Occurrence of Phthalate Esters in Coffee and Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical and Reagents
2.3. Instrumentation
2.4. Calibration Curves
2.5. Phthalic Acid Esters (PAEs) Extraction and Clean-Up
2.6. Risk Assessment
- C: Median concentrations of PAE (ng/g)
- IR: Intake rate of coffee (g/day) set to 1–6 coffee (30 mL/coffee) [12]
- BW: Body weight (kgbw) for toddlers (11.3), adolescents (52.6), and adults (69.7) [22]
- EF: Exposure frequency to the contaminant (350 days/year)
- TE: Total exposure (70 year)
- AT: Average lifetime time for non-carcinogenic risk (TE × 365 days/year)
- SF: Slope factor (μg/kgbw/day)−1.
2.7. Statistical Analysis
3. Results and Discussion
3.1. PAEs Concentrations in Coffee Powder and Coffee Beverage
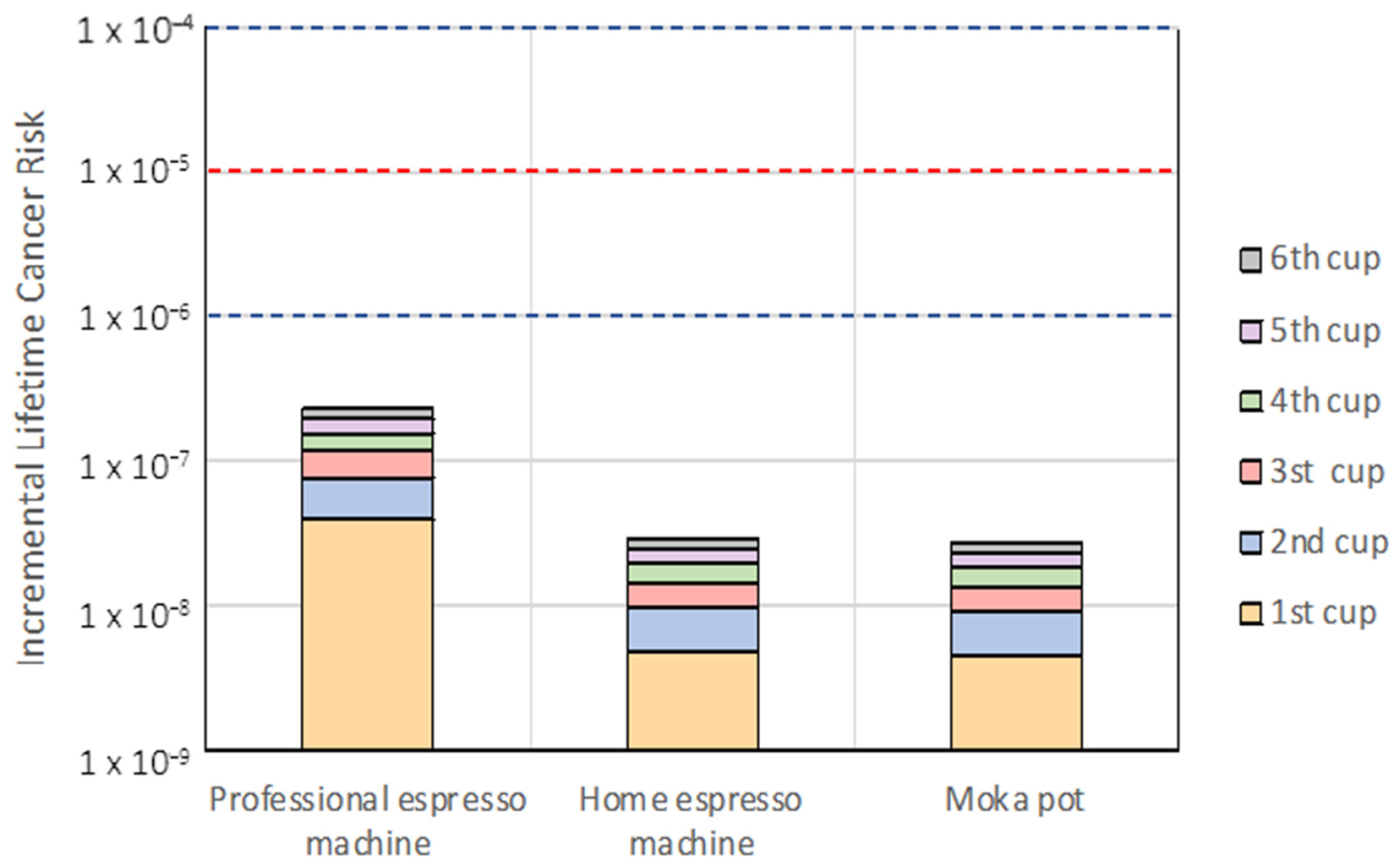
3.2. Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.J.; Guo, J.L.; Xue, J.; Bai, C.L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef] [PubMed]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Neurological Processes and Neural Health: A Literature Review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Ventrice, P.; Ventrice, D.; Russo, E.; de Sarro, G. Phthalates: European Regulation, Chemistry, Pharmacokinetic and Related Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Phthalates Action Plan; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Dhavamani, J.; Beck, A.J.; Gledhill, M.; El-Shahawi, M.S.; Kadi, M.W.; Ismail, I.M.I.; Achterberg, E.P. The Effects of Salinity, Temperature, and UV Irradiation on Leaching and Adsorption of Phthalate Esters from Polyethylene in Seawater. Sci. Total Environ. 2022, 838, 155461. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Fasano, E.; Esposito, F.; Prete, E.d.; Cocchieri, R.A. Study on the Influence of Temperature, Storage Time and Packaging Type on Di- n -Butylphthalate and Di(2-Ethylhexyl)Phthalate Release into Packed Meals. Food Addit. Contam. Part A 2013, 30, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bošnir, J.; Puntarić, D.; Galić, A.; Škes, I.; Dijanić, T.; Klarić, M.; Grgić, M.; Čurković, M.; Šmit, Z. Migration of Phthalates from Plastic Containers into Soft Drinks and Mineral Water. Food Technol. Biotechnol. 2007, 45, 91–95. [Google Scholar]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates Contamination in China: Status, Trends and Human Exposure-with an Emphasis on Oral Intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- European Coffee Federation (ECF). European Coffee Report 2018/2019; European Coffee Federation: Etterbeek, Belgium, 2019. [Google Scholar]
- Lanfranchi, M.; Giannetto, C.; Dimitrova, V. Evolutionary Aspects of Coffee Consumers’ Buying Habits: Results of a Sample Survey. Bulg. J. Agric. Sci. 2016, 22, 705–712. [Google Scholar]
- Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC; Official Journal of the European Union; European Union: Brussels, Belgium, 2004.
