Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cooking of Striploin and Tenderloin Steaks
2.2.2. Physicochemical Analysis of Meat
Cook Loss Measurements
Moisture Analysis and pH
Crude Protein (%) and Fat (%) Analysis
2.2.3. In Vitro Digestion Experiments
2.2.4. Assessment of Protein Digestibility
Ninhydrin Analysis of Digests
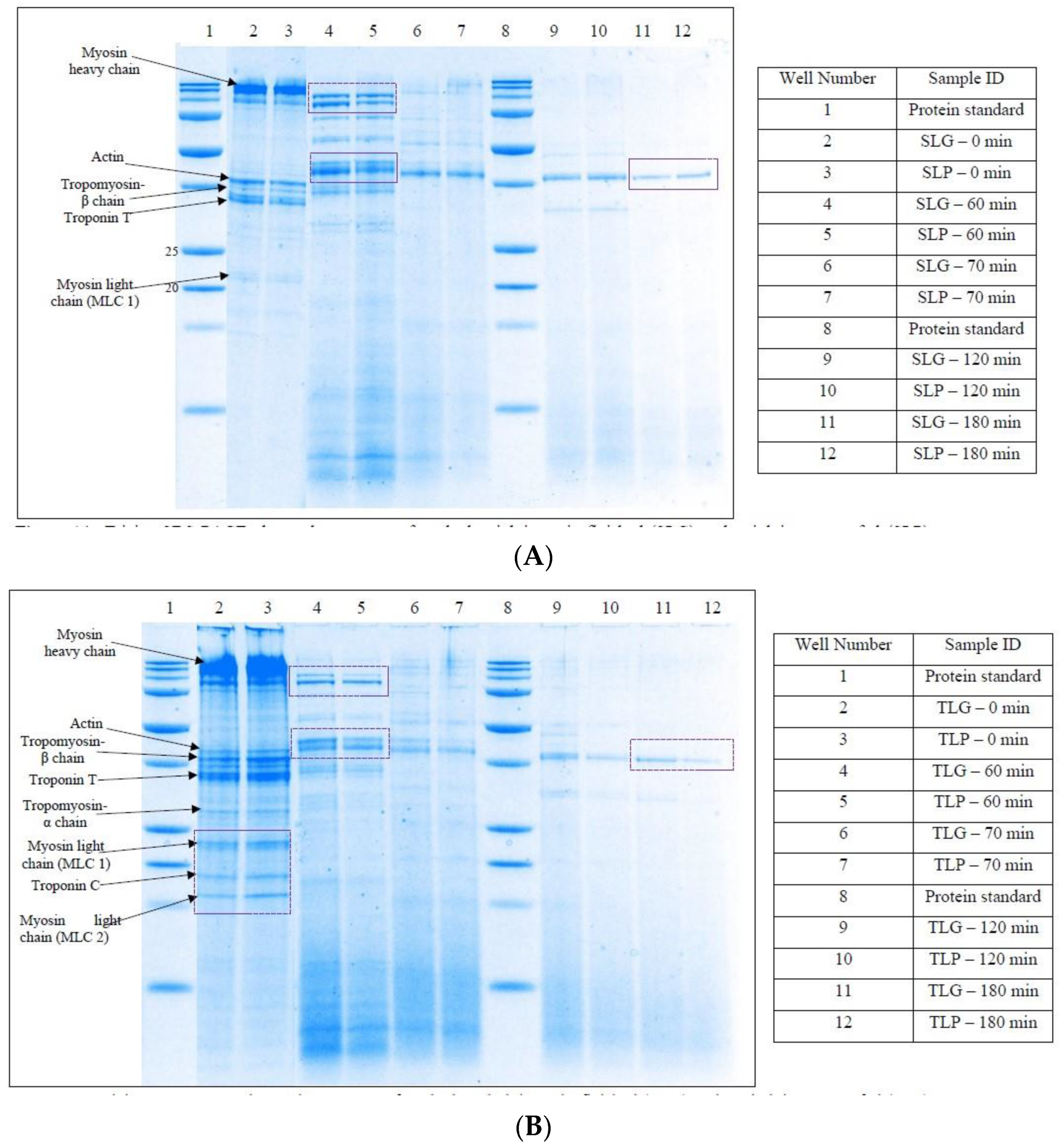
Tricine SDS-PAGE of Digests
2.2.5. Free Fatty Acid Analysis of Digests
Methylation of Total Fatty Acids (TFA)
Methylation of Ester Forms of Fatty Acids
GC Analysis of Fatty Acid Methyl Esters (FAMEs)
Calculation of Free Fatty Acids (FFA)
Desaturase Indices and n−6/n−3 Fatty Acid Ratios
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of Meat
3.1.1. Cook Loss
3.1.2. Moisture Content and pH
3.1.3. Protein Content
3.1.4. Fat Content
3.2. Protein Digestibility
3.2.1. Ninhydrin-Reactive Free Amino Nitrogen
3.2.2. Tricine SDS-PAGE
3.3. Fatty Acid Profiles of Control Meat Samples and Their Digests
3.3.1. Total Fatty Acid (TFA) Profiles of Cooked Meat
3.3.2. Free Fatty Acid (FFA) Profiles, Ratios and Desaturase Indices of Cooked Meat Digests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roseland, J.M.; Nguyen, Q.V.; Douglass, L.W.; Patterson, K.Y.; Howe, J.C.; Williams, J.R.; Thompson, L.D.; Brooks, J.C.; Woerner, D.R.; Engle, T.E.; et al. Fatty acid, cholesterol, vitamin, and mineral content of cooked beef cuts from a national study. J. Food Compos. Anal. 2018, 66, 55–64. [Google Scholar] [CrossRef]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Mechanistic and other relevant data. In Red Meat and Processed Meat: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The role of the anabolic properties of plant-versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.; Joy, M.; Ripoll, G.; Smeti, S.; Mekki, I.; Gahete, F.M.; Mahouachi, M.; Atti, N. Meat physicochemical properties, fatty acid profile, lipid oxidation and sensory characteristics from three North African lamb breeds, as influenced by concentrate or pasture finishing diets. J. Food Compos. Anal. 2016, 48, 102–110. [Google Scholar] [CrossRef]
- Jukna, V.; Jukna, Č.; Prusevičius, V.; Meškinytė-kaušilienė, E.; Pečiulaitienė, N. Meat quality of different beef cattle breeds fed high energy forage. Zemdirb. Agric. 2017, 104, 227–282. [Google Scholar] [CrossRef]
- Purchas, R.W.; Knight, T.W.; Busboom, J.R. The effect of production system and age on concentrations of fatty acids in intramuscular fat of the longissimus and triceps brachii muscles of Angus-cross heifers. Meat Sci. 2005, 70, 597–603. [Google Scholar] [CrossRef]
