Palmitic Acid Regulation of Stem Browning in Freshly Harvested Mini-Chinese Cabbage (Brassica pekinensis (Lour.) Rupr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Weight Loss and Respiration Rate
2.3. Color and Browning Index (BI)
2.4. Electrolyte Leakage and MDA Content
2.5. Total Phenolics and Total Flavonoids
2.6. CAT, APX, PPO, and POD Enzyme Activity
2.7. PAL and 4CL Enzyme Activity
2.8. Data Analysis
3. Results
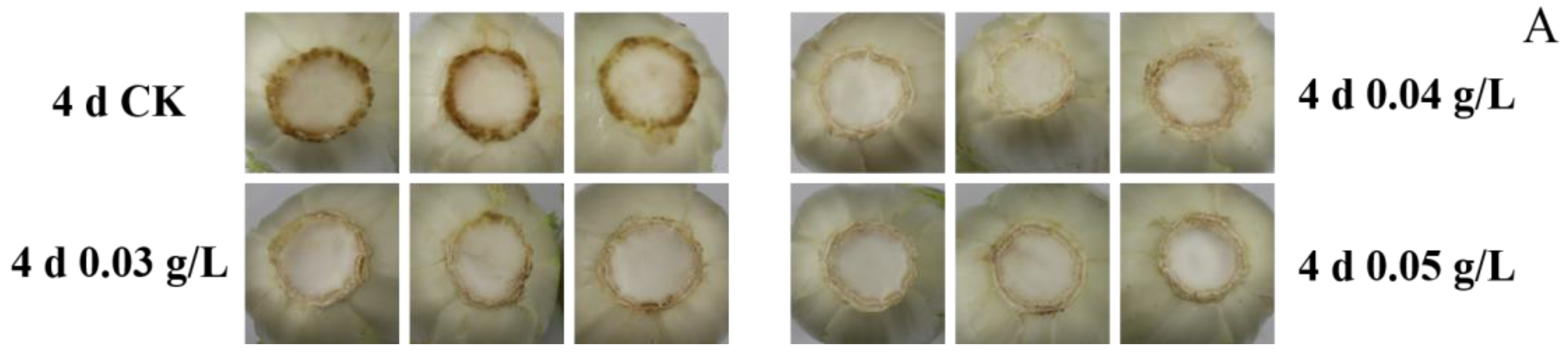
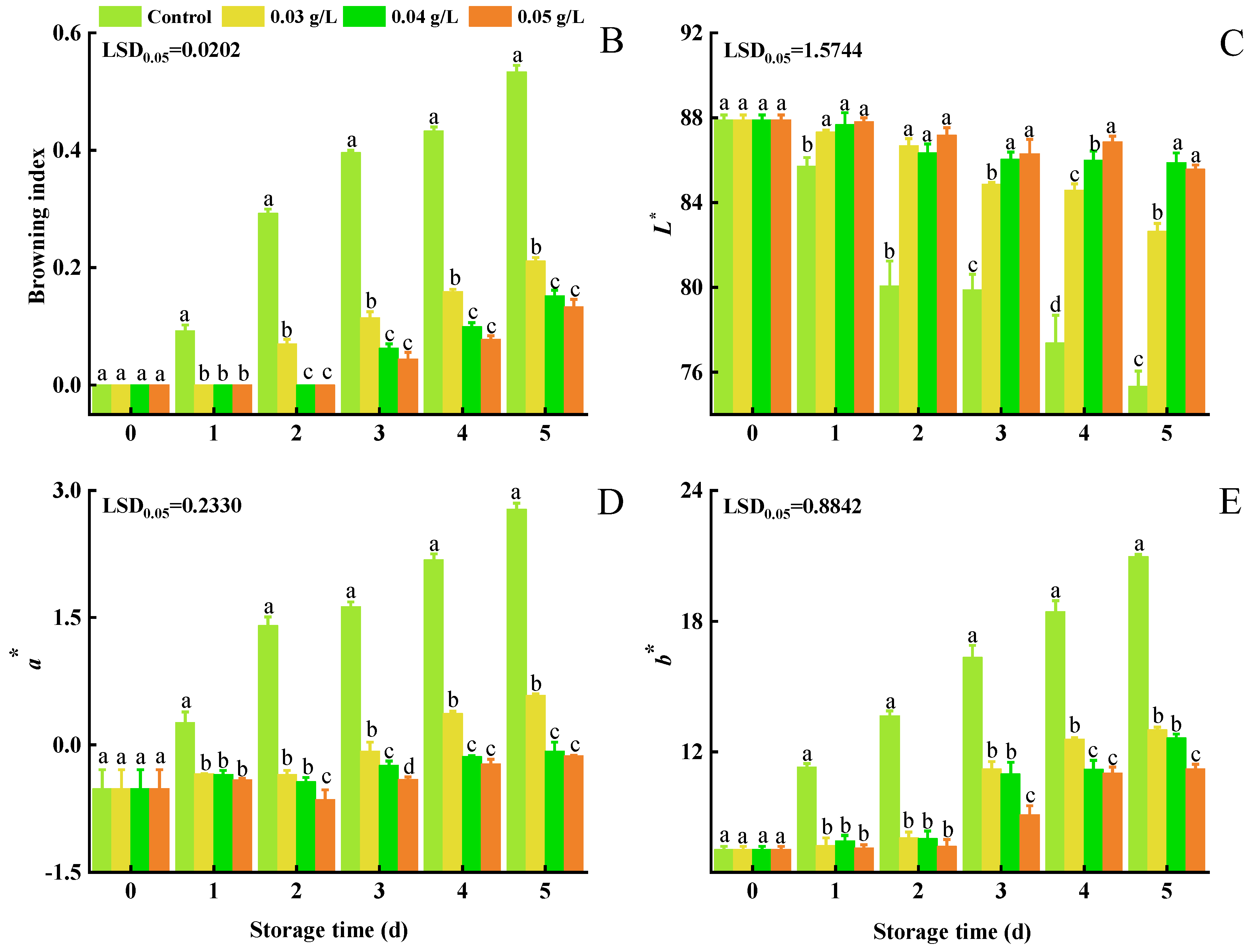
3.1. Stem Browning
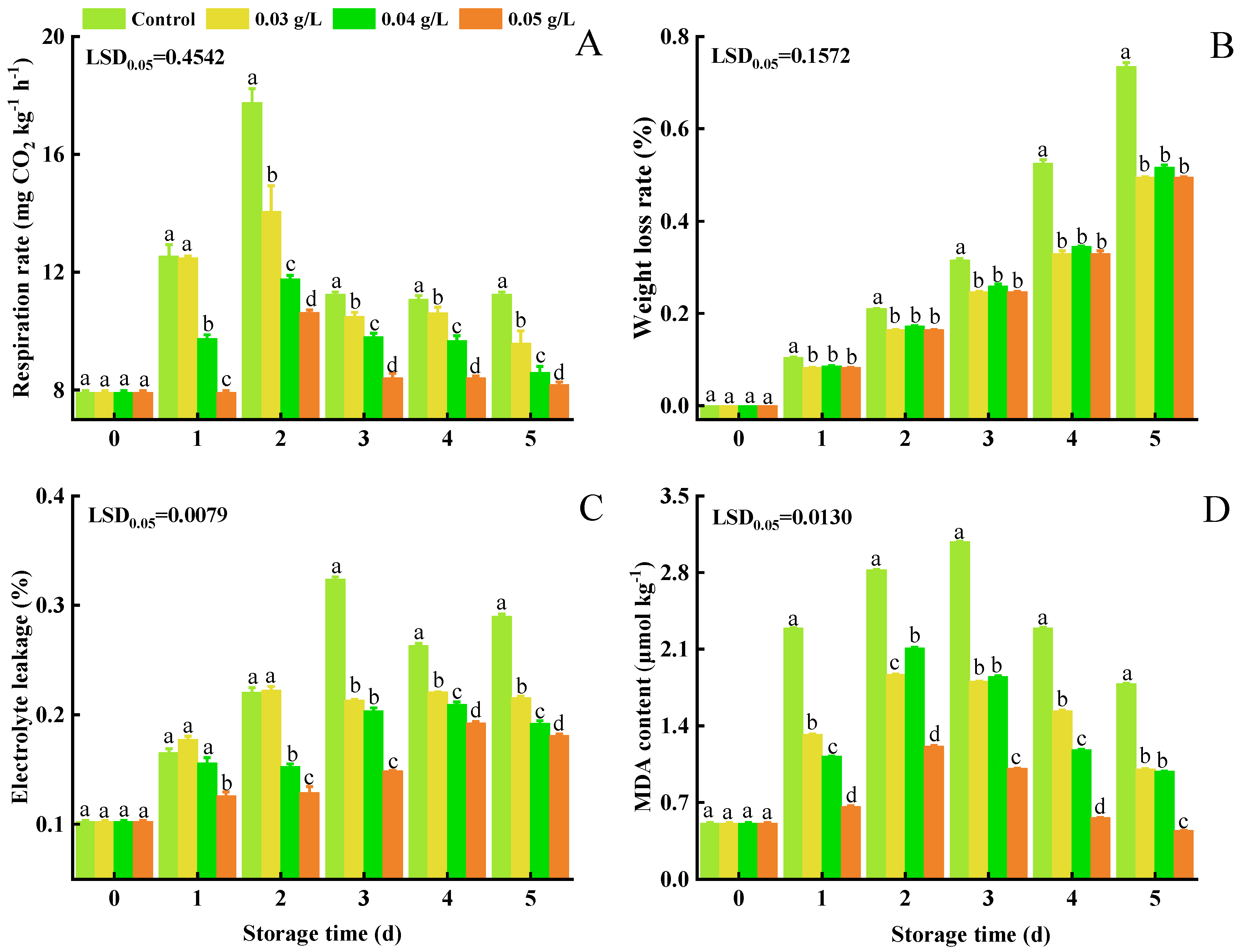
3.2. Respiration Rate, Weight Loss, Electrolyte Leakage, and MDA Content
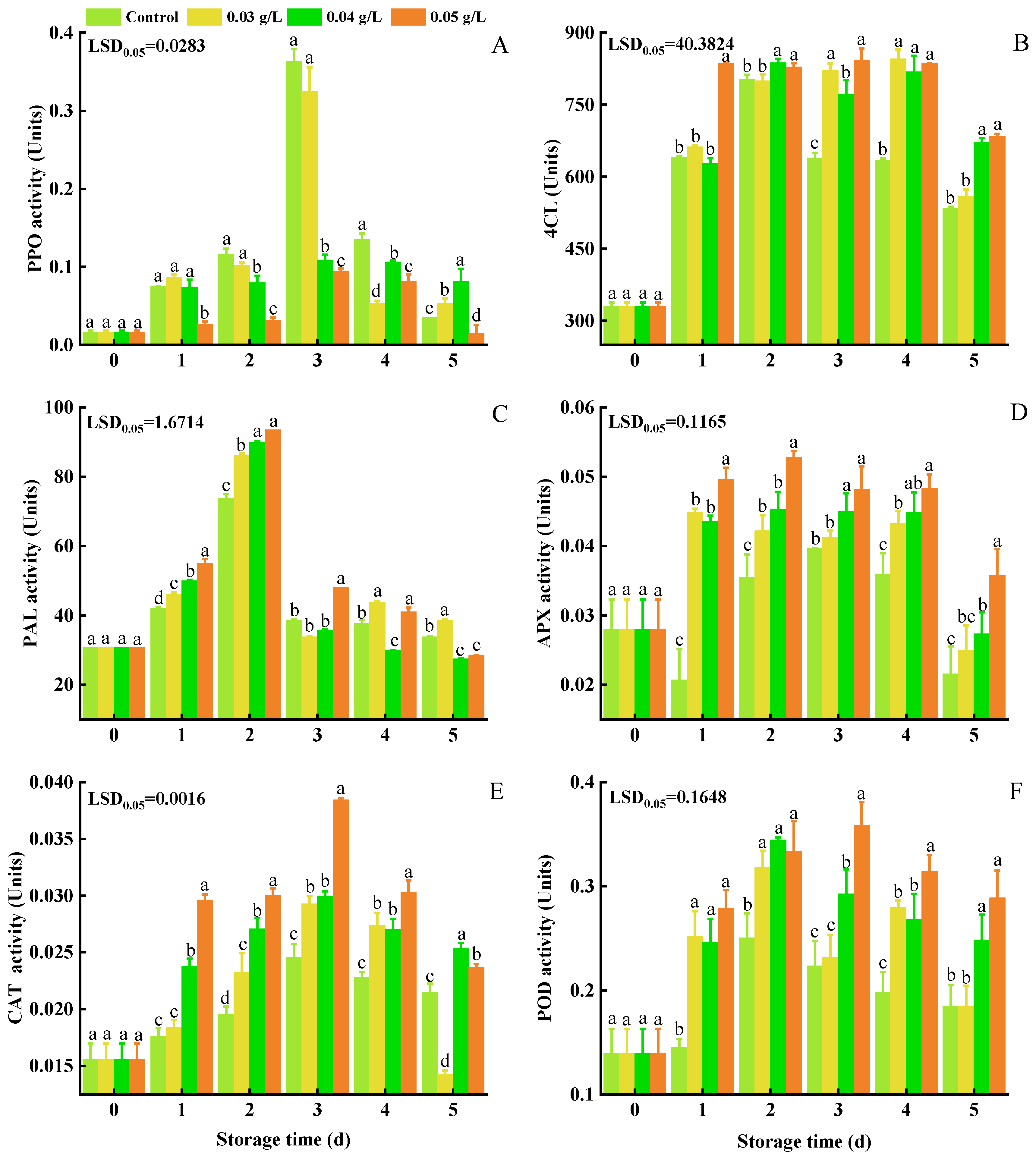
3.3. PPO, 4CL, PAL, APX, CAT, and POD Activity
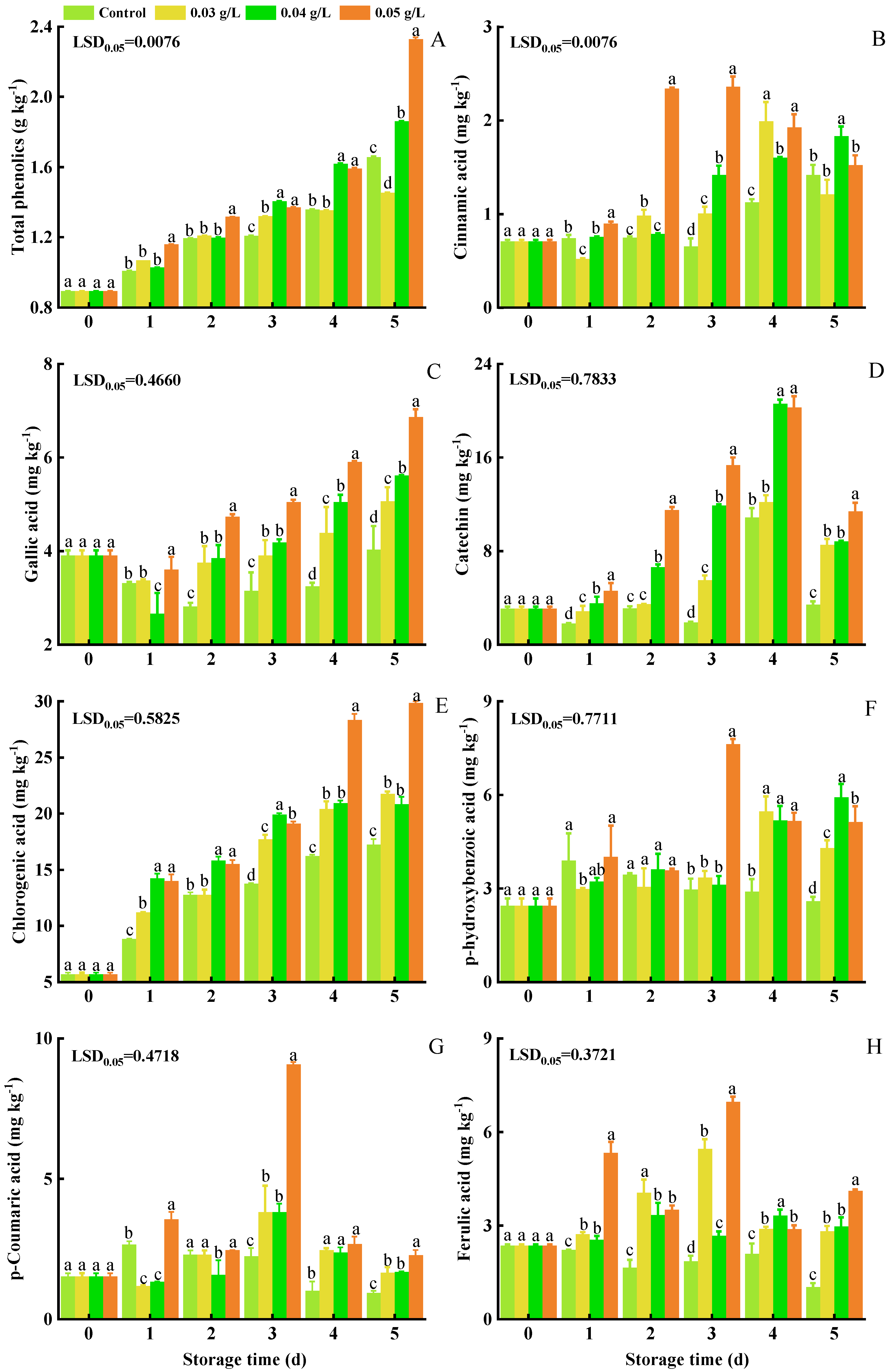
3.4. Total Phenolics, Gallic Acid, Catechin, Chlorogenic Acid, p-Hydroxybenzoic Acid, p-Coumaric Acid, Ferulic Acid, and Cinnamic Acid
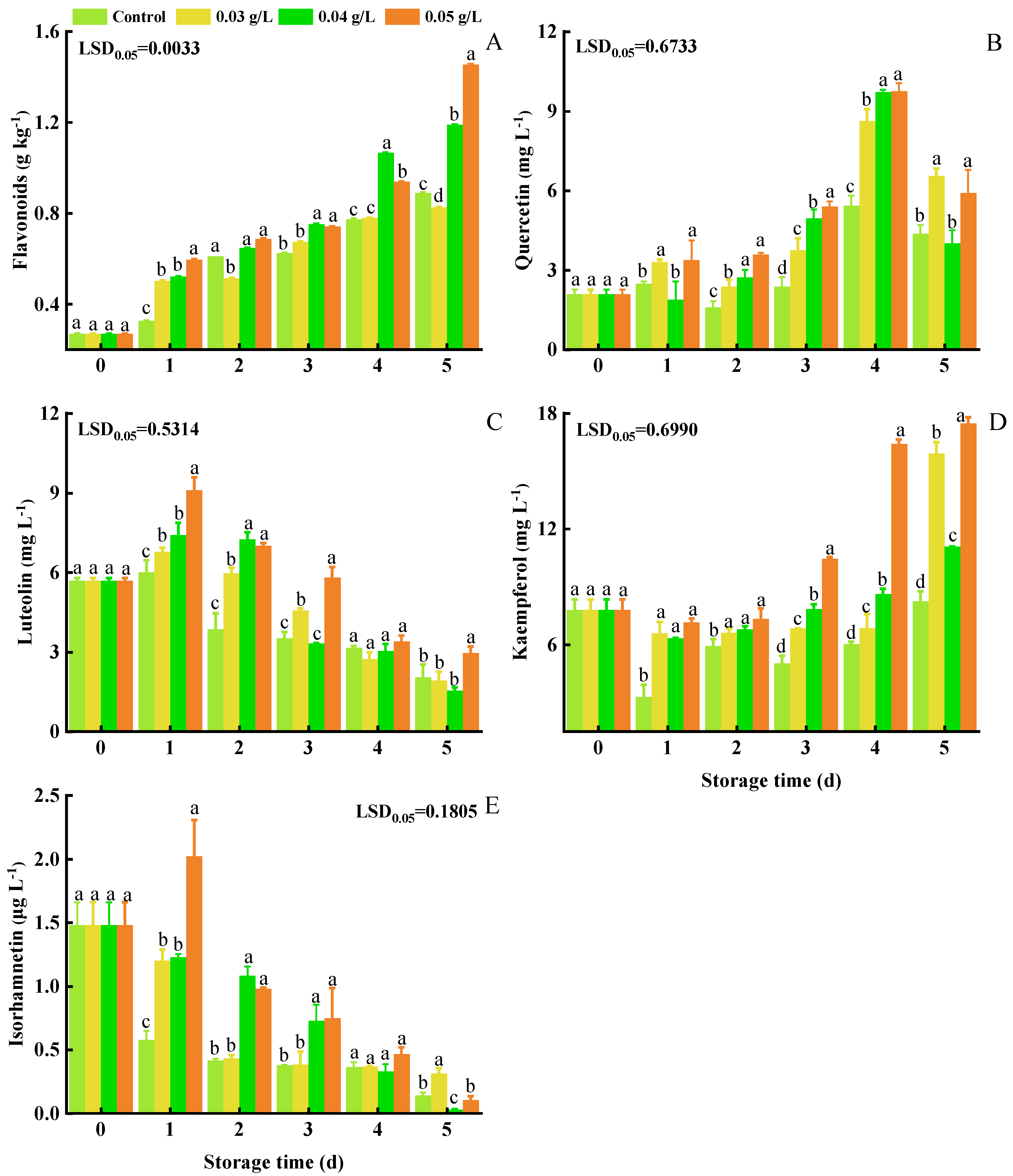
3.5. Total Flavonoids, Quercetin, Luteolin, Kaempferol, and Isorhamnetin