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance; Official Journal of the European Union; European Union: Brussels, Belgium, 2011.
- Isci, G.; Topdas, E.F.; Dagdemir, E.; Genis, H.E. Risk Assessment of Oral Exposure to Phthalates from Coffee Samples Marketed in Turkey. J. Food Compos. Anal. 2023, 115, 104913. [Google Scholar] [CrossRef]
- Sakaki, J.R.; Melough, M.M.; Provatas, A.A.; Perkins, C.; Chun, O.K. Evaluation of Estrogenic Chemicals in Capsule and French Press Coffee Using Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Toxicol. Rep. 2020, 7, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- de Toni, L.; Tisato, F.; Seraglia, R.; Roverso, M.; Gandin, V.; Marzano, C.; Padrini, R.; Foresta, C. Phthalates and Heavy Metals as Endocrine Disruptors in Food: A Study on Pre-Packed Coffee Products. Toxicol. Rep. 2017, 4, 234–239. [Google Scholar] [CrossRef] [PubMed]
- di Bella, G.; Potortì, A.G.; lo Turco, V.; Saitta, M.; Dugo, G. Plasticizer Residues by HRGC–MS in Espresso Coffees from Capsules, Pods and Moka Pots. Food Control. 2014, 41, 185–192. [Google Scholar] [CrossRef]
- Tsumura, Y.; Ishimitsu, S.; Kaihara, A.; Yoshii, K.; Nakamura, Y.; Tonogai, Y. Di(2-Ethylhexyl) Phthalate Contamination of Retail Packed Lunches Caused by PVC Gloves Used in the Preparation of Foods. Food Addit. Contam. 2001, 18, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Fasano, E.; Esposito, F.; Montuori, P.; Amodio Cocchieri, R. Di(2-Ethylhexyl)Phthalate (DEHP) and Di-n-Butylphthalate (DBP) Exposure through Diet in Hospital Patients. Food Chem. Toxicol. 2013, 51, 434–438. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment; United States Environmental Protection Agency: Washington, DC, USA, 2021.
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; le Donne, C. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main Results in Terms of Food Consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef]
- Health Canada. Contaminated Sites Division Federal Contaminated Site Risk Assessment in Canada; Part V, Guidance on Human Health Detailed Quantitative Risk Assessment for Chemicals (DQRAChem); Health Canada: Ottawa, ON, Canada, 2010; ISBN 9781100179261.
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Liu, L.; Li, Y.; Ren, N.; Kannan, K. Occurrence and Profiles of Phthalates in Foodstuffs from China and Their Implications for Human Exposure. J. Agric. Food. Chem. 2012, 60, 6913–6919. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan Coffee Brew Chemical Analysis in Comparison to Espresso, Moka and American Brews. Food Res. Int. 2014, 61, 152–160. [Google Scholar] [CrossRef]
| Packaging | DBP (ng/g) | DEHP (ng/g) |
|---|---|---|
| Multilayer | 0.303 (<LOD-2.368) | 5.014 (<LOQ-68.843) |
| Aluminum | 0.578 (<LOD-1.736) | 4.921 (<LOD-36.516) |
| Paper pod | 0.226 (<LOD-3.331) | 3.857 (<LOD-15.993) |
| Machine | DBP (ng/g) | DEHP (ng/g) |
|---|---|---|
| Professional espresso machine (PEM) | 0.07 (<LOD-0.37) | 6.65 (2.58–21.32) |
| Moka pot (MP) | 0.11 (<LOD-0.21) | 0.78 (<LOD-2.41) |
| Home espresso machine (HEM) | 0.12 (<LOD-0.25) | 0.83 (<LOD-2.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velotto, S.; Squillante, J.; Nolasco, A.; Romano, R.; Cirillo, T.; Esposito, F. Occurrence of Phthalate Esters in Coffee and Risk Assessment. Foods 2023, 12, 1106. https://doi.org/10.3390/foods12051106
Velotto S, Squillante J, Nolasco A, Romano R, Cirillo T, Esposito F. Occurrence of Phthalate Esters in Coffee and Risk Assessment. Foods. 2023; 12(5):1106. https://doi.org/10.3390/foods12051106
Chicago/Turabian StyleVelotto, Salvatore, Jonathan Squillante, Agata Nolasco, Raffaele Romano, Teresa Cirillo, and Francesco Esposito. 2023. "Occurrence of Phthalate Esters in Coffee and Risk Assessment" Foods 12, no. 5: 1106. https://doi.org/10.3390/foods12051106
APA StyleVelotto, S., Squillante, J., Nolasco, A., Romano, R., Cirillo, T., & Esposito, F. (2023). Occurrence of Phthalate Esters in Coffee and Risk Assessment. Foods, 12(5), 1106. https://doi.org/10.3390/foods12051106








