Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
2.3. Determination of the pH Value of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
2.4. Determination of the Water-Holding Capacity of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
2.5. Determination of Texture Profile Analysis of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
2.6. Determination of the Rheological Properties of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
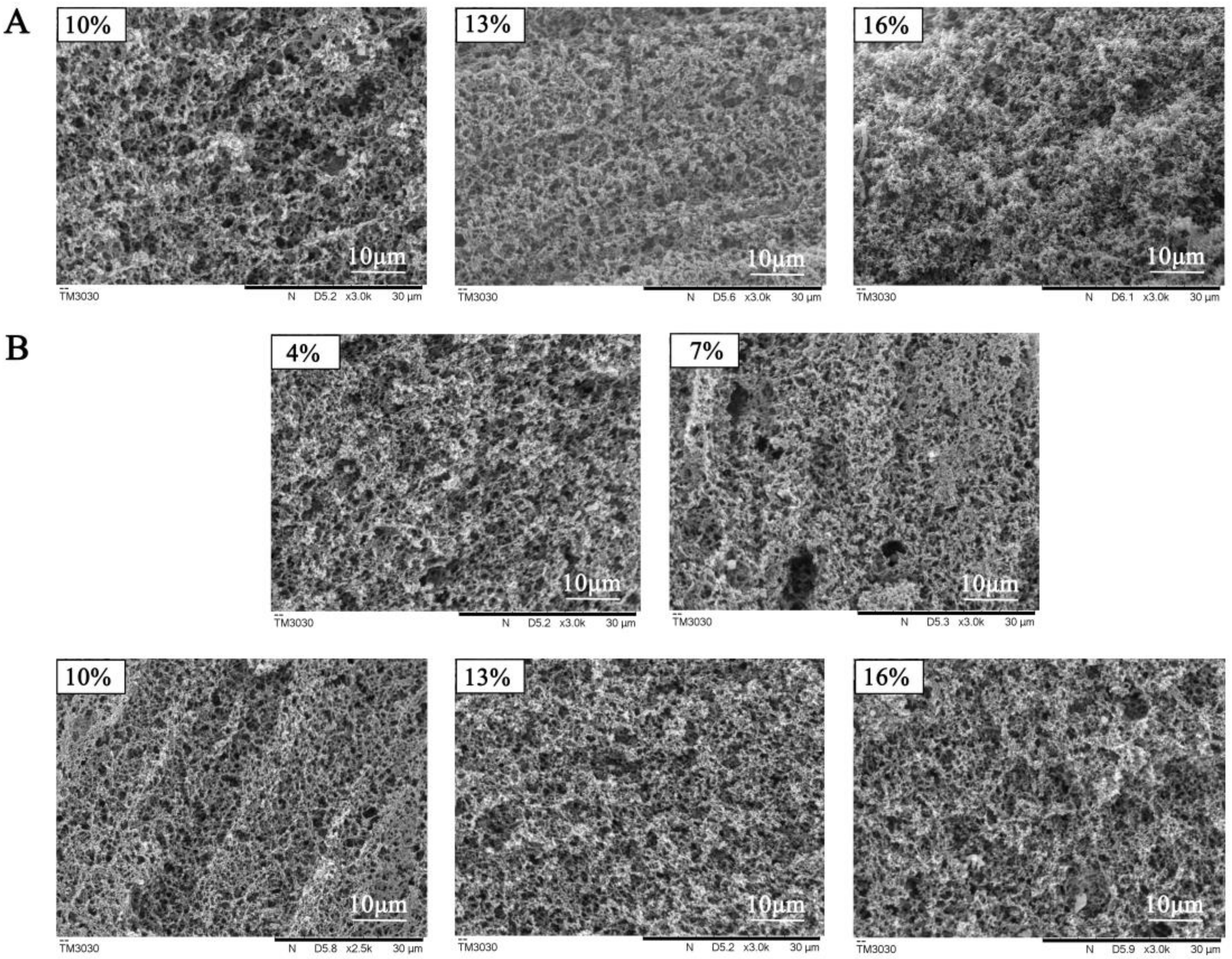
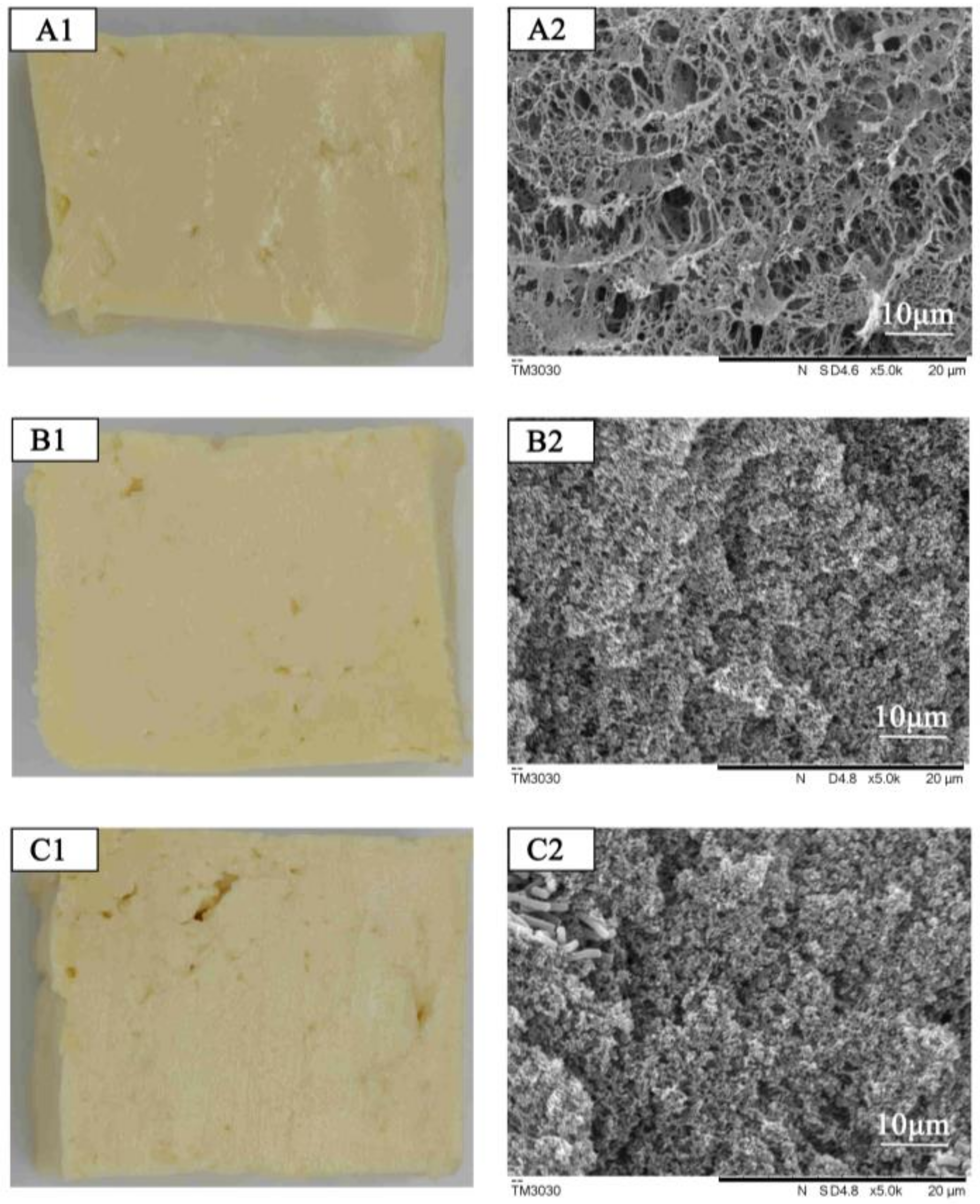
2.7. Analysis of Microstructure of Acidic Whey Tofu Gelatin and Acidic Whey Tofu
2.8. Determination of the Organic Acids in Acidic Whey Tofu
2.9. Statistical Analysis
3. Results and Discussion
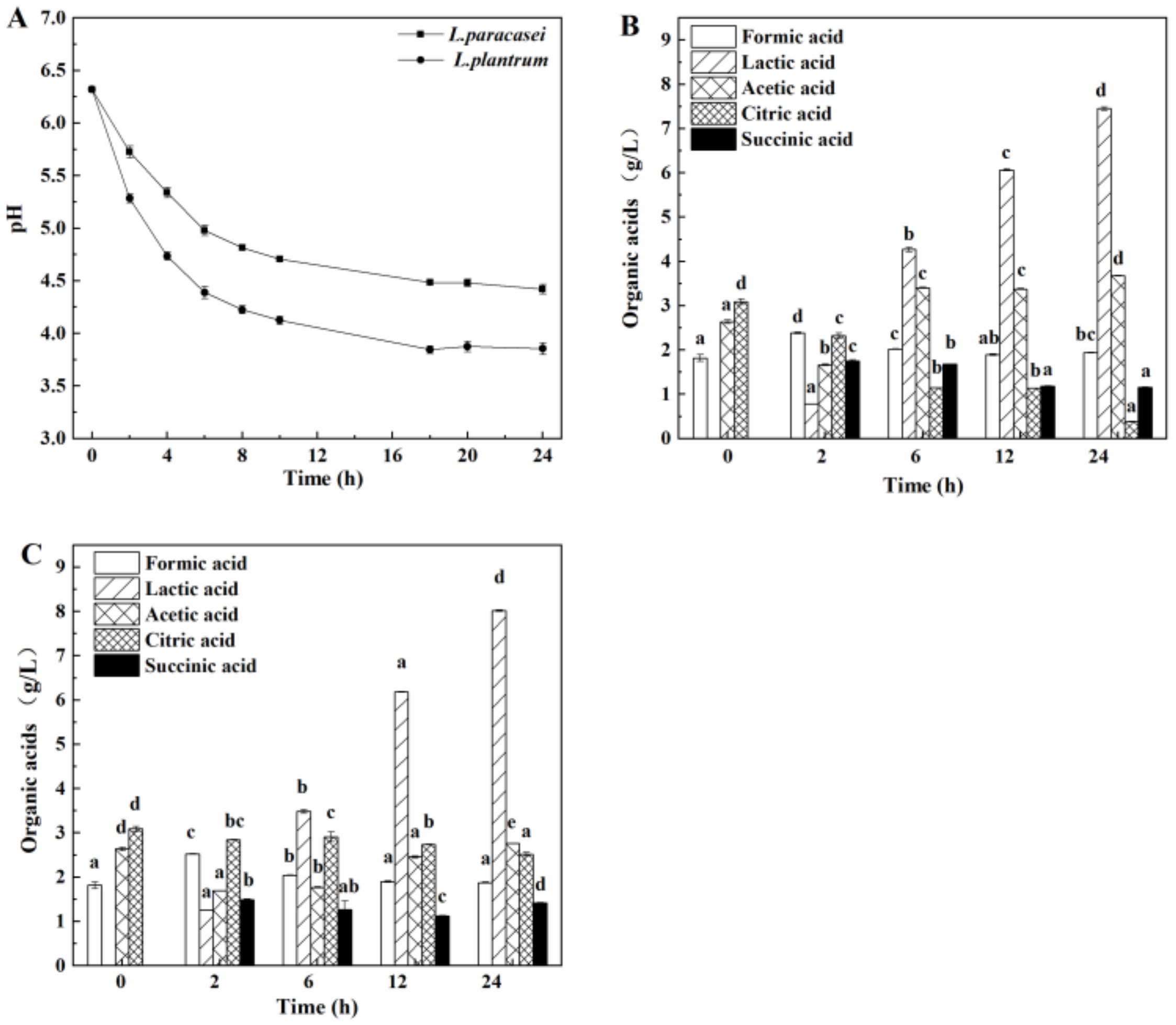
3.1. Characterization of pH and Organic Acids during the Fermentation of Soybean Whey by Lactic Acid Bacteria
3.2. Acidic Whey Tofu Gelatin
3.2.1. Effect of Holding Temperature on the Gelation Condition, pH, Hardness, Water-Holding Capacity, and Microstructure of Gelatin
3.2.2. Effect of Coagulant on the Gelation Condition, pH, Hardness, Water-Holding Capacity, and Microstructure of Gelatin
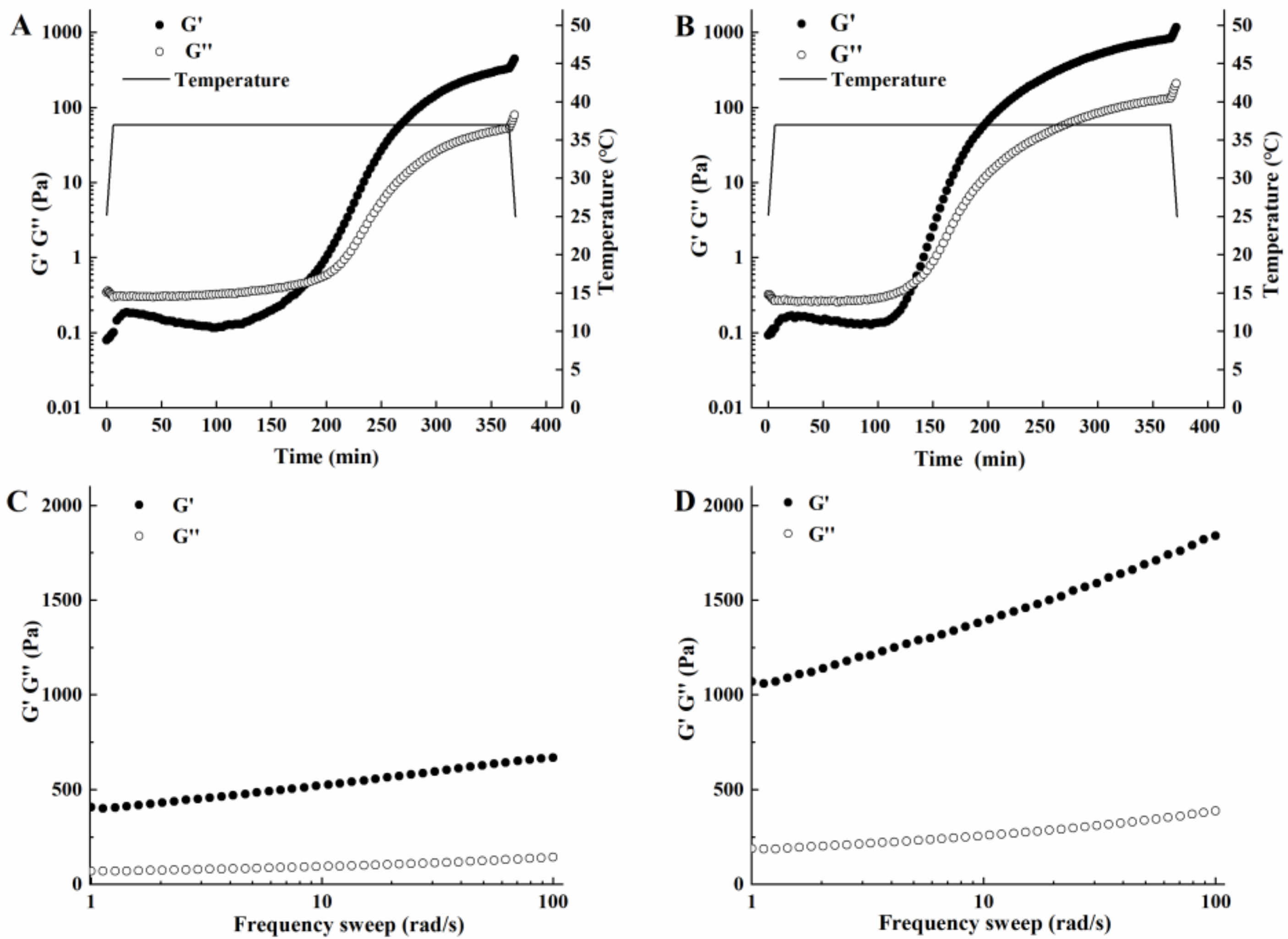
3.2.3. Study of the Rheological Properties of Acidic Whey Tofu Gelatin
3.3. Evaluation of the Quality of Acidic Whey Tofu
3.3.1. Effect of pH, Water-Holding Capacity, Texture, and Structure of Acidic Whey Tofu Fermented Using Different Strains of Bacteria