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, P.; Zhong, X.; Li, B.; Wang, W.; Yue, Z.; Lei, J.; Guo, W.; Huang, X. Genome-wide identification and characterization of aquaporin genes (AQPs) in Chinese cabbage (Brassica rapa ssp. pekinensis). Mol. Genet. Genom. 2014, 289, 1131–1145. [Google Scholar] [CrossRef]
- Hong, E.; Kim, S.J.; Kim, G.H. Identification and quantitative determination of glucosinolates in seeds and edible parts of Korean Chinese cabbage. Food Chem. 2011, 128, 1115–1120. [Google Scholar] [CrossRef]
- Kim, J.J.; John, K.; Hae-Kyung, M.; Jin, K.; Enkhtaivan, G.; Kim, D.H. Morphological and biochemical variation of Chinese cabbage (Brassica rapa spp. Pekinensis) cultivated using different agricultural practices. J. Food Compos. Anal. 2014, 36, 12–23. [Google Scholar]
- Kim, H.W.; Jang, J.J.; Kim, N.H.; Lee, N.Y.; Cho, T.J.; Kim, S.H.; Rhee, M.S. Factors that determine the microbiological quality of ready-to-use salted napa cabbage (Brassica pekinensis): Season and distribution temperature. Food Control 2018, 87, 1–8. