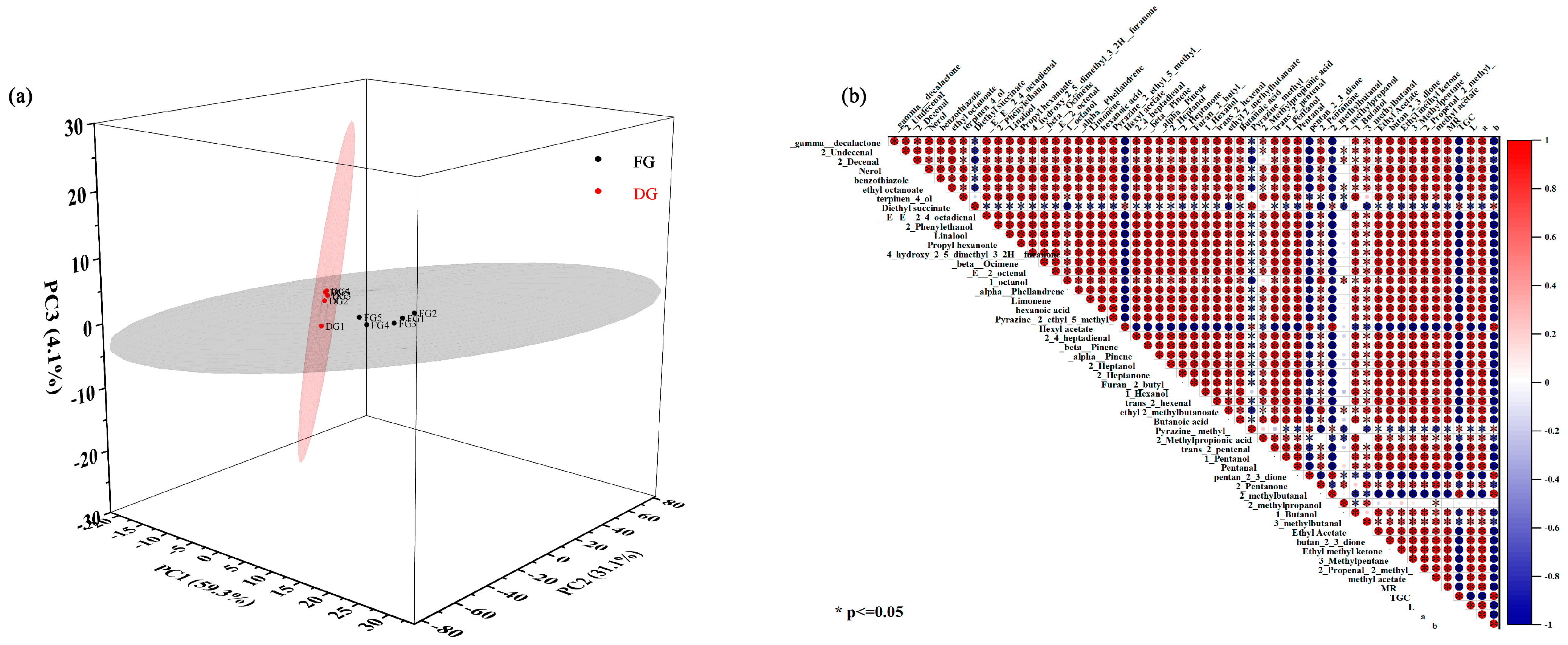
3.8. Distinction of Fresh and Dried Ginger by PCA Analysis
3.9. Correlation Analysis of Moisture and Quality Indicators Related to Drying
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zheng, Z.J.; Guo, X.F.; Yuan, J.P.; Zheng, C.C. Preparative separation of gingerols from Zingiber officinale by high-speed counter-current chromatography using stepwise elution. Food Chem. 2011, 125, 1476–1480. [Google Scholar] [CrossRef]
- He, L.; Qin, Z.; Li, M.; Chen, Z.; Zeng, C.; Yao, Z.; Yu, Y.; Dai, Y.; Yao, X. Metabolic Profiles of Ginger, A Functional Food, and Its Representative Pungent Compounds in Rats by Ultraperformance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 9010–9033. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, B.; ElGasim, A.; Yagoub, A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S.J. Recent developments in radio frequency drying of food and agricultural products: A review. Dry. Technol. 2018, 37, 271–286. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Khalloufi, S.; Mondor, M.; Ratti, C. Moisture profile analysis of food models undergoing glass transition during air-drying. J. Food Eng. 2020, 281, 109995. [Google Scholar] [CrossRef]
- Castro, A.M.; Mayorga, E.Y.; Moreno, F.L. Mathematical modelling of convective drying of fruits: A review. J. Food Eng. 2018, 223, 152–167. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, M.; Yang, P.Q. Combination of LF-NMR and BP-ANN to monitor water states of typical fruits and vegetables during microwave vacuum drying. LWT 2019, 116, 108548. [Google Scholar] [CrossRef]
- Cheng, S.S.; Li, R.R.; Yang, H.M.; Wang, S.Q.; Tan, M.Q. Water status and distribution in shiitake mushroom and the effects of drying on water dynamics assessed by LF-NMR and MRI. Dry. Technol. 2019, 38, 1001–1010. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Qiao, Y.T.; Gao, P.; Li, L.Y.; Zheng, Z.J. Potential use of low-field nuclear magnetic resonance to determine the drying characteristics and quality of Arctium lappa L. in hot-blast air. LWT 2020, 132, 109829. [Google Scholar] [CrossRef]
- Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Liu, X.; Fan, L. Simultaneous optimization of the acidified water extraction for total anthocyanin content, total phenolic content, and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L.) using response surface methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef]
