Abstract
The perception of hop-derived flavour in beer is not well understood, particularly regarding the effect that different yeast strains and fermentation parameters have on perceived hop aroma and the mechanisms responsible for these changes. To evaluate the influence of yeast strain on the sensory properties and volatile composition of beer, a standard wort, late-hopped with New Zealand Motueka hops (5 g·L−1), was fermented with one of twelve yeast strains under constant conditions (temperature and yeast inoculation rate). The bottled beers were evaluated using a free sorting sensory methodology, and their volatile organic compounds (VOC) were assessed using gas chromatography mass spectrometry (GC/MS) with headspace solid-phase microextraction (SPME) sampling. Beer fermented with SafLager W-34/70 yeast was associated with a hoppy flavour attribute, whereas WY1272 and OTA79 beers were sulfury, and WY1272 was also metallic. WB06 and WLP730 beers were perceived to be spicy, with WB06 beer also perceived as estery, whereas VIN13 beer was sour, and the WLP001 beer was astringent. Beers fermented using the twelve yeast strains had clearly distinct VOC profiles. Beer made with WLP730, OTA29, SPH, and WB06 yeasts had the highest 4-vinylguaiacol levels, which contributed to their spicy attribute. Beer made with W3470 had high levels of nerol, geraniol, and citronellol, which supported its sensory characterisation as being ‘hoppy’. This research has illustrated the important role that yeast strain has on modulating hop flavour in beer.
1. Introduction
The perception of flavour and the quality of beer is dependent on the raw materials used and the variety of reactions the happen during wort production and fermentation. Flavour generation reactions during fermentation vary depending on the nature of the ingredients, including the yeast strain used, and the fermentation conditions [1]. Yeasts are essential in beer production where the yeast strain influences the type of beer produced and its flavour profile due to the metabolites generated during fermentation [2]. Yeasts also alter the perception of hop flavour in beer by biotransforming hop flavour precursors during fermentation, with different yeast strains producing beers with differing concentrations of hop-related volatile organic compounds (VOC) [3,4].
Hop flavour in beer is dependent on complex physical, chemical, and biological changes that happen during wort production and fermentation. Hops (Humulus lupulus L.) are responsible for the characteristic bitterness of beer that counteracts the sweetness from malt and conveys drinkability to beer. Volatile compounds derived from hop essential oil are responsible for a variety of aroma attributes, particularly when hops are added late in the boil (late hopping) or added during the fermentation or maturation stages of production (dry hopping). The perception of hop flavour also changes during fermentation due to modification of hop compounds by yeast during fermentation (biotransformation reactions) [5]. These yeast–hop interactions generate a diverse range of hop flavours in beer including resinous, fruity, citrusy, floral, and spicy attributes [6]. Despite a large quantity of research on the composition of hop essential oil, much is unknown about the mechanisms by which hops contribute to beer flavour [7].
Important chemical components in hop essential oils are terpene hydrocarbons (including monoterpenes such as β-myrcene, sesquiterpenes such as α-humulene and β-caryophyllene), oxygenated compounds (alcohols, aldehydes, ketones, and esters, which may be terpene derivatives), and sulfur compounds [8,9]. Terpenoids are a diverse class of compounds derived from isopentenyl pyrophosphate precursors, which generate a range of aroma and flavour characters [10]. The biotransformation of monoterpenes by yeast during fermentation has been reported by King and Dickinson [4], where yeast activity converts monoterpene alcohols such as geraniol and linalool to a range of other terpenoid products via either isomerisation (e.g., converting geraniol to nerol), reduction (e.g., to citronellol), or esterification [4,11]. Biotransformation of hop-derived compounds by different yeast strains has been shown to impact on the VOCs profile in beer [3] and has been speculated to also impact on the perceived flavour of beer, although comprehensive studies have not been reported in the literature.
The free-sort method has been demonstrated to be an effective rapid sensory evaluation technique to describe beer characteristics [12]. Free sorting aims to measure the degree of similarity between samples by sorting the samples into groups according to their similarities and differences. The inclusion of an additional descriptive step enables the sensory characterisation of the samples [13].