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, H.; Zhang, L.; Zhou, H.; Li, P. The application of 1-methylcyclopropene preserves the postharvest quality of cabbage by inhibiting ethylene production, delaying chlorophyll breakdown and increasing antioxidant capacity. Sci. Hortic-Amst. 2021, 281, 109986. [Google Scholar] [CrossRef]
- Grzegorzewska, M.; Badeek, E.; Szczech, M.; Kosson, R.; Wrzodak, A.; Kowalska, B.; Colelli, G.; Szwejda-Grzybowska, J.; Maciorowski, R. The effect of hot water treatment on the storage ability improvement of fresh-cut Chinese cabbage. Sci. Hortic-Amst. 2022, 291, 110551. [Google Scholar] [CrossRef]
- Zeng, S.X.; Zhao, X.L.; Zuo, J.H.; Yan, Z.C.; Shi, J.Y.; Wang, Q.; Cui, J.C.; Sui, Y. Effect of kojic acid treatment on postharvest browning of baby cabbages. Food Sci. 2021, 42, 241–247, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Varzakas, T. Application of Antibrowning Agents in Minimally Processed Cabbage. J. Food Nutr. Disord. 2014, 3, 1–5. [Google Scholar] [CrossRef]
- Cabezas-Serrano, A.B.; Amodio, M.L.; Colelli, G. Effect of solution pH of cysteine-based pre-treatments to prevent browning of fresh-cut artichokes. Postharvest Biol. Technol. 2013, 75, 17–23. [Google Scholar] [CrossRef]
- Pilarska, M.; Skowron, E.; Pietra, R.; Krupinska, K.; Niewiadomska, E. Changes in lipid peroxidation in stay-green leaves of tobacco with senescence-induced synthesis of cytokinins. Plant Physiol. Biochem. 2017, 118, 161–167. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Xu, Y.; Wu, J.; Xiao, G. Effect of treatment with dimethyl dicarbonate on microorganisms and quality of Chinese cabbage. Postharvest Biol. Technol. 2013, 76, 139–144. [Google Scholar] [CrossRef]
- Li, F.; Huang, H.; Ding, X.; Liu, J.; Jiang, Y. Effect of CPPU on postharvest attributes of Chinese flowering cabbage during storage. Postharvest Biol. Technol. 2021, 174, 111438. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Wang, C.Y.; Buta, J.G. Inhibition of Browning and Decay of Fresh-cut Radishes by Natural Compounds and their Derivatives. LWT—Food Sci. Technol. 2001, 34, 324–328. [Google Scholar] [CrossRef]
