Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Characterization Methods
2.3. Protein Extraction from Oat Flakes
2.3.1. Oat-Protein-Extraction Hydrolyzing Starch by Alpha Amylase
2.3.2. Oat-protein-Extraction Hydrolyzing Starch by HTAA and Non-Starch Polysaccharides by XYL
2.3.3. Oat-Protein-Extraction Shifting-Ionic Strength of the Solution
2.4. Protein Solubility
2.5. Water and Oil-Holding Capacity
2.6. Foaming
2.7. Data Processing
3. Results and Discussion
3.1. Protein Extraction
3.1.1. Protein Recovery
3.1.2. AAs in Protein Concentrates
3.1.3. AA Redistribution among the Samples A1, AR1, AF1
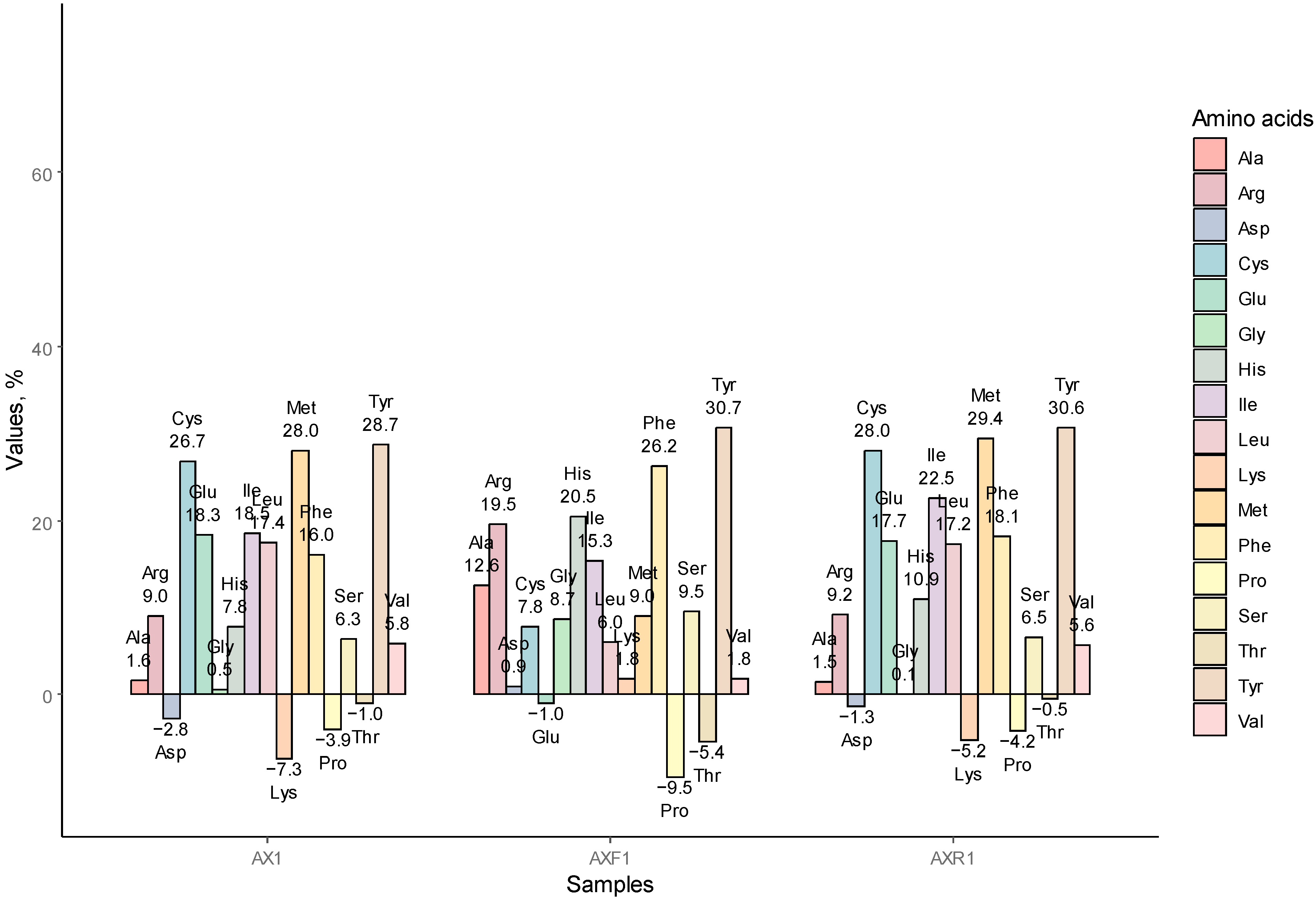
3.1.4. AA Redistribution among the AX1, AXR1, and AXF1
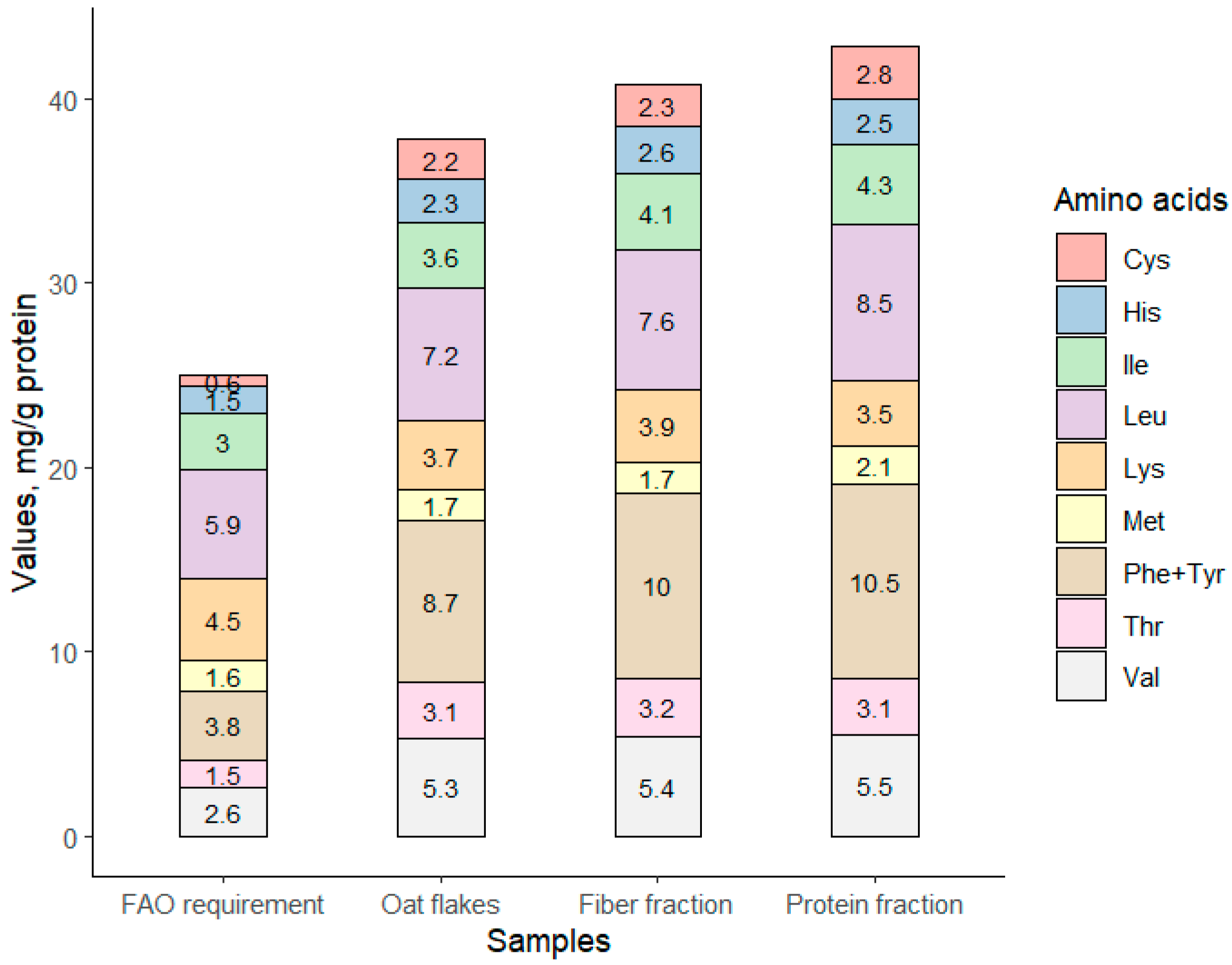
3.1.5. Nutritional Value of Fractioned Oat
3.2. Protein Solubility
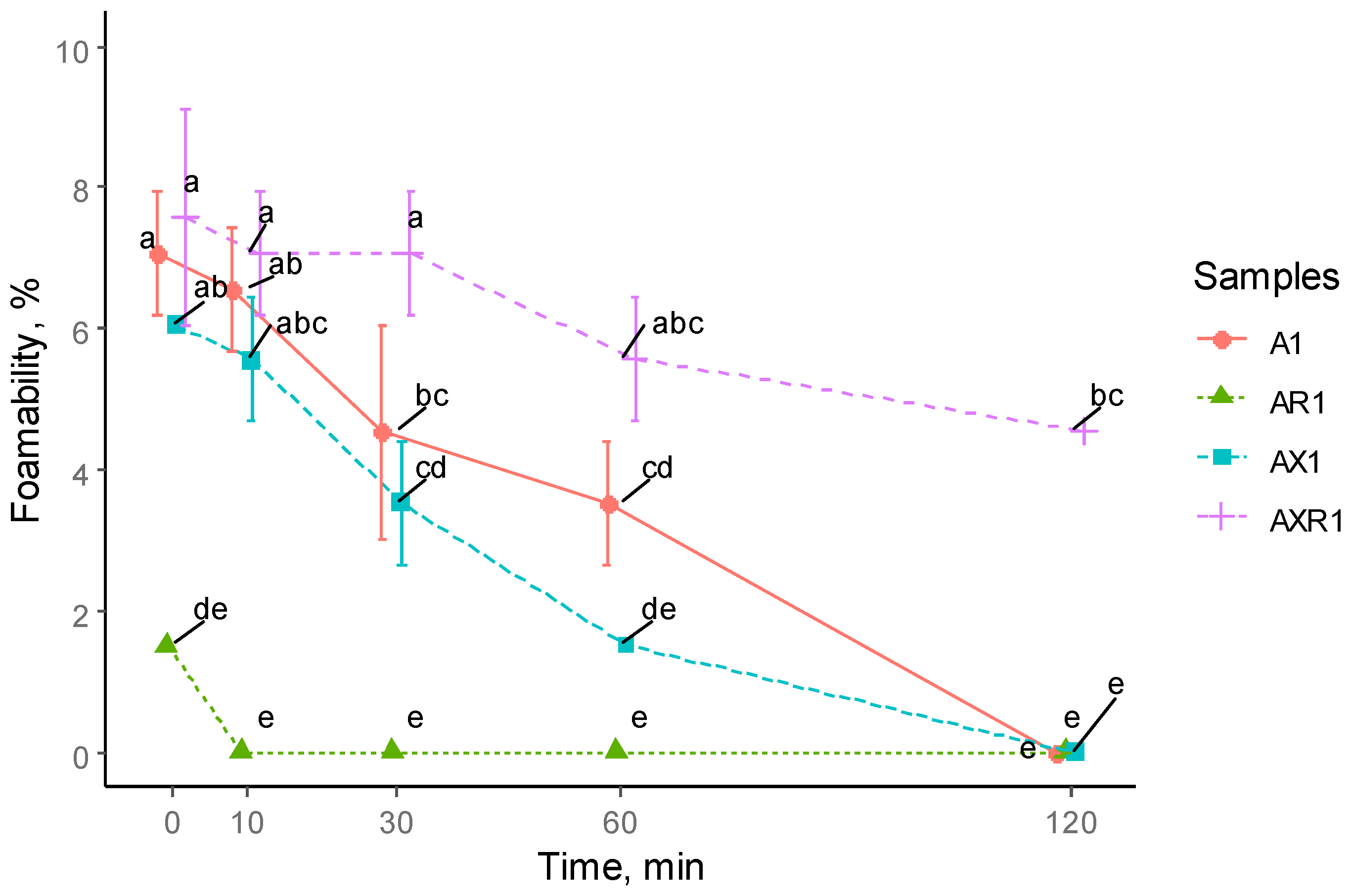
3.3. Foaming Properties
3.4. Water/Oil-Holding Capacity
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klose, C.; Arendt, E.K. Proteins in Oats; Their Synthesis and Changes during Germination: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, B. Development of High-Protein Oat for the Feed and Food Industry; Lund University: Lund, Sweden, 2016; ISBN 978-91-7623-937-7. [Google Scholar]
- Ahola, H.G.; Sontag-Strohm, T.S.; Schulman, A.H.; Tanhuanpää, P.; Viitala, S.; Huang, X. Immunochemical Analysis of Oat Avenins in an Oat Cultivar and Landrace Collection. J. Cereal Sci. 2020, 95, 103053. [Google Scholar] [CrossRef]