The aim of this study was to examine the influence of different yeast strains on the volatile composition and the sensory properties of the resulting beers. A standard wort, late-hopped with New Zealand Motueka hops, was fermented using one of 12 different yeast strains, including a selection of beer and wine yeasts, to obtain a diverse range of sensory characteristics. Sensory analysis was carried out using a free sorting task methodology with a panel of 14 assessors, including trained sensory panelists, volunteers, and brewing professionals. The VOC composition of the beers was assessed using gas chromatography mass spectrometry (GC/MS) with headspace solid-phase microextraction (HS-SPME) sampling.
2. Materials and Methods
2.1. Brewing and Fermentation
Yeast strains (Table 1) were propagated in sterile (autoclaved) malt medium (350 mL, 10% w/v) for 20 h at 20 °C. Fermentis SafAle US-05, SafAle WB-06, SafAle BE-256, SafLager W-34/70, and SafLager S-23 yeasts were provided by Fermentis (Lille, France); White Labs California Ale WLP001 (San Diego, CA, USA) was purchased from Homebrew West (Auckland, New Zealand); Anchor VIN13 hybridised wine yeast and Exotics SPH wine yeast (Anchor Oenology) were supplied by Scott Laboratories (Petaluma, CA, USA). OTA29 and White Labs Chardonnay White Wine Yeast WLP730 were propagated from agar slopes from the University of Otago yeast collection according to the protocol described below (Section 2.2). OTA79 (University of Otago yeast collection) and Wyeast American Ale II WY strain1272 (Hood River, OR, USA) were supplied as a slurry by The Emerson’s Brewing Company (Dunedin, New Zealand).

Table 1.
Yeast characteristics and pitching rates.
Wort was produced at The Emerson’s Brewing Company with an original gravity of 10°P at an efficiency of 80%. Milled malt (194 kg) was added into the mash vessel and mixed with hot water (582 L) to achieve a strike temperature of 50 °C together with CaCl2 (100 g), lactic acid (150 mL), and β-glucanase (100 mL). A temperature programmed mash with rests at 50 °C (15 min) and 67 °C (45 min) was utilised to achieve saccharification before increasing the temperature to 75 °C for mash out. The sweet wort was recirculated in the lauter tun for 15 min for clarification before transfer to the kettle for wort boiling. The pre-boil gravity and pH of the wort sample were determined to be 1.038 and 5.33, respectively.
The wort was boiled for 60 min with antifoam and bittering hops (Simcoe; 580 g) to achieve 20 IBU. Kettle finings (koppafloc; 100 g) and magiFood (100 g) were added after 45 min to reduce haze in the final beer. After 60 min, the boil was stopped, and Motueka hops (6000 g total; dose rate of 5 g·L−1) were added for a 5-min steep. The wort was transferred into a whirlpool vessel for 10 min for clarification to remove spent hops and trub. Wort was cooled to 18 °C using a heat exchanger during transfer to a holding tank with in-line oxygenation using O2 at 50 L·min−1 (pressure = 4 psi). From the final wort volume (1200 L), 12 × 10 L aliquots were added into sterile 12 L fermenters. Each wort (10 L) was inoculated with one of the 12 propagated yeasts to achieve a target pitch rate of 1 × 107 viable cells·mL−1. Fermentation took place at 20 °C for 9 days until a constant gravity was achieved in all fermenters. The beer was then matured and clarified at 4 °C for 9 days prior to bottling. Beer samples were bottled into 330-mL brown glass bottles with the addition of 1.5 g sucrose to carbonate the beer to 2.0 volumes CO2 by holding the samples in an incubator at 20 °C for 14 days.
2.2. Yeast Preparation
Yeast cultures were propagated based on the origin of each yeast strain (Table 1), before calculating the required yeast volumes to the fermenter for each yeast strain to achieve the target pitch rate.
2.2.1. Rehydration from Dry Yeast
To initially determine cell density per gram, 1 g of each dry yeast was rehydrated in filtered water (100 mL) (boiled and cooled to room temperature) and stirred for 10 min using a magnetic stirrer. Cell viability (initial cell count) was estimated to determine the amount of yeast suspension required to obtain 1 × 107 cells·mL−1 (for a 10 L ferment). For final pitching, dry yeast (11.5 g) was added to a malt solution (10°P, 1 L), incubated for 24–48 h at 20 °C, and revived 48 h prior to yeast enumeration and pitching (Section 2.2.5).
