Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives
Abstract
:1. Introduction
2. Regulatory Aspects
3. Microalgae Bioprocessing
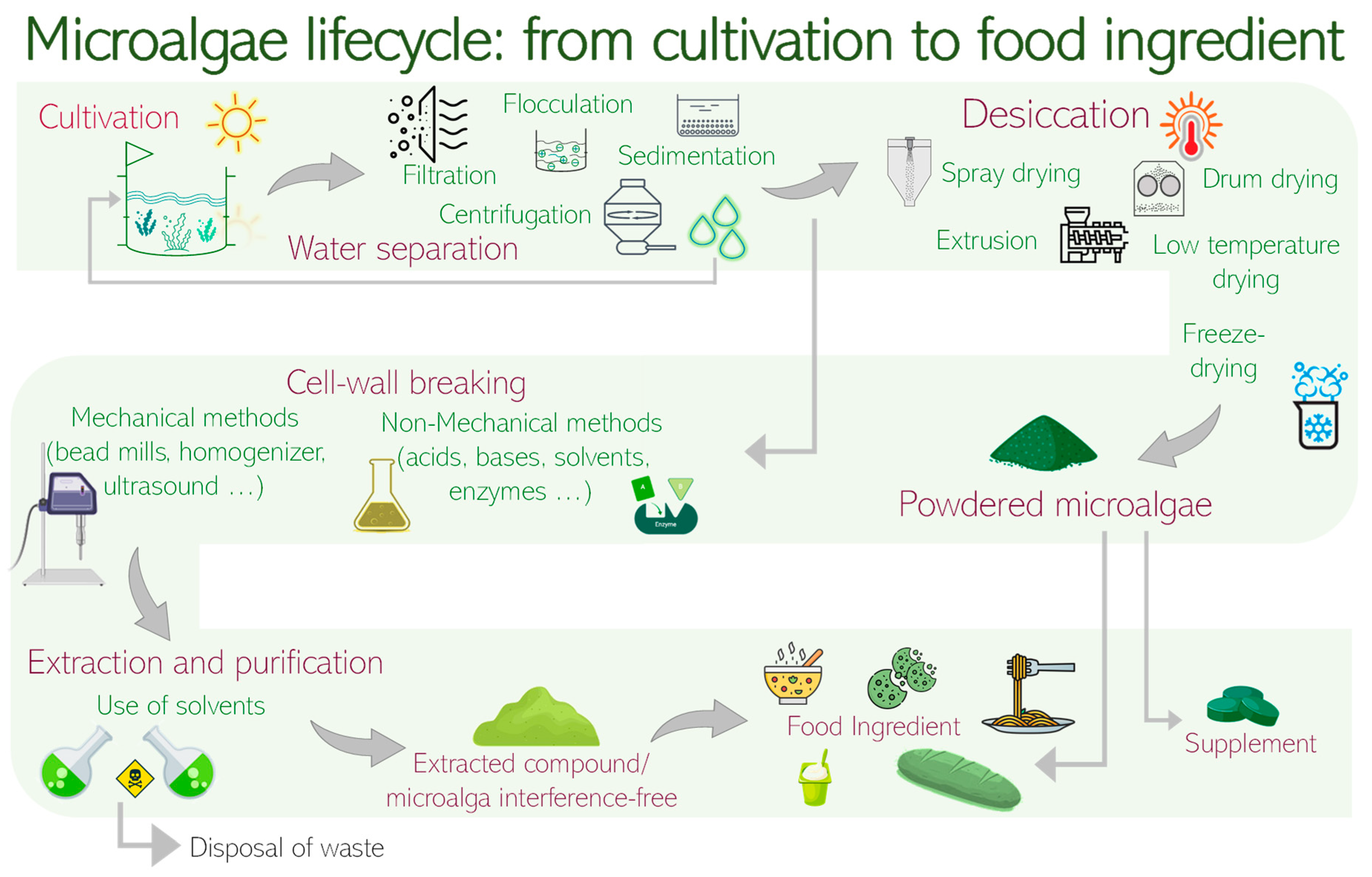
3.1. Production
3.2. Drawbacks for Human Consumption
| Functional Effect | References | |
|---|---|---|
| Proteins | ||
| Bioactive peptides | Hormonal activity, antioxidant, anticoagulant, antihypertensive, immunomodulatory, antimicrobial and cholesterol lowering functions | [30] |
| Phycobiliproteins | Anticancer, anti-inflammatory anti-oxidative, anti-viral, hepato-protective, neuroprotective effect | [38,39] |
| Pigments | ||
| Carotenoids | Anti-inflammatory, anticancer activities, antioxidant effect | [32] |
| Chlorophyll | Accelerate wound healing antioxidant properties | [40] |
| Fucoxanthin | Antioxidant, Antidiabetic, Anti-Inflammatory, and Anti-Obesity activities | [41] |
| Astaxanthin | Antioxidant and antimicrobial activities | [42] |
| Phycocyanin | Antioxidant, anticancer, anticarcinogenic, anti-inflammatory, neuroprotective, hepatoprotective, immunomodulatory, and reno-protective pharmacological effects, and antidiabetic potential | [43] |
| Lipids | ||
| Eicosapentaenoic acid and docosahexaenoic acid | Reduction of complications in cardiovascular, arthritis, and hypertension; hypolipidemic activity | [31] |
| Sterols | ||
| Phytosterol | Immunomodulatory, anti-inflammatory, anti-hypercholesterolemic, antioxidant, anticancer and antidiabetic effects | [44] |
| Polysaccharides | Immunomodulatory, antioxidant, anti-inflammatory, anti-tumor, prebiotic, and antimicrobial activities | [38,45,46] |
| Vitamins | Antioxidant activity | [47] |
| Phenolic compounds | ||
| Apigenin | Autophagy induction in leukemia cells | [48] |
| p-coumaric acid | Antioxidant activity | [49] |
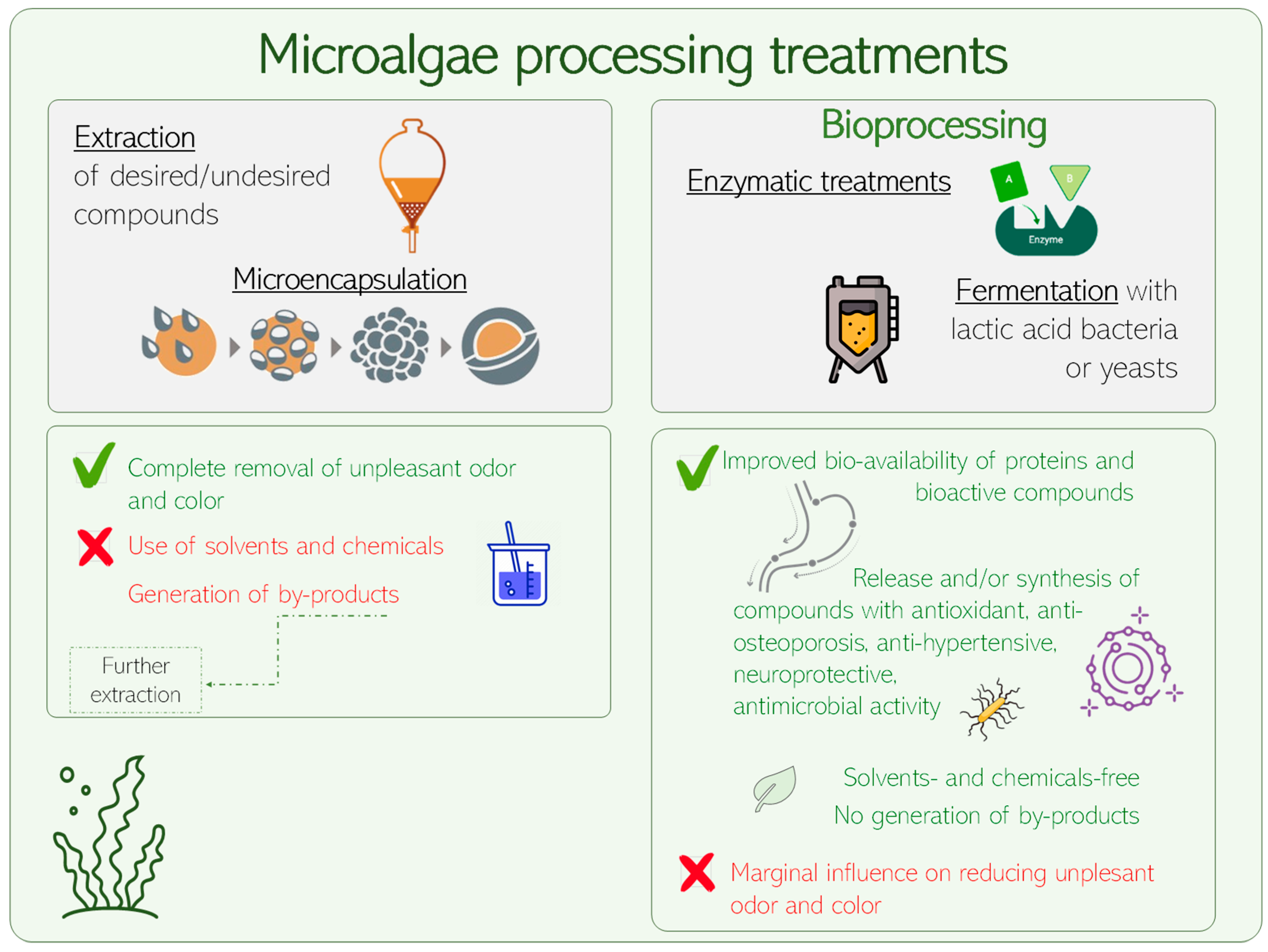
3.3. Metabolites Extraction
3.4. Bioprocessed Microalgae
3.5. Unprocessed Microalgae as Prebiotic
4. Microalgae as Ingredient in Food Making
4.1. Bread and Leavened Products
4.2. Other Baked Goods
4.3. Beverages and Soups
4.4. Snacks
4.5. Pasta
4.6. Dairy Products and Analogous