- Van Elswyk, M.A.; McNeill, S.H. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: The U.S. experience. Meat Sci. 2014, 96, 535–540. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Reis, M.G.; Subbaraj, A.K.; Cameron-Smith, D.; Fraser, K.; Jonker, A.; Craigie, C.R. Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu-dairy cross beef cattle. Meat Sci. 2018, 140, 26–37. [Google Scholar] [CrossRef]
- Lukic, M.; Trbovic, D.; Karan, D.; Petrovic, Z.; Jovanovic, J.; Milijasevic, J.B.; Nikolic, A. The nutritional and health value of beef lipids-fatty acid composition in grass-fed and grain-fed beef. IOP Conf. Series. Earth Environ. Sci. 2021, 854, 2054. [Google Scholar] [CrossRef]
- Juárez, M.; Lam, S.; Bohrer, B.M.; Dugan, M.E.R.; Vahmani, P.; Aalhus, J.; Juárez, A.; López-Campos, O.; Prieto, N.; Segura, J. Enhancing the nutritional value of red meat through genetic and feeding strategies. Foods 2021, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Milk fat: Origin of fatty acids and influence of nutritional factors thereon. In Advanced Dairy Chemistry, Volume 2: Lipids, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ministry for Primary Industries of New Zealand. Animal Status Declaration; 2016. Available online: https://www.mpi.govt.nz/dmsdocument/11509-Animal-Status-Declaration (accessed on 11 March 2023).
- Li, X.; Zhang, R.; Hassan, M.M.; Cheng, Z.; Mills, J.; Hou, C.; Realini, C.E.; Chen, L.; Day, L.; Zheng, X.; et al. Active packaging for the extended shelf-life of meat: Perspectives from consumption habits, market requirements and packaging practices in China and New Zealand. Foods 2022, 11, 2903. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.W.; Wilkinson, B.H.P. The Concentration of Selected Nutrients in New Zealand Beef and Lamb Cuts and Offal Items: A Report to Beef + Lamb New Zealand Limited; Massey University: Palmerston North, New Zealand, 2013. [Google Scholar]
- Gawat, M.; Kaur, L.; Singh, J.; Boland, M. Physicochemical and quality characteristics of New Zealand goat meat and its ultrastructural features. Food Res. Int. 2022, 161, 111736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Actinidin Treatment and Sous Vide Cooking: Effects on Tenderness and In Vitro Protein Digestibility of Beef Brisket. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2017. Massey Research Online. Available online: https://mro.massey.ac.nz/handle/10179/12752 (accessed on 11 March 2023).