- Ryu, D.H.; Cho, J.Y.; Sadiq, N.B.; Kim, J.C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.A.; Roy, D.; Ghosh, S. Process modeling and optimization for biomass steam-gasification employing response surface methodology. Biomass Bioenergy 2020, 143, 105847. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, S.; Fu, C.; Fang, F.; Liu, J.; Kan, J.; Qian, C.; Chai, Q.; Jin, C. Effects of Drying Methods on Taste Components and Flavor Characterization of Cordyceps militaris. Foods 2022, 11, 3933. [Google Scholar] [CrossRef]
- Pang, X.; Cao, J.; Wang, D.; Qiu, J.; Kong, F. Identification of Ginger (Zingiber officinale Roscoe) Volatiles and Localization of Aroma-Active Constituents by GC-Olfactometry. J. Agric. Food Chem. 2017, 65, 4140–4145. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; White, S.; Brown, P.; Naiker, M. Pungent and volatile constituents of dried Australian ginger. Curr. Res. Food Sci. 2021, 4, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.X.; Zhang, X.; Guo, S.; Yan, H.; Wang, J.M.; Zhou, J.Q.; Yang, J.; Duan, J.A. Headspace GC/MS and fast GC e-nose combined with chemometric analysis to identify the varieties and geographical origins of ginger (Zingiber officinale Roscoe). Food Chem. 2022, 396, 133672. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, J.; Wang, J.; Wang, X.Y.; Du, D.D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Xiao, N.; Xu, H.; Jiang, X.; Sun, T.; Luo, Y.; Shi, W. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res. Int. 2022, 158, 111584. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-Compound Fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a Benchtop Alternative to (1)H NMR Profiling for Assessment of the Authenticity of Honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Wang, D.; Zhang, C.J.; Zhu, X.C.; Zhu, Z.S.; Lei, Y.; Huang, M. The impact of sous vide braising on the sensory characteristics and heterocyclic amines contents of braised chicken. LWT 2022, 172, 114176. [Google Scholar] [CrossRef]
- Li, X.; Cui, W.J.; Wang, W.L.; Wang, Y.M.; Gong, Z.Q.; Xu, Z.X. Analysis of the volatile compounds associated with pickling of ginger using headspace gas chromatography—Ion mobility spectrometry. Flavour Fragr. J. 2019, 34, 485–492. [Google Scholar] [CrossRef]