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, C.; Wollenberg, E.; Benitez, M.; Newman, R.; Gardner, N.; Bellone, F. Roadmap for achieving net-zero emissions in global food systems by 2050. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2020, 531, 735885. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Micro-algal production systems, Microalgae-based products for the food and feed sector: An outlook for Europe. In JRC scientific and policy reports; JRC: Brussels, Belgium, 2014; p. 85709. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Schenk, P.M. Phycology: Algae for Food, Feed, Fuel and the Planet. Phycology 2021, 1, 76–78. [Google Scholar] [CrossRef]
- de Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Futur. Foods 2022, 6, 157. [Google Scholar] [CrossRef]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High variability in nutritional value and safety of commercially available Chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour. Technol. 2018, 275, 247–257. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a super food? J. Cell Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Bashir, S.; Sharif, M.K.; Butt, M.S.; Rizvi, S.S.; Paraman, I.; Ejaz, R. Preparation of Micronutrients Fortified Spirulina Supplemented Rice-Soy Crisps Processed Through Novel Supercritical Fluid Extrusion. J. Food Process Preserv. 2016, 41, 12986. [Google Scholar] [CrossRef]
- Batista, A.P.; Nunes, M.C.; Fradinho, P.; Gouveia, L.; Sousa, I.; Raymundo, A.; Franco, J.M. Novel foods with microalgal ingredients—Effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J. Food Eng. 2012, 110, 182–189. [Google Scholar] [CrossRef]
- Abd El-Razik, M.M.; Mohamed, A.G. Utilization of acid casein curd enriched with Chlorella vulgaris biomass as substitute of egg in mayonnaise production. World Appl. Sci. J. 2013, 26, 917–925. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2021, 62, 4929–4950. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markou, G.; Chentir, I.; Tzovenis, I. Microalgae and cyanobacteria as food: Legislative and safety aspects. In Cultured Micro-algae for the Food Industry; Academic Press: Cambridge, MA, USA, 2021; pp. 249–264. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a novel food. Potential and legal framework. Ernahr. Umsch. 2021, 68, 78–85. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Zuccaro, G.; Yousuf, A.; Pollio, A.; Steyer, J.P. Microalgae Cultivation Systems. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 11–29. [Google Scholar]
