Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Hawthorn Fruit Extract
2.3. HPLC and HPLC/ESI–MS/MS Analysis of PEHE
2.4. Determination of Antioxidants
2.4.1. Total Polyphenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Total Triterpenoid Content (TTC)
2.5. Evaluation of Antioxidant Activity
2.5.1. DPPH Free Radical-Scavenging Activity Determination
2.5.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. α-Glucosidase Inhibitory Activity Assay
2.7. Cell Experiment
2.7.1. Cell Culture
2.7.2. Cell Viability
2.7.3. Intracellular ROS Level
2.8. Gene Expression
2.8.1. Extraction of RNA from RIN-m5F Cells
2.8.2. Reverse Transcription of RNA to cDNA
2.8.3. Quantitative Analysis of mRNA Levels
2.9. Statistical Analysis
3. Results
3.1. The Contents of Total Polyphenols, Flavonoids and Triterpenoids
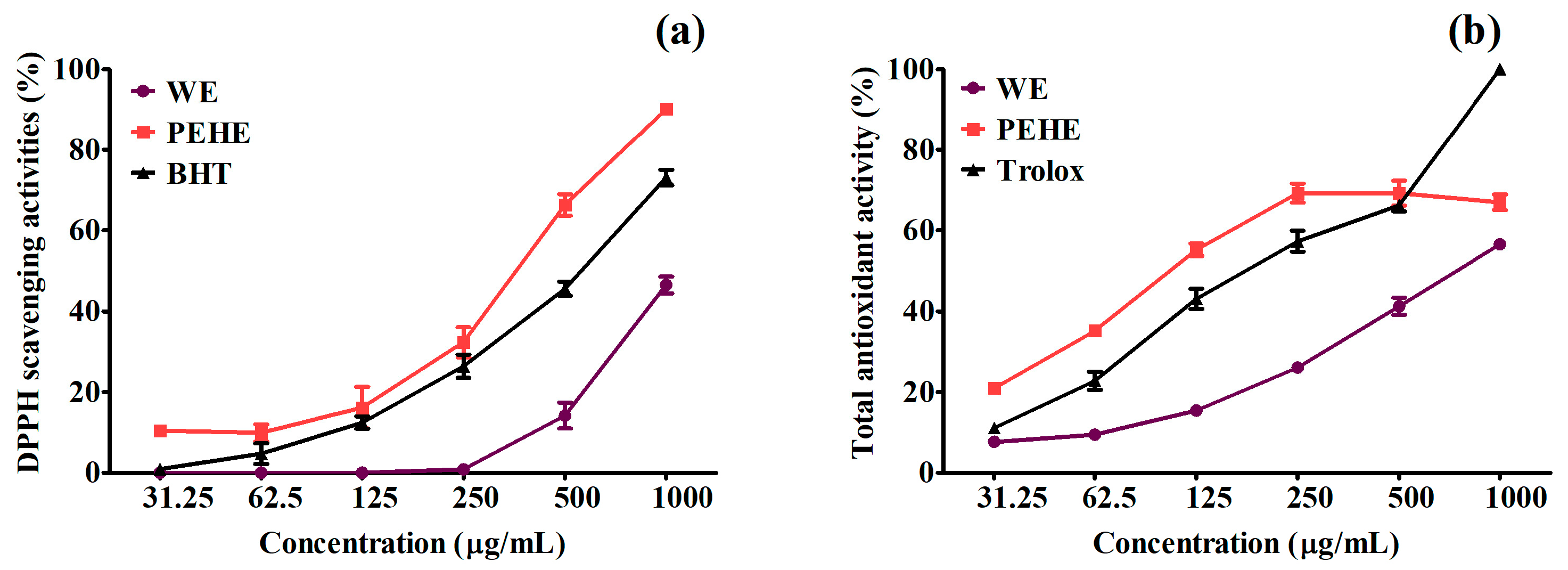
3.2. The Antioxidant Activities of WE and PEHE
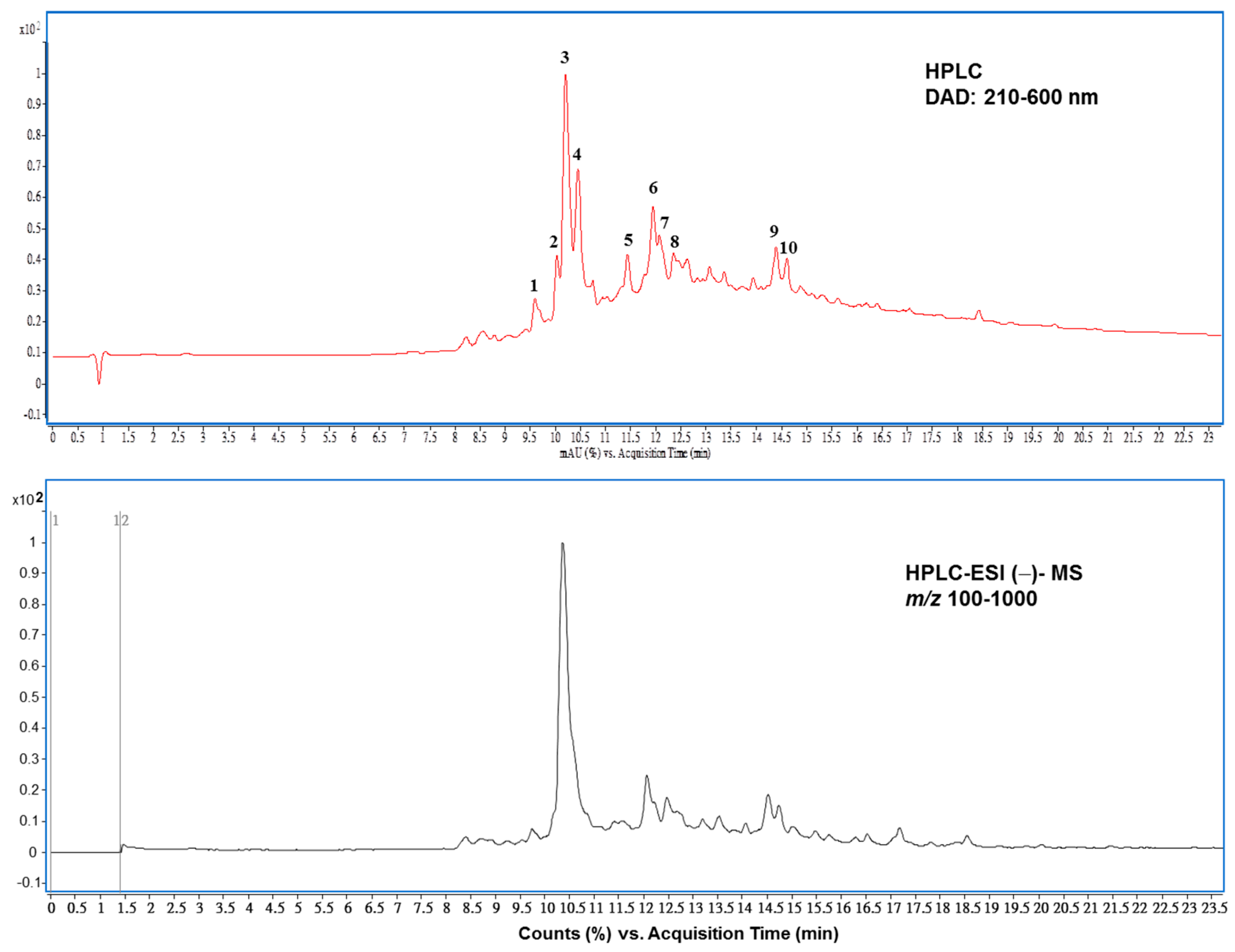
3.3. The Main Compounds in PEHE from HPLC–ESI–MS/MS Analysis
3.4. α-Glucosidase Inhibitory Activities of PEHE and WE
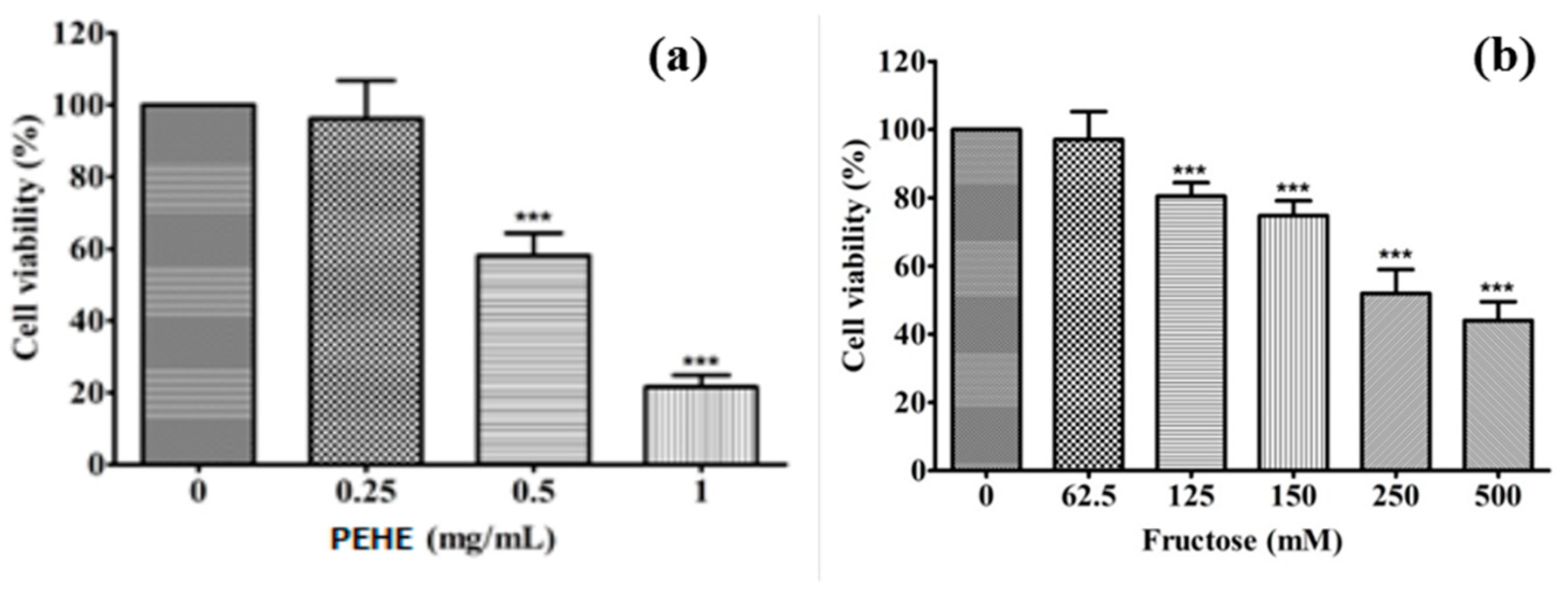
3.5. Effects of Fructose and PEHE on Cell Viability
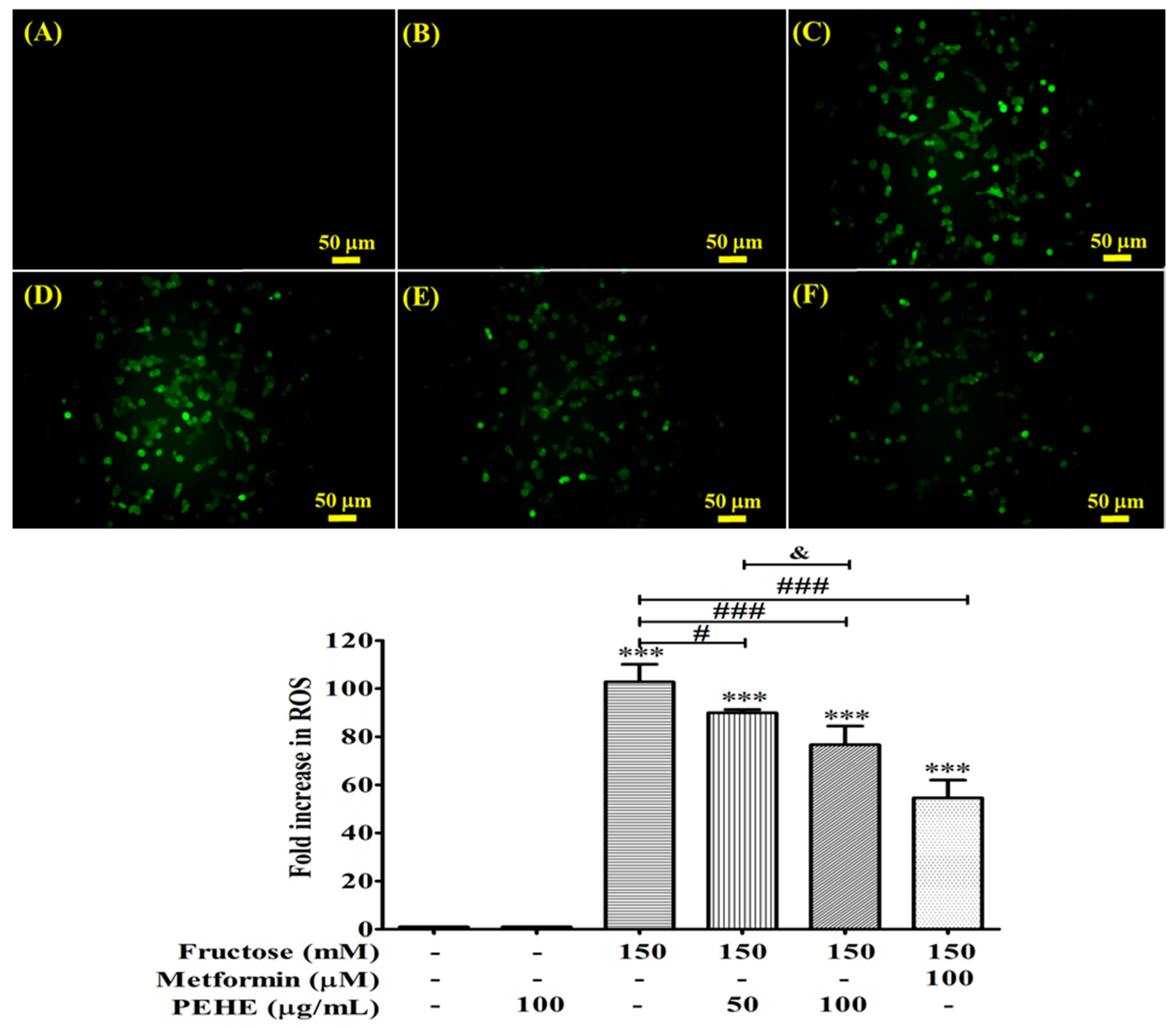
3.6. PEHE Inhibits the Production of Reactive Oxygen Species Affected by Fructose
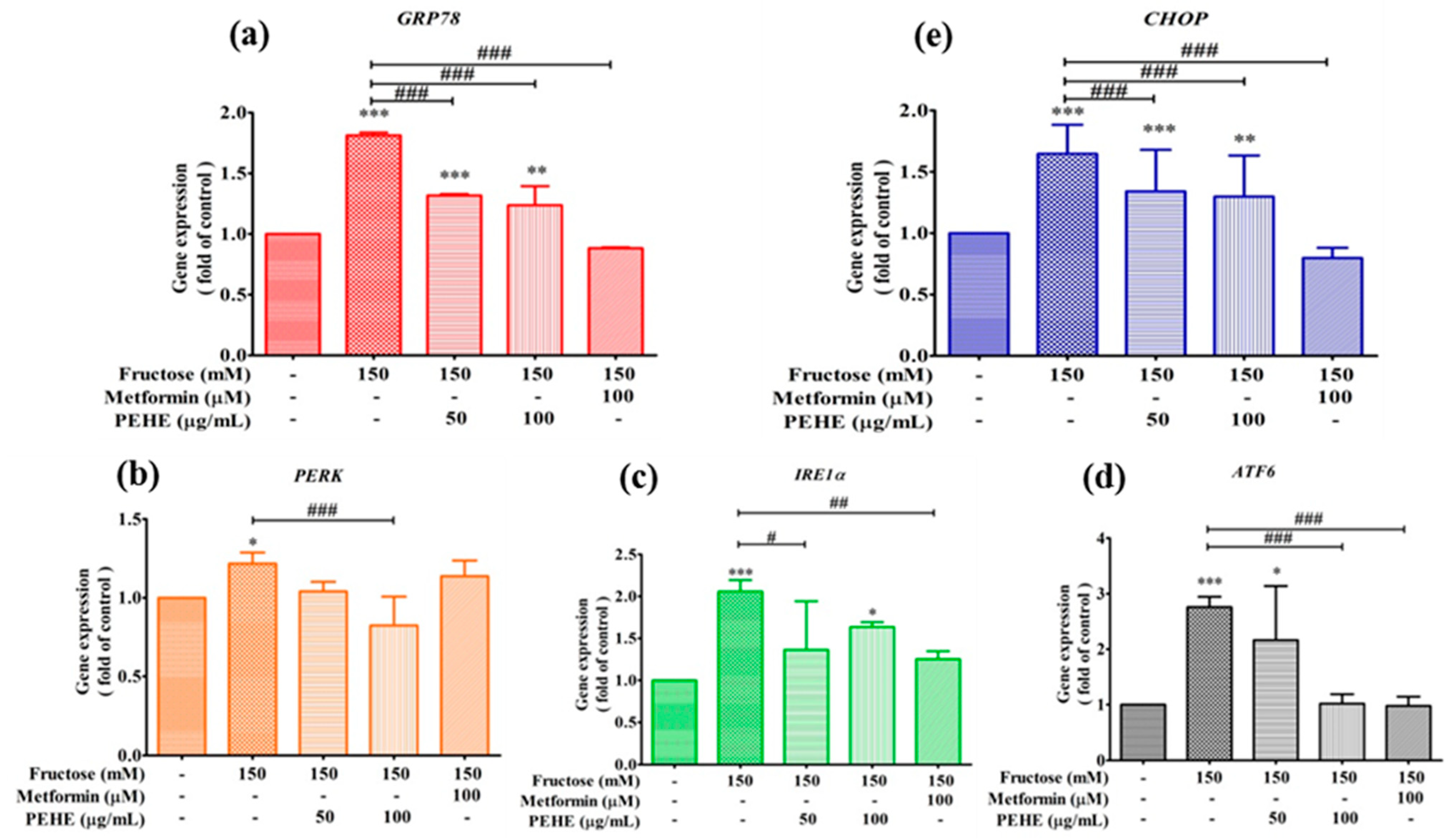
3.7. Effect of PEHE on Fructose-Induced ER Stress Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2:2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| ATF6 | activating transcription factor–6 |
| CHOP | CCAAT-enhancer-binding protein (C/EBP) homologous protein |
| DCFH-DA | dichloro-dihydro-fluorescein diacetate |
| ER | endoplasmic reticulum |