- Mahayothee, B.; Thamsala, T.; Khuwijitjaru, P.; Janjai, S. Effect of drying temperature and drying method on drying rate and bioactive compounds in cassumunar ginger (Zingiber montanum). J. Appl. Res. Med. Aromat. Plants 2020, 18, 100262. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Szumny, A.; Kaveh, M.; Rasooli Sharabiani, V.; Kumar, A.; Shimizu, N. Parboiled Paddy Drying with Different Dryers: Thermodynamic and Quality Properties, Mathematical Modeling Using ANNs Assessment. Foods 2020, 9, 86. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.H.; Wu, Z.F.; Wang, X.C.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl cellulose coating pretreatment. LWT 2020, 134, 110159. [Google Scholar] [CrossRef]
- Elmizadeh, A.; Shahedi, M.; Hamdami, N. Quality assessment of electrohydrodynamic and hot-air drying of quince slice. Ind. Crops Prod. 2018, 116, 35–40. [Google Scholar] [CrossRef]
- Feng, Y.; Ping Tan, C.; Zhou, C.; Yagoub, A.E.A.; Xu, B.; Sun, Y.; Ma, H.; Xu, X.; Yu, X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef]
- Jafari, F.; Movagharnejad, K.; Sadeghi, E. Infrared drying effects on the quality of eggplant slices and process optimization using response surface methodology. Food Chem. 2020, 333, 127423. [Google Scholar] [CrossRef]
- Jin, W.G.; Pei, J.J.; Wang, S.Q.; Chen, X.H.; Gao, R.C.; Tan, M.Q. Effect of continuous and intermittent drying on water mobility of fresh walnuts (Juglans regia L.): A LF-NMR study. Dry. Technol. 2020, 40, 254–264. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.N.; Wang, X.; Cheng, S.P.; Liu, F.; Huang, L.Q. Determination of drying kinetics and quality changes of Panax quinquefolium L. dried in hot-blast air. LWT 2019, 116, 108563. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, M.; Bhandari, B.; Zhou, L.Q. LF-NMR online detection of water dynamics in apple cubes during microwave vacuum drying. Dry. Technol. 2018, 36, 2006–2015. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Beigi, M.; Torki-Harchegani, M.; Tohidi, M. Experimental and ANN modeling investigations of energy traits for rough rice drying. Energy 2017, 141, 2196–2205. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]

| Variables | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Drying temperature (X1), °C | 60 | 70 | 80 |
| Thickness (X2), mm | 2 | 4 | 6 |
| Loading density (X3), kg/m2 | 3 | 4 | 5 |
| L* | a* | b* | ΔE | BI | RC | Gingerol Contents (mg/g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6-Gingerol | 8-Gingerol | 10-Gingerol | ||||||||