3.3.2. Comparison of the Rheological Properties of Acidic Whey Tofu
3.3.3. Comparison of the Organic Acid Content in the Acidic Whey Tofu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakshmanan, R.; De Lamballerie, M.; Jung, S. Effect of soybean-to-water ratio and pH on pressurized soymilk properties. J. Food Sci. 2006, 71, E384–E391. [Google Scholar] [CrossRef]
- Yagasaki, K.; Kousaka, F.; Kitamura, K. Potential improvement of soymilk gelation properties by using soybeans with modified protein subunit compositions. Breed. Sci. 2000, 50, 101–107. [Google Scholar] [CrossRef]
- Wei, G.; Wang, K.; Liu, Y.; Regenstein, J.M.; Liu, X.; Zhou, P. Characteristic of low-salt solid-state fermentation of Yunnan oil furu with Mucor racemosus: Microbiological, biochemical, structural, textural and sensory properties. Int. J. Food Sci. Tech. 2019, 54, 1342–1354. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Liu, S.-Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Tech. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Feng, M.; Dong, M. Effects of different coagulants on coagulation behavior of acid-induced soymilk. Food Hydrocoll. 2013, 33, 106–110. [Google Scholar] [CrossRef]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT-Food Sci. Technol. 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Ringgenberg, E.; Alexander, M.; Corredig, M. Effect of concentration and incubation temperature on the acid induced aggregation of soymilk. Food Hydrocoll. 2013, 30, 463–469. [Google Scholar] [CrossRef]