- Lian, C.; Mengyin, C.; Hetong, L.; Yihui, C.; Yifen, L.; Shaojun, C. Effects of 2,4-dinitrophenol on Lipoxygenase Activity and Fatty Acid Constituents of Membrane Lipids in Pericarp of Harvested Longan Fruit. J. Trop. Subtrop. Bot. 2009, 17, 477–482. [Google Scholar]
- Chen, Y.H.; Lin, H.T.; Lin, Y.F.; Zhao, Y.F.; Zhang, J.N. Effects of Phomopsis longanae Chi Infection on Lipoxygenase Activity and Fatty Acid Constituents of Membrane Lipids in Pericarp of Harvested Longan Fruits. J. Trop. Subtrop. Bot. 2011, 19, 260–266. [Google Scholar]
- Gianfranca, C.; Elisabetta, M.; Sebastiano, B.; Claudia, M. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar]
- Lin, H.T.; Xi, Y.F.; Chen, S.J. A review of enzymatic browning in fruit during storage. J. Fuzhou Univ. (Nat. Sci. Ed.) 2002, 30, 696–703. [Google Scholar]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Xu, D.; Gu, S.; Zhou, F.; Hu, W.; Feng, K.; Chen, C.; Jiang, A. Mechanism underlying sodium isoascorbate inhibition of browning of fresh-cut mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2021, 173, 111357. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Fza, B.; Aja, B.; Ke, F.; Sga, B.; Dxa, B.; Wha, B. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar]