- Cabanillas, B. Gluten-Related Disorders: Celiac Disease, Wheat Allergy, and Nonceliac Gluten Sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Pinto-Sánchez, M.I.; Causada-Calo, N.; Bercik, P.; Ford, A.C.; Murray, J.A.; Armstrong, D.; Semrad, C.; Kupfer, S.S.; Alaedini, A.; Moayyedi, P.; et al. Safety of Adding Oats to a Gluten-Free Diet for Patients With Celiac Disease: Systematic Review and Meta-Analysis of Clinical and Observational Studies. Gastroenterology 2017, 153, 395–409.e3. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.M. CHAPTER 8—Storage Proteins. In Oats, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; AACC International Press: Washington, DC, USA, 2011; pp. 123–142. ISBN 978-1-891127-64-9. [Google Scholar]
- Ercili-Cura, D.; Miyamoto, A.; Paananen, A.; Yoshii, H.; Poutanen, K.; Partanen, R. Adsorption of Oat Proteins to Air–Water Interface in Relation to Their Colloidal State. Food Hydrocoll. 2015, 44, 183–190. [Google Scholar] [CrossRef]
- Loponen, J.; Laine, P.; Sontag-Strohm, T.; Salovaara, H. Behaviour of Oat Globulins in Lactic Acid Fermentation of Oat Bran. Eur. Food Res. Technol. 2007, 225, 105–110. [Google Scholar] [CrossRef]
- Sterna, V.; Zute, S.; Brunava, L. Oat Grain Composition and Its Nutrition Benefice. Agric. Agric. Sci. Procedia 2016, 8, 252–256. [Google Scholar] [CrossRef]
- Ma, C.Y. Chemical Characterization and Functionality Assessment of Protein Concentrates from Oats. Cereal Chem. 1983, 60, 36–42. [Google Scholar]
- Zhang, B.; Guo, X.; Zhu, K.; Peng, W.; Zhou, H. Improvement of Emulsifying Properties of Oat Protein Isolate–Dextran Conjugates by Glycation. Carbohydr. Polym. 2015, 127, 168–175. [Google Scholar] [CrossRef]
- Sibakov, J.; Myllymäki, O.; Holopainen, U.; Kaukovirta-Norja, A.; Hietaniemi, V.; Pihlava, J.M.; Poutanen, K.; Lehtinen, P. Lipid Removal Enhances Separation of Oat Grain Cell Wall Material from Starch and Protein. J. Cereal Sci. 2011, 54, 104–109. [Google Scholar] [CrossRef]