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.-H.; Fernández, F.G.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Biological Principles of Mass Cultivation of Photoautotrophic Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 171–204. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmorieux, H.; Hernandez, F. Biochemical and physical criteria of Spirulina after different drying processes. In Proceedings of the 14th International Drying Symposium (IDS), B, São Paulo City, Brazil, 22–25 August 2004; pp. 900–907. [Google Scholar]
- Verni, M.; Dingeo, C.; Rizzello, C.G.; Pontonio, E. Lactic Acid Bacteria Fermentation and Endopeptidase Treatment Improve the Functional and Nutritional Features of Arthrospira platensis. Front. Microbiol. 2021, 12, 744437. [Google Scholar] [CrossRef] [PubMed]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Lee, T.; González-Mariño, G. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Vieira, M.; Pastrana, L.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Becker, W. 18 microalgae in human and animal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 312–351. [Google Scholar]
- Niccolai, A.; Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Nethravathy, N.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Din, N.A.S.; Alayudin, S.M.; Sofian-Seng, N.-S.; Rahman, H.A.; Razali, N.S.M.; Lim, S.J.; Mustapha, W.A.W. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Ruela-De-Sousa, R.R.; Fuhler, G.M.; Blom, N.; Ferreira, C.V.; Aoyama, H.; Peppelenbosch, M.P. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010, 1, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, L.R.; Zhu, S.-T.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermúdez, S.P.; Barba-Davila, B.; O Serna-Saldivar, S.; Parra-Saldivar, R.; Rodriguez-Rodriguez, J.; Morales-Davila, S.; Goiris, K.; Muylaert, K.; Chuck-Hernández, C. Deodorization of Arthrospira platensis biomass for further scale-up food applications. J. Sci. Food Agric. 2017, 97, 5123–5130. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaizola, M.; Huntley, M.E. Recent advances in commercial production of astaxanthin from microalgae. In Biomaterials and Bioprocessing; Fingerman, M., Nagabhushanam, R., Eds.; Science Publishers: Enfield, NH, USA, 2003; pp. 143–164. [Google Scholar]