| GRP78 | glucose-regulating protein 78 |
| IC50 | half maximal inhibitory concentration |
| IRE1α | inositol-requiring enzyme 1α |
| PERK | protein kinase R-like endoplasmic reticulum kinase |
| UPR | unfolding protein response |
References
- Kwok, C.Y.; Li, C.; Cheng, H.L.; Ng, Y.F.; Chan, T.Y.; Kwan, Y.W.; Leung, G.P.-H.; Lee, S.M.-Y.; Mok, D.K.-W.; Yu, P.H.-F.; et al. Cholesterol lowering and vascular protective effects of ethanolic extract of dried fruit of Crataegus pinnatifida, hawthorn (Shan Zha), in diet-induced hypercholesterolaemic rat model. J. Funct. Foods 2013, 5, 1326–1335. [Google Scholar] [CrossRef]
- Rigelsky, J.M.; Sweet, B.V. Hawthorn: Pharmacology and therapeutic uses. Am. J. Health Syst. Pharm. 2002, 59, 417–422. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Sun, X.L.; Yang, X.L.; Shi, P.L.; Xu, L.C.; Guo, Q.M. Botany, traditional uses, phytochemistry and pharmacological activity of Crataegus pinnatifida (Chinese hawthorn): A review. J. Pharm. Pharmacol. 2022, 74, 1507–1545. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food applications and potential health benefits of hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic effect of hawthorn in type II diabetes mellitus rat model. J. Sci. Food Agric. 2017, 97, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z.; He, X. Novel triterpenoids isolated from hawthorn berries functioned as antioxidant and antiproliferative activities. J. Funct. Foods 2015, 13, 308–313. [Google Scholar] [CrossRef]