2.2.2. Propagation of Cultures from Agar Slopes (Otago Culture Collection)
Yeast strains on agar slopes in the Otago culture collection required several propagation steps to obtain sufficient numbers for the target pitch rate. Yeasts recovered from agar slopes were inoculated into duplicate 10-mL autoclaved malt solution (10% w/v) in 20-mL Universal vials using a sterile loop and incubated for 24 h at 20 °C. These cultures (two x 10-mL volumes) were added to 180 mL of sterile malt medium (10% w/v) in a 500-mL Schott bottle and propagated for 24–48 h at 20 °C. The resulting yeast suspension was added to 2800 mL of autoclaved malt medium (10% w/v; 10°P) in a 5-L conical flask. The starter culture was stirred for 24–48 h at 20 °C with a magnetic stir bar, prior to yeast enumeration and pitching (Section 2.2.5).
2.2.3. Commercial Slurry
Fresh liquid yeast slurry (250 mL) was provided by Emerson’s from their yeast propagation tanks. The slurry was added into 1 L (in a 3-L conical flask) of fresh brewery wort at 10°P provided by the brewery and stirred for 24–48 h at 20 °C with a magnetic stir bar, prior to yeast enumeration and pitching (Section 2.2.5).
2.2.4. Commercial Yeast Slurry
A vial of White Labs WLP-001 California Ale® yeast was purchased commercially and propagated by adding the vial to 1 L (in a 3-L conical flask) of fresh brewery wort (10°P, 1 L) and stirring for 24–48 h at 20 °C with a magnetic stir bar prior to yeast enumeration and pitching (Section 2.2.5).
2.2.5. Yeast Pitching
Approximately 24 h prior to pitching, each propagated yeast culture was centrifuged (3000 rpm for 10 min at 20 °C) in 1-L bottles (Nalgene 3120-1000 Centrifuge bottle), the supernatant was discarded, Emerson’s fresh brewery wort (10°P, 200 mL) was added to the slurry, and the suspension was agitated (200 rpm, 60 min) to resuspend the yeast. Cell numbers were estimated, and the volume of yeast slurry required to achieve the target inoculation rate of 1 × 107 viable cells /mL in 10 L wort was calculated.
The number of yeast cells in each starter culture (2.2.1–2.2.4) was estimated using an Oculyze BB 1.0 (Oculyze GmbH, Hochschulring, Germany) with methylene blue (MB) as a stain (1:1 ratio). A microscopic slide (200-µL sample chamber; Gräfelfing, Germany) was prepared and analysed under 400× magnification to calculate cell numbers, budding cell values, and culture viability using the cloud-based platform [14]. Five pictures were taken of the most appropriate dilution, and the percentage cell viability (>90% was obtained) and mean yeast numbers per mL (million cells per mL) were estimated [15].
2.3. Temperature of Fermentation
S. cerevisiae was the most common (8/12) yeast used in this study (Table 1), along with one S. cerevisiae hybrid, two S. pastorianus strains, and one S. bayanus strain. The S. cerevisiae stains typically produce an ale-style beer; S. pastorianus and S. bayanus primarily produce lager-style beers [16]. The use of different yeast strains posed a question of which temperature should be used for fermentation, as this would likely impact on yeast growth rates, fermentation time, and VOC production [16]. Despite lager yeast strains being typically fermented at 8–15 °C and ale yeast strains at 14–20 °C, it was decided to remove temperature as an experimental variable and carry out all fermentations at 20 °C.
2.4. Analysis of Beer Samples Using Free Sorting Sensory Methodology
Prior to study commencement, ethics was approved by the University of Otago Human Ethics Committee (Reference 18/154). A total of 14 panelists completed the free sorting task, with eight from the University of Otago Department of Food Science sensory panel, four brewing professionals/expert beer tasters from Emerson’s Brewery, and two Department of Food Science postgraduate students. The free sorting task was completed over five sessions of 2 h each with two initial training sessions used to familiarise the panelists with the sensory space of the beer samples and the free sorting task itself, followed by three formal evaluation sessions. Session one included a taste identification test using five sample solutions (sucrose, citric acid, caffeine, sodium chloride, and alum) followed by a descriptive test where four beer samples from the twelve experimental samples were evaluated. The beers were presented in pairs, and panelists were asked to comment on the sample’s aroma, appearance, flavour, and mouthfeel, as well as their overall impression of the difference between the two beers. In session two, the sorting task protocol was explained, and a mock sorting task was carried out to familiarise the panelists with the sorting task methodology using six of the twelve beer samples.