- Zhang, Y.; Wang, X.; Xie, D.; Zou, S.; Jin, Q.; Wang, X. Synthesis and concentration of 2-monoacylglycerols rich in polyunsaturated fatty acids. Food Chem. 2018, 250, 60–66. [Google Scholar] [CrossRef]
- Estrada, J.E.P.; Bescos, P.B.; del Fresno, A.M.V. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farm. 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process Technol. 2018, 6, 00144. [Google Scholar] [CrossRef] [Green Version]
- Klejdus, B.; Kopecký, J.; Benešová, L.; Vacek, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A 2009, 1216, 763–771. [Google Scholar] [CrossRef]
- Fan, X.; Hu, S.; Wang, K.; Yang, R.; Zhang, X. Coupling of ultrasound and subcritical water for peptides production from Spirulina platensis. Food Bioprod. Process 2020, 121, 105–112. [Google Scholar] [CrossRef]
- Juárez-Oropeza, M.; Mascher, D.; Torres-Durán, P.; Farias, J.; Paredes-Carbajal, M. Effects of Dietary Spirulina on Vascular Reactivity. J. Med. Food 2009, 12, 15–20. [Google Scholar] [CrossRef]
- Sachdeva, R.; Kaur, R.; Sangha, J.K. Effect of Supplementation of Spirulina on the Haematological Profile and Intellectual Status of School Girls (7–9 years). J. Hum. Ecol. 2004, 15, 105–108. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Li, T.-T.; Zhong, R.-T.; Chen, H.-B.; Xia, X.; Gao, L.-Y.; Gao, X.-X.; Liu, B.; Zhang, H.-Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Echevarrieta, L.; Otero, C. Advantageous Preparation of Digested Proteic Extracts from Spirulina platensis Biomass. Catalysts 2019, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Akbarbaglu, Z.; Ayaseh, A.; Ghanbarzadeh, B.; Sarabandi, K. Techno-functional, biological and structural properties of Spirulina platensis peptides from different proteases. Algal Res. 2022, 66, 102755. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2020, 139, 109908. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira Platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Hosoglu, M.I.; Guneser, O.; Karagul-Yuceer, Y. Fermented Spirulina products with Saccharomyces and non- Saccharomyces yeasts: Special reference to their microbial, physico-chemical and sensory characterizations. Food Biosci. 2022, 47, 101691. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2018, 31, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Kang, K.-H.; Ngo, D.-H.; Qian, Z.-J.; Kim, S.