- Zhao, P.; Qiu, S.; Yu, X.Q.; Yao, G.D.; Huang, X.X.; Song, S.J. Three new sesquineolignans from the fruits of Crataegus pinnatifida. J. Asian Nat. Prod. Res. 2021, 23, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Leng, P.; Li, Y.; Zeng, Y.; Sun, Y. Comparative study of antioxidant compounds and antiradical properties of the fruit extracts from three varieties of Crataegus pinnatifida. J. Food Sci. Technol. 2013, 52, 430–436. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lü, D.; Zhou, C.; Yang, B. Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry. Food Chem. 2011, 127, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cao, Y.; Zhao, M. Extraction optimization, purification and antioxidant activity of procyanidins from hawthorn (C. pinnatifida Bge. var. major) fruits. Food Chem. 2010, 119, 1656–1662. [Google Scholar] [CrossRef]
- Cremonini, E.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016, 599, 13–21. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, K.; Zhu, X.; Lu, G.; Jin, D.; Qiu, J.; Zhou, F. Procyanidin B2 suppresses hyperglycemia-induced renal mesangial cell dysfunction by modulating CAV-1-dependent signaling. Exp. Ther. Med. 2022, 24, 496. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes Dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Miki, A.; Ricordi, C.; Sakuma, Y.; Yamamoto, T.; Misawa, R.; Mita, A.; Molano, R.D.; Vaziri, N.D.; Pileggi, A.; Ichii, H.; et al. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS ONE 2018, 13, e0196570. [Google Scholar] [CrossRef]
- Donath, M.Y.; Halban, P.A. Decreased beta-cell mass in diabetes: Significance, mechanisms and therapeutic implications. Diabetologia 2004, 47, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, L.; Xing, Y.; Yang, S.; Li, H.; Cao, Y. Roles and mechanisms of hawthorn and its extracts on atherosclerosis: A Review. Front. Pharmacol. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef]