The formal evaluation sessions were completed during the remaining three sessions. In each evaluation session, the panelists received a tray of all twelve beer samples presented in a balanced order according to a Williams Latin Square design. Beer samples (40 mL) were served at 10 °C ± 2 °C in 200-mL lidded plastic cups identified with random 3-digit codes. The panelists were instructed to smell and taste the samples in the order presented and sort them into groups based on their similarities of sensory attributes. The panelists were instructed to sort the samples into any number of groups, provided that a minimum of two groups and a maximum of 11 groups were formed. A group could contain up to 11 samples if preferred, and a panelist could choose any criteria (sensory attributes) to sort the samples. Panelists also separately recorded individual descriptions on each sample. Retasting was allowed for confirmation of groupings. Once the samples were sorted into groups, the panelists were asked to record the characteristic sensory attributes of each group. The groups and sensory attributes were recorded using Compusense Cloud (Compusense Inc., Guelph, ON, Canada) on Apple iPads (Apple Inc., Cupertino, CA, USA). Filtered water, plain crackers, and sliced carrots were provided as palate cleansers between samples. Maximum alcohol consumption for a panelist at each evaluation session was equivalent to a maximum of 1.50 standard drinks. Panelists were provided with food after each session.
2.5. Analysis of Beer VOC Using Headspace Solid Phase Microextraction and Gas Chromatography Mass Spectrometry
The VOC profiles of the beer samples were measured using gas chromatography mass spectrometry (GC/MS) coupled with headspace solid-phase microextraction (HS-SPME). Aliquots of each beer (8 mL) were combined with 2.5 g analytical grade sodium chloride (NaCl; BDH Laboratory Supplies, England) in 20-mL headspace vials and capped with PTFE-lined silicon septa screw caps. Blank samples were prepared with deionised water (8 mL) and NaCl (2.5 g). Each sample was incubated at 40 °C for 5 min with agitation, followed by SPME extraction for 30 min at 40 °C using a multipurpose sampler (Agilent PAL3 RSI 85 Autosampler; Palo Alto, CA, USA). Analysis was completed with an Agilent 6890 N gas chromatograph connected to an Agilent 5975 VL mass spectrometer (MSD) with triple axis detector (Agilent Technologies, USA). Helium was used as the carrier gas in constant flow mode at a rate of 1.0 mL·min−1. Separation of analytes was achieved using a Zebron ZB-Wax column (60 m, 0.32-mm i.d., 0.5-μm film thickness; Phenomenex, Torrance, CA, USA). Samples were desorbed in the inlet at 240 °C for 5 min in the splitless mode. The initial oven temperature was 50 °C for 5 min, then heated at 5 °C.min−1 to 210 °C, followed by 10 °C.min−1 to 240 °C, and held for 5 min. The MSD was operated in electron impact (EI) ionisation mode at 70 eV with an ion source temperature of 230 °C with a scan range of m/z 29–300. Analyses were completed in quadruplicate (samples, n = 12; blanks, n = 4). To prevent order effects, samples were analysed according to a modified Williams Latin Square design.
2.6. Data Analysis
2.6.1. Analysis of Sensory Data
The sorting task data, groups, and sensory attributes were exported from Compusense for all evaluation sessions. Due to the free-sort methodology enabling panelists to use their own vocabulary, textual preprocessing was required. This included correcting spelling, standardising word endings, combining synonyms, and selecting key words [17,18]. Multiple researchers confirmed synonymy of attributes and final selection of key words, with any terms used in less than three groups removed from further analysis. The data were initially analysed using the Factorial Approach for Sorting Task data (FAST) [19], which applies multiple correspondence analysis (MCA) to the group and attributes data and projects the data onto a two-dimensional map of the samples and attributes and evaluates significant associations between the samples and attributes. A contingency table of the attributes was generated (Appendix A, Table A1) and used to evaluate the relationship between the sensory and analytical data with the application of multiple factor analysis for contingency tables (MFACT) [20].
2.6.2. Analysis of GC-MS Data
Exported GC-MS data was analysed using PARADISe (PARAFAC2 based Deconvolution and Identification System), version 3.87 [21,22]. This resulted in a table of relative peak area for each detected compound for all samples. Retention indices (RIs) were determined using cubic spline interpolation [23] after running a C9–C30 saturated alkane standard using the same GC temperature program. VOCs were regarded as “unknown” if the mass-spectra match value was below 700 or the calculated RI did not match the reported RI. The relative peak area table was analysed by one-way analysis of variance (ANOVA) with a level of confidence of 95% (Appendix A, Table A2) followed by Tukey post-hoc testing to identify significant groupings.