- AOAC. AOAC Official Method 950.46. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC. Official Method 981.10. Protein/crude protein in meat and meat products. Block digestion method. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- AOAC. Official Method 922.06. Fat in flour. Acid hydrolysis method. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effect of pulsed electric fields (PEF) on the ultrastructure and in vitro protein digestibility of bovine longissimus thoracis. LWT-Food Sci. Technol. 2019, 103, 253–259. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, A.; Verrier, T.; Singh, H. Free fatty acid profiles of emulsified lipids during in vitro digestion with pancreatic lipase. Food Chem. 2013, 139, 398–404. [Google Scholar] [CrossRef]
- Carriere, F.; Barrowman, J.A.; Verger, R.; Laigier, R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology 1993, 105, 878–888. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N. Infogest consensus method. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Alarcón, G.; Roco, J.; Medina, A.; Van Nieuwenhove, C.; Medina, M.; Jerez, S. Stearoyl-CoA desaturase indexes and n−6/n−3 fatty acids ratio as biomarkers of cardiometabolic risk factors in normal-weight rabbits fed high fat diets. J. Biomed. Sci. 2016, 23, 13. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Cooking-induced protein modifications in meat. Compr. Rev. Food Sci. Food Saf. 2017, 16, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt, H.C.; Strydom, P.E. Effect of age and cut on cooking loss, juiciness and flavour of South African beef. Meat Sci. 2011, 87, 180–190. [Google Scholar] [CrossRef]
- Jeremiah, L.E.; Gibson, L.L. Cooking influences on the palatability of roasts from the beef hip. Food Res. Int. 2003, 36, 1–9. [Google Scholar] [CrossRef]
- Pereira, P.M.d.C.C.; Vicente, A.F.d.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Purchas, R.W.; Wilkinson, B.H.; Carruthers, F.; Jackson, F. A comparison of the nutrient content of uncooked and cooked lean from New Zealand beef and lamb. J. Food Compos. Anal. 2014, 35, 75–82. [Google Scholar] [CrossRef]
- Smith, M.E.; Morton, D.G. The Digestive System: Systems of the Body Series; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kaur, L.; Maudens, E.; Haisman, D.R.; Boland, M.J.; Singh, H. Microstructure and protein digestibility of beef: The effect of cooking conditions as used in stews and curries. LWT-Food Sci. Technol. 2014, 55, 612–620. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and flavor chemistry characteristics of Australian beef: Influence of intramuscular fat, feed, and breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Lucherk, L.W.; O’Quinn, T.G.; Legako, J.F.; Shackelford, S.D.; Brooks, J.C.; Miller, M. Palatability of New Zealand grass-finished and American grain-finished beef strip steaks of varying USDA quality grades and wet-aging treatments. Meat Muscle Biol. 2022, 6, 12601. [Google Scholar]
- Clancy, K. Greener Pastures: How Grass-Fed Beef and Milk Contribute to Healthy Eating; Union of Concerned Scientists: Cambridge, MA, USA, 2006; pp. 1–4. Available online: https://www.ucsusa.org/sites/default/files/2019-10/greener-pastures.pdfhttp://www.ucsusa.org/assets/documents/food_and_agriculture/greener-pastures.pdf (accessed on 13 March 2023).