- Wang, Q.; Ding, T.; Zuo, J.H.; Gao, L.P.; Fan, L.L. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biol. Technol. 2016, 112, 114–120. [Google Scholar] [CrossRef]
- Kamdee, C.; Ketsa, S.; Doorn, W. Effect of heat treatment on ripening and early peel spotting in cv. Sucrier banana. Postharvest Biol. Technol. 2009, 52, 288–293. [Google Scholar] [CrossRef]
- Giménez, M.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Membrane lipid peroxidation and its conflict of interest: The two faces of oxidative stress. Curr. Sci. 2014, 107, 1811–1823. [Google Scholar]
- Gürbüz, G.; Heinonen, M. LC–MS investigations on interactions between isolated β-lactoglobulin peptides and lipid oxidation product malondialdehyde. Food Chem. 2015, 175, 300–305. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic-Amst. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Sreenivas, K.M.; Singhal, R.S.; Lele, S.S. Chemical pretreatments and partial dehydration of ash gourd (Benincasa hispida) pieces for preservation of its quality attributes. LWT—Food Sci. Technol. 2011, 44, 2281–2284. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Palmisano, F.; Zambonin, P.G. Characterization of caffeic acid enzymatic oxidation by-products by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2006, 1102, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Gu, S.; Zuo, J.; Gao, L.; Wang, Q.; Jiang, A. LED irradiation delays the postharvest senescence of garland chrysanthemum (Chrysanthemum carinatum Schousb.). J. Food Meas. Charact. 2019, 13, 3005–3014. [Google Scholar] [CrossRef]
- Yan, J.; Song, Y.; Li, J.; Jiang, W. Forced-air precooling treatment enhanced antioxidant capacities of apricots. J. Food Process. Preserv. 2018, 42, e13320. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Korbes, A.P.; Garighan, J.A.; Jardim-Messeder, D.; Carvalho, F.E.L.; Sousa, R.H.V.; Caverzan, A.; Teixeira, F.K.; Silveira, J.A.G.; Margis-Pinheiro, M. Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Sci. 2017, 263, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zeng, S.; Yue, X.; Yuan, S.; Zuo, J.; Wang, Q. Palmitic Acid Regulation of Stem Browning in Freshly Harvested Mini-Chinese Cabbage (Brassica pekinensis (Lour.) Rupr.). Foods 2023, 12, 1105. https://doi.org/10.3390/foods12051105
Gao H, Zeng S, Yue X, Yuan S, Zuo J, Wang Q. Palmitic Acid Regulation of Stem Browning in Freshly Harvested Mini-Chinese Cabbage (Brassica pekinensis (Lour.) Rupr.). Foods. 2023; 12(5):1105. https://doi.org/10.3390/foods12051105
Chicago/Turabian StyleGao, Hongdou, Shixian Zeng, Xiaozhen Yue, Shuzhi Yuan, Jinhua Zuo, and Qing Wang. 2023. "Palmitic Acid Regulation of Stem Browning in Freshly Harvested Mini-Chinese Cabbage (Brassica pekinensis (Lour.) Rupr.)" Foods 12, no. 5: 1105. https://doi.org/10.3390/foods12051105
APA StyleGao, H., Zeng, S., Yue, X., Yuan, S., Zuo, J., & Wang, Q. (2023). Palmitic Acid Regulation of Stem Browning in Freshly Harvested Mini-Chinese Cabbage (Brassica pekinensis (Lour.) Rupr.). Foods, 12(5), 1105. https://doi.org/10.3390/foods12051105