-K. Statistical optimization of microalgae Pavlova lutheri cultivation conditions and its fermentation conditions by yeast, Candida rugopelliculosa. Bioresour. Technol. 2012, 107, 307–313. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Jamnik, P.; Mahnič, N.; Mrak, A.; Pogačnik, L.; Jeršek, B.; Niccolai, A.; Rutar, J.M.; Ogrinc, N.; Dušak, L.; Ferjančič, B.; et al. Fermented Biomass of Arthrospira platensis as a Potential Food Ingredient. Antioxidants 2022, 11, 216. [Google Scholar] [CrossRef]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT 2020, 135, 110029. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kang, D.H.; Heo, S.-J.; Lee, H.Y. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Appl. Sci. 2018, 8, 2469. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.-J.; Jung, W.-K.; Kang, K.-H.; Ryu, B.; Kim, S.-K.; Je, J.-Y.; Heo, S.-J.; Oh, C.; Kang, D.-H.; Park, W.S.; et al. In vitro antioxidant activities of the fermented marine microalga pavlova lutheri (Haptophyta) with the yeast hansenula polymorpha1. J. Phycol. 2012, 48, 475–482. [Google Scholar] [CrossRef]
- Qian, Z.; Ryu, B.; Kang, K.; Heo, S.; Kang, D.; Bae, S.Y.; Park, S.; Kim, J.; Kim, Y.; Kim, Y.; et al. Cellular properties of the fermented microalgae Pavlova�lutheri and its isolated active peptide in osteoblastic differentiation of MG-63 cells. Mol. Med. Rep. 2017, 17, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Lim, S.U.; Kim, I.H. Effect of Fermented Chlorella Supplementation on Growth Performance, Nutrient Digestibility, Blood Characteristics, Fecal Microbial and Fecal Noxious Gas Content in Growing Pigs. Asian-Australasian J. Anim. Sci. 2012, 25, 1742–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic efficiency of Spirulina platensis-stimulating growth of lactic acid bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, S.; Xiao, Z. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: Research advances and application challenges. Crit. Rev. Food Sci. Nutr. 2017, 59, 207–227. [Google Scholar] [CrossRef]
- Achour, H.Y.; Doumandji, A.; Sadi, S.; Saadi, S. Evaluation of nutritional and sensory properties of bread enriched with Spir-ulina. An. Rev. Food Sci. Technol. 2014, 15, 270–275. Available online: http://www.afst.valahia.ro/images/documente/2014/issue2/full/section1/s01_w08_full.pdf (accessed on 22 January 2023).