- Yuen, K.-H. The transit of dosage forms through the small intestine. Int. J. Pharm. 2010, 395, 9–16. [Google Scholar] [CrossRef]
- Smith, S.B.; Johnson, B.J. Marbling: Management of cattle to maximize the deposition of intramuscular adipose tissue. J. Anim. Sci. 2016, 94, 382. [Google Scholar] [CrossRef]
- Horman, T.; Fernandes, M.F.; Tache, M.C.; Hucik, B.; Mutch, D.M.; Leri, F. Dietary n−6/n−3 ratio influences brain fatty acid composition in adult rats. Nutrients 2020, 12, 1847. [Google Scholar] [CrossRef]
- Gupta, R.; Lakshmy, R.; Abraham, R.A.; Reddy, K.S.; Jeemon, P.; Prabhakaran, D. Serum omega-6/omega-3 ratio and risk markers for cardiovascular disease in an industrial population of Delhi. Food Nutr. Sci. 2013, 4, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Importance of the omega-6/omega-3 balance in health and disease: Evolutionary aspects of diet. World Rev. Nutr. Diet. 2011, 102, 10–21. [Google Scholar] [PubMed]
- Warensjö, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Risérus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.; Sierra-Johnson, J.; Gertow, K.; Rosell, M.; Vessby, B.; De Faire, U.; Hamsten, A.; Hellenius, M.-L.; Fisher, R.M. Fatty acid desaturases in human adipose tissue: Relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008, 51, 328–335. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Berglund, L. Indices of fatty acid desaturase activity in healthy human subjects: Effects of different types of dietary fat. Br. J. Nutr. 2013, 110, 871–879. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Muscle Type | Production System | SEM | Meat Cut | Production System | Interaction | |
|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | ||||||
| Cook loss (%) | SL | 14.58 aY | 15.74 aX | 0.50 | p < 0.001 | p > 0.05 | p > 0.05 |
| TL | 18.67 aX | 18.00 aX | 0.49 | ||||
| Moisture content (%) | SL | 66.67 aX | 63.49 bX | 0.01 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 64.14 aY | 61.47 bX | 0.01 | ||||
| Protein content (%) | SL | 28.12 bX | 29.28 aX | 0.31 | p < 0.05 | p > 0.05 | p < 0.05 |
| TL | 28.39 aX | 27.13 aY | 0.43 | ||||
| Fat content (%) | SL | 4.97 bX | 7.70 aX | 0.64 | p < 0.05 | p < 0.001 | p > 0.05 |
| TL | 6.43 bX | 10.30 aX | 0.87 | ||||
| pH | SL | 5.97 aX | 5.94 aX | 0.03 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 5.87 aX | 5.85 aX | 0.02 | ||||
| Free Amino Nitrogen (%) | Muscle Type | Production System | SEM | Meat Cut | Production System | Interaction | |
|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | ||||||
| 0 min | SL | 1.65 aX | 1.66 aX | 0.06 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 2.22 aY | 2.09 aY | 0.14 | ||||
| 60 min | SL | 4.24 aX | 4.42 bX | 0.12 | p < 0.001 | p < 0.05 | p > 0.05 |
| TL | 4.53 aY | 5.42 bY | 0.16 | ||||
| 180 min | SL | 13.06 aX | 12.88 aX | 0.29 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 13.55 aY | 14.79 aY | 0.34 | ||||
| Fatty Acids | Muscle Type | Total Fatty Acid (mg/g Cooked Meat) | SEM | Meat Cut | Production System | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | |||||||
| Individual SFA | ||||||||
| C14:0 | Myristic acid | SL | 1.08 bX | 1.68 aX | 0.13 | p > 0.05 | p < 0.05 | p > 0.05 |
| TL | 1.08 bX | 1.85 aX | 0.20 | |||||
| C16:0 | Palmitic acid | SL | 13.69 bX | 15.09 aX | 0.62 | p > 0.05 | p < 0.05 | p > 0.05 |