- Spaen, J.; Silva, J.V.C. Oat Proteins: Review of Extraction Methods and Techno-Functionality for Liquid and Semi-Solid Applications. LWT 2021, 147, 111478. [Google Scholar] [CrossRef]
- Cluskey, J.E.; Wu, Y.V.; Inglett, G.E.; Wall, J.S. Oat Protein Concentrates for Beverage Fortification. J. Food Sci. 1976, 41, 799–804. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Noma, A.T. Factors Governing Lysinoalanine Formation in Soy Proteins. J. Food Sci. 1984, 49, 1282–1288. [Google Scholar] [CrossRef]
- Liu, K. Fractionation of Oats into Products Enriched with Protein, Beta-Glucan, Starch, or Other Carbohydrates. J. Cereal Sci. 2014, 60, 317–322. [Google Scholar] [CrossRef]
- Guan, X.; Yao, H. Optimization of Viscozyme L-Assisted Extraction of Oat Bran Protein Using Response Surface Methodology. Food Chem. 2008, 106, 345–351. [Google Scholar] [CrossRef]
- Jodayree, S.; Smith, J.C.; Tsopmo, A. Use of Carbohydrase to Enhance Protein Extraction Efficiency and Antioxidative Properties of Oat Bran Protein Hydrolysates. Food Res. Int. 2012, 46, 69–75. [Google Scholar] [CrossRef]
- Prosekov, A.; Babich, O.; Kriger, O.; Ivanova, S.; Pavsky, V.; Sukhikh, S.; Yang, Y.; Kashirskih, E. Functional Properties of the Enzyme-Modified Protein from Oat Bran. Food Biosci. 2018, 24, 46–49. [Google Scholar] [CrossRef]
- van Hee, P.; Hoeben, M.A.; van der Lans, R.G.J.M.; van der Wielen, L.A.M. Strategy for Selection of Methods for Separation of Bioparticles from Particle Mixtures. Biotechnol. Bioeng. 2006, 94, 689–709. [Google Scholar] [CrossRef]