- Figueira, F.D.S.; Crizel, T.D.M.; Silva, C.R.; Salas-Mellado, M.D.L.M. Pão sem glúten enriquecido com a microalga Spirulina platensis. Braz. J. Food Technol. 2011, 14, 308–316. [Google Scholar] [CrossRef]
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT 2018, 89, 466–474. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Singh, B. Evaluation and quality assessment of defatted microalgae meal of Chlorella as an alternative food ingredient in cookies. Nutr. Food Sci. 2019, 49, 221–231. [Google Scholar] [CrossRef]
- Bolanho, B.C.; Egea, B.C.B.; Campos, A.L.M.; De Carvalho-Eliane, J.C.M.; Danesi, D.G. Antioxidant and nutritional potential of cookies enriched with Spirulina platensis and sources of fibre. J. Food Nutr. Res. 2014, 53, 171–179. [Google Scholar]
- Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Santos, T.D.; de Freitas, B.C.B.; Moreira, J.B.; Zanfonato, K.; Costa, J.A.V. Development of powdered food with the addition of Spirulina for food supplementation of the elderly population. Innov. Food Sci. Emerg. Technol. 2016, 37, 216–220. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Goffi, V.; A Gómez, P.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Natural vitamin B12 and fucose supplementation of green smoothies with edible algae and related quality changes during their shelf life. J. Sci. Food Agric. 2017, 98, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Niccolai, A.; Bažec, K.; Rodolfi, L.; Biondi, N.; Zlatić, E.; Jamnik, P.; Tredici, M.R. Lactic Acid Fermentation of Arthrospira platensis (Spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Tańska, M.; Konopka, I.; Ruszkowska, M. Sensory, Physico-Chemical and Water Sorption Properties of Corn Extrudates Enriched with Spirulina. Plant Foods Hum. Nutr. 2017, 72, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Zouari, N.; Abid, M.; Fakhfakh, N.; Ayadi, M.A.; Zorgui, L.; Ayadi, M.; Attia, H. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: Free radical scavenging activity, sensory and cooking characteristics evaluation. Int. J. Food Sci. Nutr. 2011, 62, 811–813. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef] [Green Version]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Khosravi-Darani, K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef]
- Ak, B.; Avşaroğlu, E.; Işık, O.; Özyurt, G.; Kafkas, E.; Etyemez, M.; Uslu, L. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 12, 30–38. [Google Scholar]
- Saharan, V.; Jood, S. Vitamins, minerals, protein digestibility and antioxidant activity of bread enriched with spirulina platensis powder. Int. J. Agric. Sci. 2017, 7, 1292–1297. Available online: www.internationalscholarsjournals.org (accessed on 22 January 2023).