| TL | 13.56 bX | 19.53 aX | 1.65 | |||||
| C17:0 | Heptadecanoic acid | SL | 0.57 bX | 0.99 aX | 0.09 | p > 0.05 | p < 0.001 | p > 0.05 |
| TL | 0.70 bX | 1.29 aX | 0.13 | |||||
| C18:0 | Stearic acid | SL | 10.05 aX | 8.10 aX | 0.48 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 12.49 aY | 12.75 aY | 0.87 | |||||
| Individual MUFA | ||||||||
| C14:1 | Myristoleic acid | SL | 0.17 bX | 0.42 aX | 0.05 | p > 0.05 | p < 0.001 | p > 0.05 |
| TL | 0.15 bX | 0.38 aX | 0.05 | |||||
| C16:1 | Palmitoleic acid | SL | 1.20 bX | 1.95 aX | 0.15 | p > 0.05 | p < 0.001 | p > 0.05 |
| TL | 0.99 bX | 2.08 aX | 0.23 | |||||
| C18:1 c9 | Oleic acid | SL | 19.32 bX | 28.57 aX | 1.75 | p > 0.05 | p < 0.001 | p > 0.05 |
| TL | 18.34 bX | 32.89 aX | 3.26 | |||||
| C18:1 c11 | cis-Vaccenic acid | SL | 1.70 bX | 0.72 aX | 0.19 | p > 0.05 | p < 0.001 | p > 0.05 |
| TL | 1.39 bX | 0.58 aX | 0.14 | |||||
| Individual PUFA | ||||||||
| C18:2 n−6 | Linoleic acid | SL | 0.88 bY | 1.49 aY | 0.11 | p < 0.001 | p < 0.001 | p > 0.05 |
| TL | 1.65 bX | 2.46 aX | 0.18 | |||||
| C18:2 c9, t11 | Conjugated linoleic acid | SL | 0.25 aY | 0.13 aY | 0.02 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 0.26 aX | 0.31 aX | 0.04 | |||||
| C18:3 n−3 | α-Linolenic acid | SL | 0.52 bY | 0.23 aY | 0.06 | p < 0.001 | p < 0.001 | p > 0.05 |
| TL | 0.91 bX | 0.45 aX | 0.09 | |||||
| C20:5 n−3 | Eicosapentaenoic acid (EPA) | SL | 0.20 bY | 0.15 aY | 0.01 | p < 0.001 | p < 0.001 | p > 0.05 |
| TL | 0.31 bX | 0.20 aX | 0.02 | |||||
| C22:5 n−3 | Docosapentaenoic acid (DPA) | SL | 0.31 bY | 0.27 aY | 0.01 | p < 0.001 | p < 0.001 | p > 0.05 |
| TL | 0.47 bX | 0.34 aX | 0.02 | |||||
| C22:6 n−3 | Docosahexaenoic acid (DHA) | SL | 0.03 aX | 0.03 aX | 0.00 | p < 0.001 | p > 0.05 | p > 0.05 |
| TL | 0.05 aY | 0.05 aY | 0.00 | |||||
| Fatty Acids | Muscle Type | Free Fatty Acid Released (mg/g Cooked Meat) | SEM | Meat Cut | Production System | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | |||||||
| Individual SFA | ||||||||
| C14:0 | Myristic acid | SL | 0.22 bY | 0.47 aX | 0.04 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 0.41 bX | 0.90 aX | 0.13 | |||||
| C16:0 | Palmitic acid | SL | 3.75 bY | 5.48 aY | 0.34 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 5.61 bX | 10.47 aX | 1.20 | |||||
| C17:0 | Heptadecanoic acid | SL | 0.14 bY | 0.33 aY | 0.04 | p < 0.05 | p < 0.001 | p > 0.05 |
| TL | 0.27 bX | 0.67 aX | 0.09 | |||||
| C18:0 | Stearic acid | SL | 2.33 aY | 2.50 aY | 0.17 | p < 0.001 | p < 0.05 | p > 0.05 |
| TL | 4.43 aX | 6.35 aX | 0.62 | |||||
| Individual MUFA | ||||||||
| C14:1 | Myristoleic acid | SL | 0.05 bX | 0.13 aX | 0.01 | p > 0.05 | p < 0.05 | p > 0.05 |
| TL | 0.06 bX | 0.15 aX | 0.03 | |||||
| C16:1 | Palmitoleic acid | SL | 0.31 bX | 0.70 aX | 0.07 | p > 0.05 | p < 0.05 | p > 0.05 |
| TL | 0.41 bX | 1.03 aX | 0.15 | |||||
| C18:1 c9 | Oleic acid | SL | 4.88 bY | 8.92 aX | 0.78 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 7.32 bX | 16.76 aX | 2.39 | |||||
| C18:1 c11 | cis-Vaccenic acid | SL | 0.18 bY | 0.45 aY | 0.05 | p < 0.05 | p < 0.001 | p > 0.05 |
| TL | 0.29 bX | 0.84 aX | 0.12 | |||||
| Individual PUFA | ||||||||
| C18:2 n−6 | Linoleic acid | SL | 0.56 bY | 0.68 aY | 0.03 | p < 0.001 | p < 0.05 | p > 0.05 |