- Ali, F.; Tian, K.; Wang, Z.-X. Modern techniques efficacy on tofu processing: A review. Trends Food Sci. Tech. 2021, 116, 766–785. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Influence of processing parameters on the quality of soycurd (tofu). J. Food Sci. Technol. 2013, 50, 176–180. [Google Scholar] [CrossRef]
- Chen, C.; Rui, X.; Lu, Z.; Li, W.; Dong, M. Enhanced shelf-life of tofu by using bacteriocinogenic Weissella hellenica D1501 as bioprotective cultures. Food Control 2014, 46, 203–209. [Google Scholar] [CrossRef]
- Riciputi, Y.; Serrazanetti, D.I.; Verardo, V.; Vannini, L.; Caboni, M.F.; Lanciotti, R. Effect of fermentation on the content of bioactive compounds in tofu-type products. J. Funct. Foods 2016, 27, 131–139. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Ndagijimana, M.; Miserocchi, C.; Perillo, L.; Guerzoni, M.E. Fermented tofu: Enhancement of keeping quality and sensorial properties. Food Control 2013, 34, 336–346. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chen, X.-H.; Li, W.; Jiang, M.; Rui, X.; Dong, M.-S. Textural Properties and Microstructure of Tofu Coagulated by Plum Juice. Food Sci. 2014, 35, 40–43. [Google Scholar]
- Yuan, S.; Chang, S.K. Texture profile of tofu as affected by instron parameters and sample preparation, and correlations of instron hardness and springiness with sensory scores. J. Food Sci. 2007, 72, 136–145. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Li, W.; Qin, F.; Chen, J. Effects of oligosaccharides and soy soluble polysaccharide on the rheological and textural properties of calcium sulfate-induced soy protein gels. Food Bioprocess Tech. 2017, 10, 556–567. [Google Scholar] [CrossRef]
- Bi, C.-H.; Li, D.; Wang, L.-J.; Gao, F.; Adhikari, B. Effect of high shear homogenization on rheology, microstructure and fractal dimension of acid-induced SPI gels. J. Food Eng. 2014, 126, 48–55. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological characteristics and gelation mechanism of tofu (soybean curd). J. Agric. Food Chem. 1995, 43, 1808–1812. [Google Scholar] [CrossRef]
- Noh, E.; Park, S.; Pak, J.; Hong, S.; Yun, S. Coagulation of soymilk and quality of tofu as affected by freeze treatment of soybeans. Food Chem. 2005, 91, 715–721. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, F.; Saito, M.; Tatsumi, E.; Yin, L. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204. [Google Scholar] [CrossRef]
- Kao, F.J.; Su, N.W.; Lee, M.H. Effect of calcium sulfate concentration in soymilk on the microstructure of firm tofu and the protein constitutions in tofu whey. Agric. Food Chem. 2003, 51, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, S. Effects of endogenous small molecular compounds on the rheological properties, texture and microstructure of soymilk coagulum: Removal of phytate using ultrafiltration. Food Chem. 2016, 211, 521–529. [Google Scholar] [CrossRef]