- Shin, I.S.; Lee, M.Y.; Lim, H.S.; Ha, H.; Seo, C.S.; Kim, J.C.; Shin, H.K. An extract of Crataegus pinnatifida fruit attenuates airway inflammation by modulation of matrix metalloproteinase-9 in ovalbumin induced asthma. PLoS ONE 2012, 7, e45734. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luan, F.; Zhao, Y.; Wu, M.; Lu, Y.; Tao, C.; Zhu, L.; Zhang, C.; Wan, L. Crataegus pinnatifida: A botanical, ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 2023, 301, 115819. [Google Scholar] [CrossRef] [PubMed]
- Committee on Chinese Medicine and Pharmacy. Taiwan Herbl Pharmacopeia English Verion, 3rd ed.; Ministry of Health and Welfare, Executive Yuan, Taiwan, Republic of China: Taipei, Taiwan, 2019.
- Silvia Taga, M.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez-Ruiz, J.; Garcia-Canovas, F.; Acosta, M. Inhibition by l-ascorbic acid and other antioxidants of the 2.2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Wu, S.H.; Hsieh, J.F. Isolation and characterization of α-glucosidase inhibitory constituents from Rhodiola crenulata. Food Res. Int. 2014, 57, 8–14. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and practical applications of 2′,7′-dichlorodihydrofluorescein in redox assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Chyau, C.C.; Wu, H.L.; Peng, C.C.; Huang, S.H.; Chen, C.C.; Chen, C.H.; Peng, R.Y. Potential protection effect of ER homeostasis of N6-(2-hydroxyethyl)adenosine isolated from Cordyceps cicadae in nonsteroidal anti-inflammatory drug-stimulated human proximal tubular cells. Int. J. Mol. Sci. 2021, 22, 1577. [Google Scholar] [CrossRef]