| TL | 0.76 bX | 1.14 aX | 0.08 | |||||
| C18:2 c9, t11 | Conjugated linoleic acid | SL | 0.05 aY | 0.05 aX | 0.01 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 0.08 aX | 0.12 aX | 0.02 | |||||
| C18:3 n−3 | α-Linolenic acid | SL | 0.13 aY | 0.05 bY | 0.01 | p < 0.001 | p < 0.001 | p > 0.05 |
| TL | 0.28 aX | 0.19 bX | 0.03 | |||||
| C20:5 n−3 | Eicosapentaenoic acid (EPA) | SL | 0.05 aX | 0.03 bX | 0.00 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 0.08 aX | 0.05 bX | 0.01 | |||||
| C22:5 n−3 | Docosapentaenoic acid (DPA) | SL | 0.07 aX | 0.06 aX | 0.00 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 0.10 aX | 0.06 bX | 0.01 | |||||
| C22:6 n−3 | Docosahexaenoic acid (DHA) | SL | 0.02 aX | 0.01 aX | 0.00 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 0.02 aX | 0.02 aX | 0.00 | |||||
| Free Fatty Acids | Muscle Type | Free Fatty Acid Released (mg/g Cooked Meat) | SEM | Meat Cut | Production System | Interaction | |
|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | ||||||
| Σ SFA | SL | 6.50 bY | 8.89 aY | 0.53 | p < 0.001 | p > 0.05 | p < 0.05 |
| TL | 10.87 bX | 18.65 aX | 2.05 | ||||
| Σ MUFA | SL | 5.49 bY | 10.51 aX | 0.94 | p < 0.05 | p > 0.05 | p < 0.05 |
| TL | 8.19 bX | 19.27 aX | 2.76 | ||||
| Σ PUFA | SL | 1.09 aY | 1.08 aY | 0.04 | p < 0.001 | p > 0.05 | p > 0.05 |
| TL | 1.54 aX | 1.80 aX | 0.11 | ||||
| Σ n−6 PUFA | SL | 0.78 aY | 0.87 aY | 0.03 | p < 0.001 | p > 0.05 | p > 0.05 |
| TL | 0.98 bX | 1.35 aX | 0.09 | ||||
| Σ n−3 PUFA | SL | 0.27 aY | 0.15 bY | 0.02 | p < 0.001 | p > 0.05 | p < 0.001 |
| TL | 0.47 aX | 0.32 bX | 0.04 | ||||
| Σ LCn−3 PUFA | SL | 0.14 aX | 0.10 bX | 0.01 | p < 0.05 | p < 0.05 | p > 0.05 |
| TL | 0.19 aX | 0.13 bX | 0.02 | ||||
| Σ EPA, DHA | SL | 0.06 aX | 0.04 bX | 0.00 | p < 0.05 | p > 0.05 | p > 0.05 |
| TL | 0.09 aX | 0.07 bX | 0.01 | ||||
| Free Fatty Acids | Muscle Type | Free Fatty Acid Released (mg/g Cooked Meat) | SEM | Meat Cut | Production System | Interaction | |
|---|---|---|---|---|---|---|---|
| Pasture-Raised | Grain-Finished | ||||||
| FA ratios and DI | |||||||
| n−6/n−3 PUFA | SL | 2.88 bX | 5.69 aX | 0.48 | p < 0.05 | p > 0.05 | p < 0.001 |
| TL | 2.11 bY | 4.24 aY | 0.36 | ||||
| DI16 | SL | 0.08 bX | 0.13 aX | 0.01 | p < 0.001 | p > 0.05 | p < 0.001 |
| TL | 0.07 bX | 0.10 aY | 0.01 | ||||
| DI18 | SL | 2.14 bX | 3.63 aX | 0.29 | p < 0.001 | p < 0.05 | p < 0.001 |
| TL | 1.66 bY | 2.56 aY | 0.18 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, L.; Elamurugan, A.; Chian, F.M.; Zhu, X.; Boland, M. Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison. Foods 2023, 12, 1239. https://doi.org/10.3390/foods12061239
Kaur L, Elamurugan A, Chian FM, Zhu X, Boland M. Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison. Foods. 2023; 12(6):1239. https://doi.org/10.3390/foods12061239
Chicago/Turabian StyleKaur, Lovedeep, Amrutha Elamurugan, Feng Ming Chian, Xianqian Zhu, and Mike Boland. 2023. "Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison" Foods 12, no. 6: 1239. https://doi.org/10.3390/foods12061239
APA StyleKaur, L., Elamurugan, A., Chian, F. M., Zhu, X., & Boland, M. (2023). Protein and Lipid Digestibility of Pasture-Raised and Grain-Finished Beef: An In Vitro Comparison. Foods, 12(6), 1239. https://doi.org/10.3390/foods12061239