- Durand, D.; Christophe Gimel, J.; Nicolai, T. Aggregation, Gelation and Phase Separation of Heat Denatured Globular Proteins. Phys. A Stat. Mech. Its Appl. 2002, 304, 253–265. [Google Scholar] [CrossRef]
- Li, R.; Xiong, Y.L. Sensitivity of Oat Protein Solubility to Changing Ionic Strength and PH. J. Food Sci. 2021, 86, 78–85. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Buren, J.P.V.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of Different Alcoholic Extraction Conditions on Soybean Oil Yield, Fatty Acid Composition and Protein Solubility of Defatted Meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of Succinylation and Deamidation on Functional Properties of Oat Protein Isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. JOSS 2021, 6, 3167. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 16 December 2022).
- Posit Team. RStudio: Integrated Development. 2022. Environment for R. Posit Software, PBC, Boston, MA.. Available online: http://www.posit.co/ (accessed on 16 December 2022).
- Immonen, M.; Myllyviita, J.; Sontag-Strohm, T.; Myllärinen, P. Oat Protein Concentrates with Improved Solubility Produced by an Enzyme-Aided Ultrafiltration Extraction Method. Foods 2021, 10, 3050. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Yi, C.; Hua, Y.; Yang, C.; Cui, S. Effect of Ionic Strength on the Heat-Induced Soy Protein Aggregation and the Phase Separation of Soy Protein Aggregate/Dextran Mixtures. Food Hydrocoll. 2009, 23, 1015–1023. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Zhang, T.; Ju, X.; He, R. Effects of Acylation and Glycation Treatments on Physicochemical and Gelation Properties of Rapeseed Protein Isolate. RSC Adv. 2018, 8, 40395–40406. [Google Scholar] [CrossRef]