- Schmidt, J. Negative ion electrospray high-resolution tandem mass spectrometry of polyphenols. J. Mass Spectrom. 2016, 51, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.G.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2015, 1, 102. [Google Scholar]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef]
- Kohara, Y.; Ikai, S.; Yoshihara, A.; Murao, K.; Sugiyama, Y. Effect of chronic exposure to ketohexoses on pancreatic β-cell function in INS-1 rat insulinoma cells. Biosci. Biotechnol. Biochem. 2023, 87, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Fujii, J.; Myint, T.; Miyazawa, N.; Islam, K.N.; Kawasaki, Y.; Suzuki, K.; Nakamura, M.; Tatsumi, H.; Yamasaki, Y.; et al. Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells by provoking oxidative stress through the glycation reaction. Biochem. J. 1996, 320, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Sung, P.J.; Kuo, Y.H.; Cheng, M.J.; Chen, J.J. Antioxidant and anti-α-glucosidase activities of various solvent extracts and major bioactive components from the fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef]
- Kicel, A.; Magiera, A.; Skrzywanek, M.; Malczuk, M.; Olszewska, M.A. The inhibition of α-glucosidase, α-amylase and protein glycation by phenolic extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus leaves and fruits: Focus on anti-hyperglycemic activity and kinetic parameters. Molecules 2022, 27, 7081. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guo, X.; Liu, R.H.; You, L.; Abbasi, A.M.; Fu, X. Phenolic contents and cellular antioxidant activity of Chinese hawthorn “Crataegus pinnatifida”. Food Chem. 2015, 186, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. Research on antioxidant activity of flavonoids from natural materials. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lou, X.; Xu, H.; Hanna, M.; Long, Y. Identification and quantification of free, esterified, glycosylated and insoluble-bound phenolic compounds in hawthorn berry fruit (Crataegus pinnatifida) and antioxidant activity evaluation. LWT 2020, 130, 109643. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Prins, J.B.; McGuckin, M.A. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J. Mol. Endocrinol. 2016, 56, R33–R54. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.I.; Wright, B.J.; Hurst, W.J.; van Breemen, R.B. Screening antioxidants using LC-MS: Case study with cocoa. J. Agric. Food Chem. 2009, 57, 5693–5699. [Google Scholar] [CrossRef] [PubMed]
| Content (mg/g) | WE | PEHE |
|---|---|---|
| Total polyphenol (mg GAE/g dw) 1 | 56.53 ± 3.66 b | 122.27 ± 5.02 a |
| Total flavonoid (mg QUE/g dw) 2 | 24.98 ± 1.09 b | 192.47 ± 13.96 a |
| Total triterpenoid (mg GLE/g dw) 3 | 6.04 ± 0.12 b | 6.61 ± 0.02 a |
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| Free Radicals | WE | PEHE | BHT | Trolox |
| DPPH | 1057.36 ± 41.98 a | 379.62 ± 23.21 c | 547.47 ± 9.69 b | -- |
| ABTS•+ | 784.93 ± 19.11 a | 108.99 ± 3.05 c | -- | 186.04 ± 22.60 b |
| Peak No. a | RT (min) | Compound Name | λmax (nm) | [M−H]−1 m/z | MS/MS d m/z | Area % e |
|---|---|---|---|---|---|---|
| 1 | 9.58 | Epicatechin- 4,8′-epicatechin C-hexoside c | 280 | 739 c | 289, 449, 329, 467 | 3.86 |
| 2 | 10.03 | Procyanidin B2 b | 282 | 577 | 407, 289, 125, 245 | 5.25 |