| Drying temperature, °C | 60 | 73.43 ± 0.11 b | 1.19 ± 0.06 b | 28.10 ± 0.18 a | 8.36 ± 0.13 b | 48.09 ± 0.50 b | 4.26 ± 0.09 a | 5.78 ± 0.24 b | 1.27 ± 0.11 c | 2.48 ± 0.25 b |
| 70 | 74.50 ± 0.07 a | 0.73 ± 0.05 c | 28.27 ± 0.01 a | 7.22 ± 0.07 c | 47.09 ± 0.11 c | 4.29 ± 0.06 a | 7.97 ± 0.63 a | 1.79 ± 0.13 b | 3.66 ± 0.25 a | |
| 80 | 72.10 ± 0.03 c | 1.83 ± 0.05 a | 28.10 ± 0.20 a | 9.83 ± 0.05 a | 49.91 ± 0.50 a | 4.36 ± 0.17 a | 7.51 ± 0.04 a | 2.05 ± 0.01 a | 3.85 ± 0.01 a | |
| Thickness, mm | 2 | 73.57 ± 0.06 b | 0.32 ± 0.03 c | 29.24 ± 0.19 a | 8.13 ± 0.04 a | 49.43 ± 0.40 a | 5.57 ± 0.16 a | 8.75 ± 0.12 ab | 2.96 ± 0.90 a | 4.65 ± 0.09 a |
| 4 | 73.48 ± 0.19 c | 1.09 ± 0.07 a | 28.60 ± 0.47 b | 8.32 ± 0.22 a | 48.98 ± 1.14 a | 4.43 ± 0.45 b | 9.08 ± 0.38 a | 2.38 ± 0.09 a | 4.24 ± 0.18 b | |
| 6 | 75.93 ± 0.03 a | 0.81 ± 0.07 b | 28.28 ± 0.09 b | 5.89 ± 0.04 b | 46.07 ± 0.26 b | 3.34 ± 0.36 c | 8.25 ± 0.11 b | 2.55 ± 0.04 a | 4.89 ± 0.07 a | |
| Loading densities, kg/m2 | 3 | 75.97 ± 0.11 b | 0.60 ± 0.03 a | 29.45 ± 0.20 a | 6.00 ± 0.05 b | 48.18 ± 0.35 a | 4.08 ± 0.03 b | 7.06 ± 0.03 a | 2.03 ± 0.01 a | 3.95 ± 0.02 a |
| 4 | 76.41 ± 0.07 a | 0.37 ± 0.02 c | 28.38 ± 0.10 b | 5.30 ± 0.06 c | 45.46 ± 0.17 b | 4.63 ± 0.03 a | 6.95 ± 0.46 a | 1.92 ± 0.13 a | 3.75 ± 0.24 a | |
| 5 | 75.21 ± 0.18 c | 0.47 ± 0.01 b | 26.97 ± 0.19 b | 6.49 ± 0.20 a | 43.64 ± 0.23 c | 4.14 ± 0.04 b | 7.23 ± 0.73 a | 2.01 ± 0.16 a | 3.88 ± 0.26 a | |
| Run | Independent Variables | Responses | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Ekg (kw·h/kg) | TGC (mg/g) | |
| 1 | 0 | −1 | 1 | 1.56 | 21.37 |
| 2 | −1 | −1 | 0 | 1.64 | 17.75 |
| 3 | 1 | 0 | −1 | 1.92 | 16.71 |
| 4 | 0 | −1 | −1 | 1.72 | 20.12 |
| 5 | 1 | −1 | 0 | 1.54 | 13.07 |
| 6 | 0 | 0 | 0 | 1.73 | 11.88 |
| 7 | 0 | 0 | 0 | 1.75 | 10.16 |
| 8 | 0 | 1 | 1 | 1.72 | 12.59 |
| 9 | 0 | 0 | 0 | 1.77 | 11.35 |
| 10 | 1 | 0 | 1 | 1.7 | 15.74 |
| 11 | 1 | 1 | 0 | 1.94 | 13.69 |
| 12 | −1 | 1 | 0 | 1.74 | 10.07 |
| 13 | 0 | 1 | −1 | 2.15 | 11.26 |
| 14 | −1 | 0 | 1 | 1.74 | 10.33 |
| 15 | −1 | 0 | −1 | 1.78 | 9.23 |
| NO | Compounds | CAS | Molecule Formula | MW | RI | Rt | Dt |
|---|---|---|---|---|---|---|---|
| 1 | γ-decalactone | 706-14-9 | C10H18O2 | 170.3 | 1480.7 | 2285.21 | 1.4903 |
| 2 | 2-Undecenal | 2463-776 | C11H20O | 168.3 | 1383.1 | 1578.444 | 1.4865 |