- Casciano, F.; Nissen, L.; Gianotti, A. Effect of formulations and fermentation processes on volatile organic compounds and prebiotic potential of gluten-free bread fortified by spirulina (Arthrospira platensis). Food Funct. 2021, 12, 10226–10238. [Google Scholar] [CrossRef] [PubMed]
- Montevecchi, G.; Santunione, G.; Licciardello, F.; Köker, Ö.; Masino, F.; Antonelli, A. Enrichment of wheat flour with Spirulina. Evaluation of thermal damage to essential amino acids during bread preparation. Food Res. Int. 2022, 157, 111357. [Google Scholar] [CrossRef] [PubMed]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Onopiuk, A.; Zalewska, M.; Ciepłoch, A.; Barotti, L. The effect of different level of Spirulina powder on the chosen quality parameters of shortbread biscuits. J. Food Process Preserv. 2017, 42, e13561. [Google Scholar] [CrossRef]
- Rabelo, S.F.; Lemes, A.C.; Takeuchi, K.P.; Frata, M.T.; de Carvalho, J.C.M.; Danesi, E.D.G. Development of cassava doughnuts enriched with Spirulina platensis biomass. Braz. J. Food Technol. 2013, 16, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Viñas, I.; Villaró, S.; Acién-Fernández, F.G.; Castellari, M.; Aguiló-Aguayo, I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT 2019, 115, 108439. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Ghanbarzadeh, B.; Galali, Y.; Bagheri, H. The physicochemical properties of the spirulina-wheat germ-enriched high-protein functional beverage based on pear-cantaloupe juice. Food Sci. Nutr. 2022, 10, 3651–3661. [Google Scholar] [CrossRef]
- Sadeghi, T.; Mehdi Marvizadeh, M.; Ebrahimi, F.; Mafi, S.; Foughani, O. Assessment of nutritional and antioxidant activity of sport drink enriched with Spirulina platensis. J. Chem. Health Risks 2022, 12, 00. [Google Scholar] [CrossRef]
- Tork, M.B.; Vazifedoost, M.; Hesarinejad, M.A.; Didar, Z.; Zenoozian, M.S. Fabrication of Dragee Containing Spirulina platensis Microalgae to Enrich Corn Snack and Evaluate Its Sensorial, Physicochemical and Nutritional Properties. Foods 2022, 11, 1909. [Google Scholar] [CrossRef]
- da Silva, P.C.; Toledo, T.; Brião, V.; Bertolin, T.E.; Costa, J.A.V. Development of extruded snacks enriched by bioactive peptides from microalga Spirulina sp. LEB 18. Food Biosci. 2021, 42, 101031. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of Spirulina (Arthrospira platensis) Supplementation on Physical and Chemical Properties of Semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef] [PubMed]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2019, 45, 101743. [Google Scholar] [CrossRef]
- De Marco, E.R.; Steffolani, M.E.; Martínez, M.; León, A.E. The use of Nannochloropsis sp. as a source of omega-3 fatty acids in dry pasta: Chemical, technological and sensory evaluation. Int. J. Food Sci. Technol. 2017, 53, 499–507. [Google Scholar] [CrossRef]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of Microalgal Biomass for Innovative Food Production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef]
- Ścieszka, S.; Gorzkiewicz, M.; Klewicka, E. Innovative fermented soya drink with the microalgae Chlorella vulgaris and the probiotic strain Levilactobacillus brevis ŁOCK 0944. LWT 2021, 151, 112131. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Influence of incorporated Spirulina platensis on the growth of microflora and physicochemical properties of ayran as a functional food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Dubey, R.P.; Kumari, P. Preparation of low fat and high protein frozen yoghurt enriched with papaya pulp and Spirulina. Trends Biosci. 2011, 4, 182–184. [Google Scholar]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2018, 102, 230–236. [Google Scholar] [CrossRef]
- Liu, J.-G.; Hou, C.-W.; Lee, S.-Y.; Chuang, Y.; Lin, C.-C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
| Product | Microalgae Used | Nutritional Effect | Sensory and Technological Effect | References |
|---|---|---|---|---|
| Bread | S. platensis | Increase of proteins, amino acids, and ashes content | Volume decrease, increase in crumb hardness and colour modification | [86,87] |
| C. vulgaris | Positive impact on viscoelastic characteristics, with strengthening of the gluten network | [88] | ||
| Crostini | A. platensis | Increase of phycocyanin, total phenolic content and radical scavenging activity on DPPH radical | Appropriate volume after fermentation | [89] |