- Lin, H.F.; Lu, C.P.; Hsich, J.F.; Kuo, M.I. Effect of ultrasonic treatment on the rheological property and microstructure of tofu made from different soybean cultivars. Innov. Food Sci. Emerg. Technol. 2016, 37, 98–105. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Veronicaa, O. Effect of different coagulants on yield and quality of tofu from soymilk. Eur. Food Res. Technol. 2008, 226, 467–472. [Google Scholar]
- Zuo, F.; Chen, Z.; Shi, X.; Wang, R.; Guo, S. Yield and textural properties of tofu as affected by soymilk coagulation prepared by a high-temperature pressure cooking process. Food Chem. 2016, 213, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-S.; Luo, S.-Z.; Cai, J.; Zhong, X.-Y.; Jiang, S.-T.; Zhao, Y.-Y.; Zheng, Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016, 31, 590–597. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. New studies on basil (Ocimum bacilicum L.) seed gum: Part III–Steady and dynamic shear rheology. Food Hydrocoll. 2017, 67, 243–250. [Google Scholar] [CrossRef]


| Temperature/°C | pH | Water-Holding Capacity (%) | Hardness (g) | |||
|---|---|---|---|---|---|---|
| L. paracasei | L. plantarum | L. paracasei | L. plantarum | L. paracasei | L. plantarum | |
| 27 | 6.34 ± 0.03 c | 5.51 ± 0.04 c | - | 76.6 ± 1.7 bc | - | 23 ± 1 b |
| 37 | 5.64 ± 0.02 a | 4.50 ± 0.02 a | 79 ± 4 | 70.2 ± 0.4 a | 20.2 ± 0.6 | 57 ± 2 c |
| 47 | 5.70 ± 0.03 b | 5.26 ± 0.01 b | 84 ± 2 | 75.4 ± 2.8 ab | 14.7 ± 2.2 | 41 ± 4 d |