- Franks, F.; Eagland, D.; Lumry, R. The Role of Solvent Interactions in Protein Conformatio. CRC Crit. Rev. Biochem. 1975, 3, 165–219. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Rout, M.K.; Chan, W.-M.; Phillips, D.L. Raman Spectroscopic Study of Oat Globulin Conformation. J. Agric. Food Chem. 2000, 48, 1542–1547. [Google Scholar] [CrossRef]
- Paulson, A.T.; Tung, M.A. Solubility, Hydrophobicity and Net Charge of Succinylated Canola Protein Isolate. J. Food Sci. 1987, 52, 1557–1561. [Google Scholar] [CrossRef]
- Jing, X.; Yang, C.; Zhang, L. Characterization and Analysis of Protein Structures in Oat Bran: Characterization of Protein Structures. J. Food Sci. 2016, 81, C2337–C2343. [Google Scholar] [CrossRef]
- Burgess, S.R.; Shewry, P.R.; Matlashewski, G.J.; Altosaar, I.; Miflin, B.J. Characteristics Of Oat (Avena sativa L.) Seed Globulins. J. Exp. Bot. 1983, 34, 1320–1332. [Google Scholar] [CrossRef]
- McDonald, A.G.; Tipton, K.F. Enzyme Nomenclature and Classification: The State of the Art. FEBS J. 2021. [Google Scholar] [CrossRef]
- FAO; Joint Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition; Weltgesundheitsorganisation; United Nations University. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- Averina, E.; Konnerth, J.; D’Amico, S.; van Herwijnen, H.W.G. Protein Adhesives: Alkaline Hydrolysis of Different Crop Proteins as Modification for Improved Wood Bonding Performance. Ind. Crops Prod. 2021, 161, 113187. [Google Scholar] [CrossRef]
- Feng, J.; Berton-Carabin, C.C.; Ataç Mogol, B.; Schroën, K.; Fogliano, V. Glycation of Soy Proteins Leads to a Range of Fractions with Various Supramolecular Assemblies and Surface Activities. Food Chem. 2021, 343, 128556. [Google Scholar] [CrossRef]