| 3 | 2-Decenal | 3913-71-1 | C10H18O | 154.3 | 1278.8 | 1063.094 | 1.4749 |
| 4 | Nerol | 106-25-2 | C10H18O | 154.3 | 1230.9 | 886.403 | 1.2046 |
| 5 | benzothiazole | 95-16-9 | C7H5NS | 135.2 | 1230.9 | 886.403 | 1.6216 |
| 6 | ethyl octanoate | 106-32-1 | C10H20O2 | 172.3 | 1208 | 812.781 | 1.4672 |
| 7 | terpinen-4-ol | 562-74-3 | C10H18O | 154.3 | 1174.4 | 715.601 | 1.2239 |
| 8 | Diethyl succinate | 123-25-1 | C8H14O4 | 174.2 | 1179.8 | 730.325 | 1.3012 |
| 9 | (2E,4E)-2,4-octadienal | 30361-28-5 | C8H12O | 124.2 | 1109.3 | 559.081 | 1.2677 |
| 10 | propyl | 60-12-8 | C8H10O | 122.2 | 1107.4 | 555.006 | 1.3064 |
| 11 | Linalool | 78-70-6 | C10H18O | 154.3 | 1092.5 | 524.443 | 1.7565 |
| 12 | Propyl hexanoate | 626-77-7 | C9H18O2 | 158.2 | 1098 | 535.649 | 1.9235 |
| 13 | 4-hydroxy-2,5-dimethyl-3(2H)-furanone | 3658-77-3 | C6H8O3 | 128.1 | 1078.3 | 496.936 | 1.1927 |
| 14 | β-Ocimene | 13877-91-3 | C10H16 | 136.2 | 1053.9 | 453.128 | 1.2193 |
| 15 | (E)-2-octenal | 2548-87-0 | C8H14O | 126.2 | 1056.3 | 457.203 | 1.3355 |
| 16 | 1-octanol | 111-87-5 | C8H18O | 130.2 | 1063.8 | 470.447 | 1.4661 |
| 17 | α-Phellandrene | 99-83-2 | C10H16 | 136.2 | 1012.2 | 386.907 | 1.2226 |
| 18 | Limonene | 138-86-3 | C10H16 | 136.2 | 1025.1 | 406.264 | 1.7326 |
| 19 | hexanoic acid | 142-62-1 | C6H12O2 | 116.2 | 1019.1 | 397.095 | 1.6439 |
| 20 | Pyrazine, 2-ethyl-5-methyl- | 13360-64-0 | C7H10N2 | 122.2 | 997.2 | 365.512 | 1.1734 |
| 21 | Hexyl acetate | 142-92-7 | C8H16O2 | 144.2 | 1007.3 | 379.775 | 1.4148 |
| 22 | 2,4-heptadienal | 5910-85-0 | C7H10O | 110.2 | 998 | 366.531 | 1.6193 |
| 23 | β-Pinene | 127-91-3 | C10H16 | 136.2 | 972.4 | 340.77 | 1.6457 |
| 24 | α-Pinene | 80-56-8 | C10H16 | 136.2 | 929.2 | 302.153 | 1.2181 |
| 25 | 2-Heptanol | 543-49-7 | C7H16O | 116.2 | 897.9 | 276.918 | 1.7266 |
| 26 | 2-Heptanone | 110-43-0 | C7H14O | 114.2 | 887 | 269.271 | 1.6372 |
| 27 | Furan, 2-butyl | 4466-24-4 | C8H12O | 124.2 | 888.2 | 270.036 | 1.1777 |
| 28 | 1-Hexanol | 111-27-3 | C6H14O | 102.2 | 861 | 253.595 | 1.333 |
| 29 | trans-2-hexenal | 6728-26-3 | C6H10O | 98.1 | 841.6 | 242.507 | 1.182 |
| 30 | ethyl 2-methylbutanoate | 7452-79-1 | C7H14O2 | 130.2 | 847 | 245.566 | 1.2369 |
| 31 | Butyric acid | 107-92-6 | C4H8O2 | 88.1 | 833.3 | 237.919 | 1.164 |
| 32 | Pyrazine, methyl | 109-08-0 | C5H6N2 | 94.1 | 819.9 | 230.655 | 1.0868 |