| 57 | 6.39 ± 0.01 d | 6.18 ± 0.02 d | - | 80.2 ± 2.8 bc | - | 17 ± 2 a |
| 67 | 6.28 ± 0.02 c | 6.27 ± 0.02 d | - | 81.6 ± 1.6 c | - | 13 ± 4 a |
| Percentage (%) | pH | Water-Holding Capacity (%) | Hardness (g) | |||
|---|---|---|---|---|---|---|
| L. paracasei | L. plantarum | L. paracasei | L. paracasei | L. plantarum | L. paracasei | |
| 4 | 6.36 ± 0.01 d | 5.55 ± 0.01 d | - | 75.5 ± 0.3 a | - | 46 ± 2 a |
| 7 | 6.09 ± 0.02 c | 5.17 ± 0.02 c | - | 72.0 ± 1.4 a | - | 49 ± 3 ab |
| 10 | 5.64 ± 0.03 b | 4.50 ± 0.02 b | 79 ± 3 a | 70.2 ± 0.4 a | 20.2 ± 0.6 a | 57 ± 2 ab |
| 13 | 5.59 ± 0.03 b | 4.42 ± 0.01 a | 77 ± 1 a | 72.2 ± 2.8 a | 21.5 ± 0.5 a | 48 ± 5 ab |
| 16 | 5.51 ± 0.01 a | 4.35 ± 0.03 a | 76 ± 2 a | 70.5 ± 7.1 a | 21.5 ± 0.2 a | 54 ± 5 b |
| Index | L. paracasei | L. plantarum | Natural Bacteria |
|---|---|---|---|
| pH | 5.62 ± 0.03 c | 4.64 ± 0.04 b | 4.23 ± 0.03 a |
| Water-holding capacity (%) | 89.8 ± 1.4 a | 92.1 ± 0.8 b | 88.6 ± 0.8 a |
| Hardness (g) | 212 ± 5 a | 338 ± 38 b | 337 ± 21 b |
| Springiness | 0.93 ± 0.02 a | 0.92 ± 0.01 a | 0.93 ± 0.03 a |
| Cohesiveness | 0.83 ± 0.01 b | 0.79 ± 0.01 a | 0.79 ± 0.01 a |
| Chewingness (g) | 164 ± 1 a | 246 ± 26 b | 247 ± 9 b |
| G0/×103 Pa | 0.81 ± 0.01 a | 6.49 ± 0.07 b | 7.76 ± 0.13 c |
| G1/×103 Pa | 0.85 ± 0.01 a | 9.12 ± 0.14 c | 6.93 ± 0.11 b |
| λ/s | 36.8 ± 1.3 b | 27.7 ± 0.9 a | 38.3 ± 1.5 b |
| μ0/×105 Pa | 3.23 ± 0.08 a | 36.34 ± 0.66 c | 9.43 ± 0.09 b |