- Walters, M.E.; Udenigwe, C.C.; Tsopmo, A. Structural Characterization and Functional Properties of Proteins from Oat Milling Fractions. J. Am. Oil Chem. Soc. 2018, 95, 991–1000. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and Functional Evaluation of Oat Protein Isolate-Pleurotus Ostreatus β-Glucan Conjugates Formed via Maillard Reaction. Food Hydrocoll. 2019, 87, 459–469. [Google Scholar] [CrossRef]
- Brückner-Gühmann, M.; Heiden-Hecht, T.; Sözer, N.; Drusch, S. Foaming Characteristics of Oat Protein and Modification by Partial Hydrolysis. Eur. Food Res. Technol. 2018, 244, 2095–2106. [Google Scholar] [CrossRef]
- Yue, J.; Gu, Z.; Zhu, Z.; Yi, J.; Ohm, J.-B.; Chen, B.; Rao, J. Impact of Defatting Treatment and Oat Varieties on Structural, Functional Properties, and Aromatic Profile of Oat Protein. Food Hydrocoll. 2021, 112, 106368. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, Secondary Structure, and Self-Assembly of Oat Protein Isolate. J. Agric. Food Chem. 2009, 57, 4552–4558. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring Colloidal Oat and Faba Bean Protein Particles via Enzymatic Modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, O.; Sontag-Strohm, T.; Salovaara, H.; Lampi, A.-M.; Sibakov, J.; Loponen, J. Foaming of Differently Processed Oats: Role of Nonpolar Lipids and Tryptophanin Proteins. Cereal Chem. J. 2011, 88, 239–244. [Google Scholar] [CrossRef]
- Runyon, J.R.; Nilsson, L.; Alftrén, J.; Bergenståhl, B. Characterization of Oat Proteins and Aggregates Using Asymmetric Flow Field-Flow Fractionation. Anal. Bioanal. Chem. 2013, 405, 6649–6655. [Google Scholar] [CrossRef] [PubMed]