| 3 | 10.20 | Epicatechin b | 230, 278 | 289 | 109, 245, 203, 123 | 35.64 |
| 4 | 10.44 | Epicatechin-4,8′-epi catechin C-hexoside c | 224, 280 | 739 | 289, 197, 449, 167 | 18.91 |
| 5 | 11.43 | Sinapic acid O-hexoside c | 280 | 385 | 223, 179 | 3.06 |
| 6 | 11.94 | Epicatechin derivative | 280 | 401 | 289, 245, 203, 109 | 6.19 |
| 7 | 12.06 | Procyanidin C1 c | 280 | 865 | 287, 695, 245, 407 | 2.99 |
| 8 | 12.35 | Epicatechin derivative | 280 | 647 | 289, 221, 245, 137 | 1.18 |
| 9 | 14.39 | Quercein-3-O-galactoside b | 270, 352 | 463 | 300, 301, 271, 255 | 3.88 |
| 10 | 14.60 | Quercetin-3-O-glucoside b | 270, 352 | 463 | 300, 301, 271, 255 | 2.11 |
| 83.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, H.-M.; Lin, H.-T.; Huang, S.-H.; Chen, Y.-R.; Huang, C.-L.; Chen, C.-C.; Chyau, C.-C. Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells. Foods 2023, 12, 1130. https://doi.org/10.3390/foods12061130
Lien H-M, Lin H-T, Huang S-H, Chen Y-R, Huang C-L, Chen C-C, Chyau C-C. Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells. Foods. 2023; 12(6):1130. https://doi.org/10.3390/foods12061130
Chicago/Turabian StyleLien, Hsiu-Man, Hsin-Tang Lin, Shiau-Huei Huang, Yìng-Ru Chen, Chao-Lu Huang, Chia-Chang Chen, and Charng-Cherng Chyau. 2023. "Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells" Foods 12, no. 6: 1130. https://doi.org/10.3390/foods12061130
APA StyleLien, H.-M., Lin, H.-T., Huang, S.-H., Chen, Y.-R., Huang, C.-L., Chen, C.-C., & Chyau, C.-C. (2023). Protective Effect of Hawthorn Fruit Extract against High Fructose-Induced Oxidative Stress and Endoplasmic Reticulum Stress in Pancreatic β-Cells. Foods, 12(6), 1130. https://doi.org/10.3390/foods12061130