| Organic Acids/g/L | L. paracasei | L. plantarum | Natural Bacteria |
|---|---|---|---|
| Formic acid | 0.07 ± 0.01 a | 0.12 ± 0.01 b | 0.15 ± 0.01 c |
| Malic acid | 0.24 ± 0.01 c | 0.18 ± 0.00 b | 0.11 ± 0.02 a |
| Lactic acid | 3.60 ± 0.09 a | 6.37 ± 0.01 b | 3.72 ± 0.03 a |
| Acetic acid | 1.70 ± 0.02 a | 1.98 ± 0.02 b | 2.43 ± 0.02 c |
| Citric acid | 0.19 ± 0.01 a | 0.24 ± 0.03 b | 0.19 ± 0.01 a |
| Succinic acid | 0.21 ± 0.00 a | 0.23 ± 0.01 b | 0.73 ± 0.00 c |
| Propionic acid | 6.86 ± 0.01 a | 6.70 ± 0.01 a | 8.06 ± 0.77 a |
| Total contents | 12.87 ± 0.01 a | 15.81 ± 0.01 b | 15.39 ± 0.75 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Z.; Zhang, J.; Zhang, S.; He, Y.; Li, Y.; Regenstein, J.M.; Xie, Y.; Zhou, P. Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu. Foods 2023, 12, 918. https://doi.org/10.3390/foods12050918
Guan Z, Zhang J, Zhang S, He Y, Li Y, Regenstein JM, Xie Y, Zhou P. Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu. Foods. 2023; 12(5):918. https://doi.org/10.3390/foods12050918
Chicago/Turabian StyleGuan, Ziyu, Jie Zhang, Shitong Zhang, Yun He, Yadi Li, Joe M. Regenstein, Yuan Xie, and Peng Zhou. 2023. "Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu" Foods 12, no. 5: 918. https://doi.org/10.3390/foods12050918
APA StyleGuan, Z., Zhang, J., Zhang, S., He, Y., Li, Y., Regenstein, J. M., Xie, Y., & Zhou, P. (2023). Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu. Foods, 12(5), 918. https://doi.org/10.3390/foods12050918