| 33 | 2-Methylpropionic acid | 79-31-2 | C4H8O2 | 88.1 | 791.7 | 216.126 | 1.3677 |
| 34 | trans-2-pentenal | 1576-87-0 | C5H8O | 84.1 | 743.2 | 194.332 | 1.1061 |
| 35 | Pentanol | 71-41-0 | C5H12O | 88.1 | 758.3 | 200.832 | 1.2583 |
| 36 | Pentanal | 110-62-3 | C5H10O | 86.1 | 704.3 | 178.596 | 1.1866 |
| 37 | pentan-2,3-dione | 600-14-6 | C5H8O2 | 100.1 | 692.1 | 173.952 | 1.297 |
| 38 | Propyl methyl ketone | 107-87-9 | C5H10O | 86.1 | 699.2 | 176.648 | 1.3769 |
| 39 | 2-methylbutanal | 96-17-3 | C5H10O | 86.1 | 645.5 | 163.468 | 1.4048 |
| 40 | 2-methylpropanol | 78-83-1 | C4H10O | 74.1 | 654.8 | 165.415 | 1.1671 |
| 41 | 1-Butanol | 71-36-3 | C4H10O | 74.1 | 661.9 | 166.913 | 1.1832 |
| 42 | 3-methyl butyraldehyde | 590-86-3 | C5H10O | 86.1 | 638.9 | 162.12 | 1.1846 |
| 43 | Ethyl Acetate | 141-78-6 | C4H8O2 | 88.1 | 614.4 | 157.177 | 1.0974 |
| 44 | butan-2,3-dione | 431-03-8 | C4H6O2 | 86.1 | 603 | 154.93 | 1.1765 |
| 45 | Ethyl methyl ketone | 78-93-3 | C4H8O | 72.1 | 600.7 | 154.481 | 1.253 |
| 46 | 3-Methylpentane | 96-14-0 | C6H14 | 86.2 | 582.9 | 151.036 | 1.2154 |
| 47 | 2-Propenal, 2-methyl- | 78-85-3 | C4H6O | 70.1 | 559.7 | 146.693 | 1.2167 |
| 48 | methyl acetate | 79-20-9 | C3H6O2 | 74.1 | 521.7 | 139.803 | 1.1957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Sun, J.; Qiao, X.; Zheng, Z.; Li, M.; Zhang, B. Hot Air Convective Drying of Ginger Slices: Drying Behaviour, Quality Characteristics, Optimisation of Parameters, and Volatile Fingerprints Analysis. Foods 2023, 12, 1283. https://doi.org/10.3390/foods12061283
Bai R, Sun J, Qiao X, Zheng Z, Li M, Zhang B. Hot Air Convective Drying of Ginger Slices: Drying Behaviour, Quality Characteristics, Optimisation of Parameters, and Volatile Fingerprints Analysis. Foods. 2023; 12(6):1283. https://doi.org/10.3390/foods12061283
Chicago/Turabian StyleBai, Ruoxi, Jieru Sun, Xuguang Qiao, Zhenjia Zheng, Meng Li, and Bin Zhang. 2023. "Hot Air Convective Drying of Ginger Slices: Drying Behaviour, Quality Characteristics, Optimisation of Parameters, and Volatile Fingerprints Analysis" Foods 12, no. 6: 1283. https://doi.org/10.3390/foods12061283
APA StyleBai, R., Sun, J., Qiao, X., Zheng, Z., Li, M., & Zhang, B. (2023). Hot Air Convective Drying of Ginger Slices: Drying Behaviour, Quality Characteristics, Optimisation of Parameters, and Volatile Fingerprints Analysis. Foods, 12(6), 1283. https://doi.org/10.3390/foods12061283