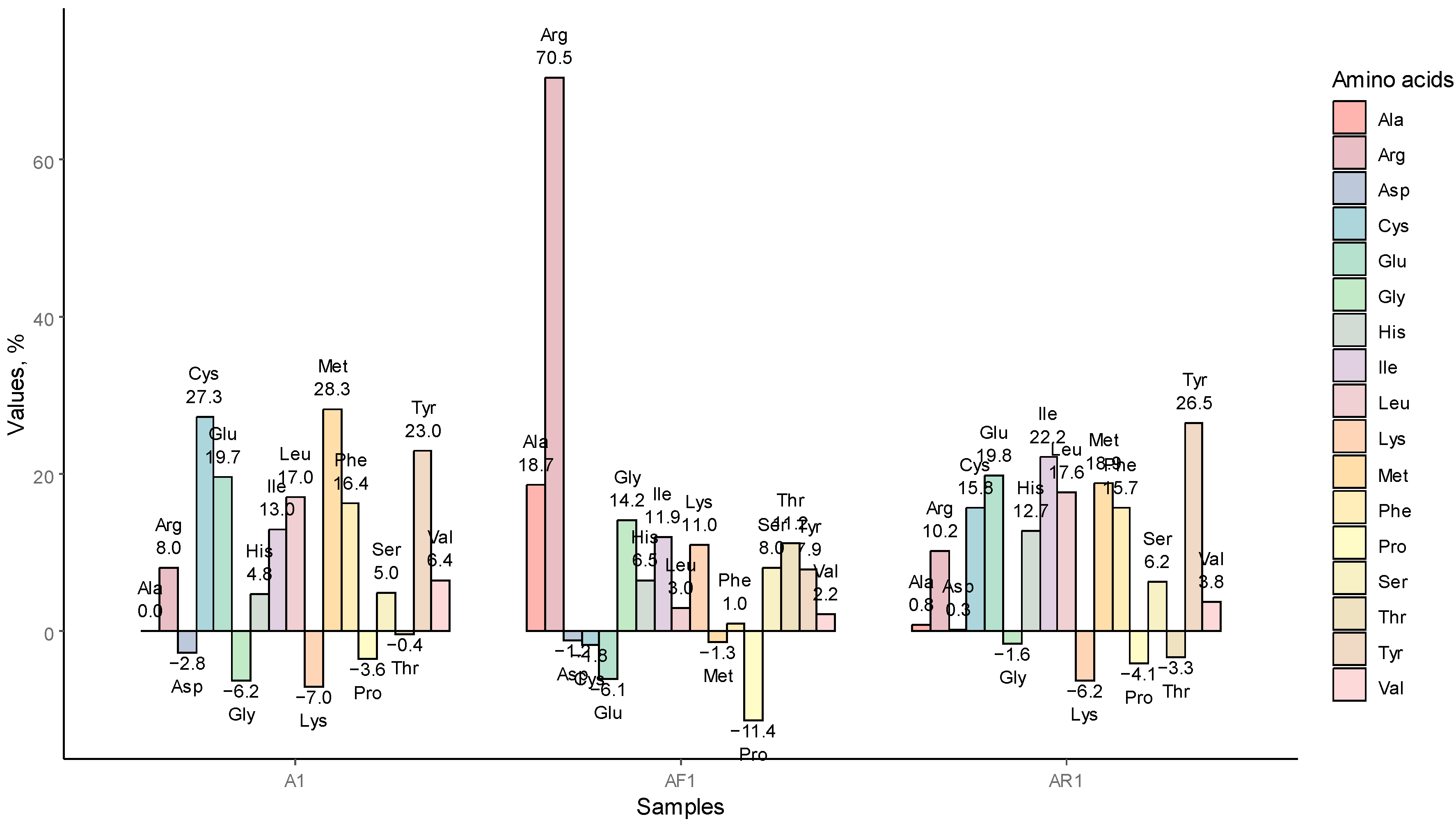


| Samples | Protein | Crude Oil | Fiber | Protein Yield * |
|---|---|---|---|---|
| FL1 | 17.56 ± 0.03 d | 5.7 ± 0.11 d | 2.1 ± 0.1 b | NA |
| A1 | 84.64 ± 1.64 a | 3.0 ± 0.14 e | 1.4 ± 0.08 c | 35.9 ± 0.70 c |
| AR1 | 84.2 ± 1.89 a | 5.2 ± 0.06 d | NA | 44.8 ± 0.83 b |
| AX1 | 85.86 ± 1.80 a | 5.7 ± 0.08 d | 1.1 ± 0.05 c | 28.1 ± 0.56 e |
| AXR1 | 86.46 ± 2.23 a | 6.3 ± 0.20 c | NA | 33.1 ± 0.51 d |
| AF1 | 30.30 ± 0.62 c | 7.6 ± 0.16 b | 5.5 ± 0.37 a | 35.9 ± 0.73 c |
| AXF1 | 39.36 ± 0.76 b | 9.0 ± 0.40 a | 5.2 ± 0.34 a | 47.6 ± 1.02 a |
| FL1 | A1 | AR1 | AX1 | AXR1 | AF1 | AXF1 | FL1 |
|---|---|---|---|---|---|---|---|
| Ala | 3.77 ± 0.13 b | 3.77 ± 0.05 b | 3.8 ± 0.1 b | 3.83 ± 0.05 b | 3.83 ± 0.05 b | 4.48 ± 0.14 a | 4.25 ± 0.11 a |
| Arg | 6.55 ± 0.17 d | 7.08 ± 0.19 cd | 7.22 ± 0.08 c | 7.14 ± 0.16 c | 7.16 ± 0.34 c | 11.17 ± 0.21 a | 7.83 ± 0.15 b |
| Asp | 7.54 ± 0.28 a | 7.33 ± 0.33 a | 7.57 ± 0.2 a | 7.33 ± 0.17 a | 7.44 ± 0.2 a | 7.45 ± 0.17 a | 7.61 ± 0.32 a |
| Cys | 2.23 ± 0.05 c | 2.83 ± 0.12 a | 2.58 ± 0.08 b | 2.82 ± 0.03 a | 2.85 ± 0.03 a | 2.18 ± 0.09 c | 2.4 ± 0.12 bc |
| Phe | 5.07 ± 0.18 c | 5.9 ± 0.08 b | 5.86 ± 0.22 b | 5.88 ± 0.06 b | 5.99 ± 0.07 ab | 5.12 ± 0.25 c | 6.4 ± 0.17 a |
| Gly | 4.39 ± 0.14 bc | 4.12 ± 0.14 c | 4.32 ± 0.19 c | 4.41 ± 0.19 bc | 4.39 ± 0.12 bc | 5.01 ± 0.15 a | 4.77 ± 0.08 ab |
| Glu | 19.91 ± 0.42 b | 23.84 ± 0.65 a | 23.85 ± 0.79 a | 23.55 ± 0.31 a | 23.44 ± 0.47 a | 18.7 ± 0.3 b | 19.71 ± 0.76 b |
| His | 2.29 ± 0.05 c | 2.4 ± 0.04 bc | 2.58 ± 0.12 ab | 2.46 ± 0.07 bc | 2.54 ± 0.05 b | 2.44 ± 0.1 bc | 2.76 ± 0.08 a |
| Ile | 3.59 ± 0.16 c | 4.05 ± 0.06 b | 4.38 ± 0.14 a | 4.25 ± 0.13 ab | 4.39 ± 0.06 a | 4.01 ± 0.06 b | 4.14 ± 0.15 ab |