| Cookies | S. platensis, C. vulgaris, Tetraselmis suecica, and Phaeodactylum tricornutum | High protein and total phenolic content and in vitro antioxidant capacity | Colour modification depending on the microalga, decrease of hardness | [90] |
| Chlorophyll extracted from Chlorella sp. | Increase of ash content. | Increase in weight, thickness, and moisture content. Decrease in pasting viscosities. Dark colour and increased hardness | [91] | |
| S. platensis | Increase of protein, ash, fibre, total phenolic content, and antioxidant activity | Interference in elastic net, colour modification | [92] | |
| Astaxanthin from H. pluvialis | Reduction of glucose released during in vitro digestion, increase in the total phenolic content, and antioxidant capacity | Reduction of height and diameter gain, and weight loss | [93] | |
| C. vulgaris | Poor colour stability during storage, increased firmness | [94] | ||
| Shake-type powdered food | Spirulina sp. | Increase of protein content, reduction of carbohydrates and lipid content | Shelf-life reduction | [95] |
| Smoothies with grapes, broccoli, cucumber | Several ground dried powder of edible algae, including C. vulgaris and Spirulina spp | Supplied 50–60% of the recommended intake of vitamin C | Stronger marine odour and flavour | [96] |
| Broccoli soup | Spirulina sp., Chlorella sp., or Tetraselmis sp. | Increase of polyphenols content and higher antioxidant capacity | Low acceptability scores | [97] |
| Vegetal soybean drink | A. platensis | Increase of in vitro and in vivo antioxidant activity, reduction of intracellular oxidation level | [98] | |
| Extruded snacks | Spirulina | Increase of protein, lipid, and minerals content | High acceptability index | [99] |
| Spirulina | Increase of nutritional qualities, phycocyanin was destroyed. | Decrease in lightness and expansion indices, increase in softness, greenness, and yellowness. Low acceptability scores | [100] | |
| Artisanal wheat crackers | A. platensis, C. vulgaris, T. suecica, and Phaeodactylum tricornutum | Higher digestibility, antioxidant activity and protein content | Darker colour, reduction of crackers’ width and thickness | [101] |
| Pasta | Spirulina | Higher antioxidant activity, protein, and phenolic compounds content. Lower protein digestibility | Structure little compromised compared to control | [102] |
| C. vulgaris and S. maxima | Decrease in protein content after cooking | Stability of colour after cooking, increase in firmness. higher acceptance scores than the control pasta. | [103] | |
| A. platensis (spray-dried or lyophilized biomass) | Increase in antioxidant activity. In vitro protein digestibility showed opposite trends for pastas obtained with either microalgae biomass | Accentuated green tonality, low cooking loss, increase in swelling index | [104] | |
| Fermented milk | Microencapsulated S. platensis | Anti-inflammatory activity Antimicrobial activity against Pseudomonas aeruginosa | Absence of unpleasant fish-like odor, homogeneous appearance | [105] |
| C. vulgaris and A. platensis | Higher viability of probiotic | Unpleasant flavour, colour changes to greenish or bluish | [106] | |
| Ice-cream | A. platensis | Higher protein and fat content | Lower melting time, shorter first drop times, higher overrun, lower viscosity | [107] |
| Cheese analogous | C. vulgaris or A. platensis | Higher protein content, higher antioxidant activity | Lower melting index, higher firmness values | |
| Cheese | C. vulgaris or A. platensis | Higher antioxidant activity | Granular texture, bitter aroma and taste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verni, M.; Demarinis, C.; Rizzello, C.G.; Pontonio, E. Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives. Foods 2023, 12, 983. https://doi.org/10.3390/foods12050983
Verni M, Demarinis C, Rizzello CG, Pontonio E. Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives. Foods. 2023; 12(5):983. https://doi.org/10.3390/foods12050983
Chicago/Turabian StyleVerni, Michela, Chiara Demarinis, Carlo Giuseppe Rizzello, and Erica Pontonio. 2023. "Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives" Foods 12, no. 5: 983. https://doi.org/10.3390/foods12050983
APA StyleVerni, M., Demarinis, C., Rizzello, C. G., & Pontonio, E. (2023). Bioprocessing to Preserve and Improve Microalgae Nutritional and Functional Potential: Novel Insight and Perspectives. Foods, 12(5), 983. https://doi.org/10.3390/foods12050983