| Leu | 7.23 ± 0.13 b | 8.46 ± 0.17 a | 8.51 ± 0.19 a | 8.49 ± 0.1 a | 8.47 ± 0.36 a | 7.45 ± 0.3 b | 7.66 ± 0.17 b |
| Lys | 3.71 ± 0.05 bc | 3.45 ± 0.08 d | 3.48 ± 0.06 cd | 3.44 ± 0.05 d | 3.51 ± 0.16 cd | 4.12 ± 0.04 a | 3.78 ± 0.11 b |
| Met | 1.67 ± 0.08 c | 2.14 ± 0.02 a | 1.99 ± 0.04 ab | 2.14 ± 0.08 a | 2.16 ± 0.06 a | 1.65 ± 0.08 c | 1.82 ± 0.05 bc |
| Pro | 6.18 ± 0.14 a | 5.96 ± 0.15 ab | 5.93 ± 0.26 ab | 5.94 ± 0.25 ab | 5.93 ± 0.11 ab | 5.48 ± 0.25 b | 5.6 ± 0.16 b |
| Ser | 4.08 ± 0.05 a | 4.28 ± 0.17 a | 4.33 ± 0.15 a | 4.34 ± 0.13 a | 4.34 ± 0.17 a | 4.41 ± 0.13 a | 4.47 ± 0.2 a |
| Tyr | 3.59 ± 0.12 b | 4.41 ± 0.2 a | 4.54 ± 0.12 a | 4.61 ± 0.2 a | 4.68 ± 0.07 a | 3.87 ± 0.12 b | 4.69 ± 0.16 a |
| Thr | 3.09 ± 0.08 b | 3.08 ± 0.14 b | 2.99 ± 0.12 b | 3.06 ± 0.1 b | 3.08 ± 0.05 b | 3.44 ± 0.07 a | 2.92 ± 0.04 b |
| Val | 5.25 ± 0.26 a | 5.59 ± 0.11 a | 5.45 ± 0.08 a | 5.56 ± 0.2 a | 5.55 ± 0.21 a | 5.37 ± 0.17 a | 5.35 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargautis, D.; Kince, T. Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties. Foods 2023, 12, 965. https://doi.org/10.3390/foods12050965
Sargautis D, Kince T. Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties. Foods. 2023; 12(5):965. https://doi.org/10.3390/foods12050965
Chicago/Turabian StyleSargautis, Darius, and Tatjana Kince. 2023. "Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties" Foods 12, no. 5: 965. https://doi.org/10.3390/foods12050965
APA StyleSargautis, D., & Kince, T. (2023). Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties. Foods, 12(5), 965. https://doi.org/10.3390/foods12050965