2.6.3. Analysis of the Relationship between the Sensory and GC-MS Data
Due to the ability of multiple factor analysis (MFA) to analyse multiple data sets of variables collected from the same set of samples, it is particularly useful when investigating the relationship between different experimental measures [24]. MFA produces sample and attribute projections that represent the similarities between the samples and between the different data sets of variables. The extension of MFA to include contingency tables (MFACT) [25] allows for the investigation of relationships between the sensory characterisation completed using the free-sort method and volatile analysis using GC-MS. Due to the use of contingency data for the sensory attributes, means of the four replicates of each sample were calculated for the volatile data, which were unit scaled as part of the MFACT analysis. Only the VOCs determined to be significantly different across the beer samples were included in the MFACT.
All data analysis was completed using R version 3.5.3 [26] and the RStudio IDE [27] with the tidyverse suite of packages [28], plus additional packages agricolae [29], SensoMineR [30], and FactoMineR [31].
3. Results and Discussion
3.1. Summary of Beer Groups Formed
In the sorting task, panelists created between three and ten groups from the twelve beer samples (Figure 1a) with four, five, and six groups being most common. The groups most frequently contained two beers (Figure 1b), with one sample per group being the next most frequent, showing that the sensory attributes of the beers were being perceived by the panelists as being distinctly different.
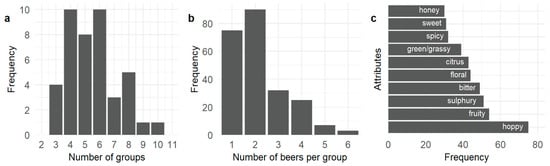
Figure 1.
(a) Frequency of the number of groups formed by the panelists during the free sorting task (14 panelists × 3 sessions); (b) frequency of the number of beers sorted into each group; (c) frequency of the ten most commonly used attributes.
3.2. Representation of Beers and Sensory Attributes
A total of 96 distinct attributes were generated by the panelists, which were refined through textual processing to 41. Hoppy was the most commonly used attribute to describe the twelve beers, followed by fruity, sulfury, bitter, floral, citrus, green/grassy, spicy, sweet, and honey (Figure 1c). From the FAST analysis, hoppy was significantly (p < 0.05) associated with beer W3470 (Table 2) while sulfury was significantly (p < 0.05) associated with samples WY1272 and OTA79. WY1272 was also significantly associated with metallic. WB06 was significantly associated with the terms spicy and estery while WLP730 was significantly associated with spicy. The use of sour was significantly associated with the VIN13 beer sample while astringent was significantly associated with the WLP001 beer. No terms were significantly associated with US05, BE256, S23, OTA29, or SPH.

Table 2.
Significant association of beer samples to sensory attributes using FAST analysis.
3.3. Representation of Similarity Co-Occurrence Matrix of Beers
The co-occurrence matrix reflects the perceived similarity of the different beers (Table 3). The most similar beers were WB06 and WLP730, associated together 17 times (40.5%); WLP730 and SPH, associated 15 times (35.7%); WB06 and SPH, associated 13 times (31.0%); S23 and WY1272, associated 13 times (31.0%); and US05 was associated with WY1272 and OTA29 12 times each (28.6%). The least similar beers were OTA79 and WLP730, which were not grouped together at all. Samples were grouped alone a total of 75 times, with each sample grouped alone between three and nine times (Table 3). BE256 and VIN13 were most frequently grouped alone nine times (21.4%) while S23 was alone only three times (7.1%), indicating it was perceived as more similar to the other samples.

Table 3.
Co-occurrences of beer samples in the sorting task.
3.4. Relationship of Beer VOCs with Sensory Attributes
The twelve beers were projected similarly in the FAST and MFACT, so to prevent duplication, only the MFACT is presented to illustrate the relationships between the twelve beers and their sensory attributes and VOC profile (Figure 2). Factors 1 (21.82%), 2 (14.32%), and 3 (12.50%) in the MFACT show that the beers were distributed in all dimensions with a total explained variance for these three factors of 48.64%.
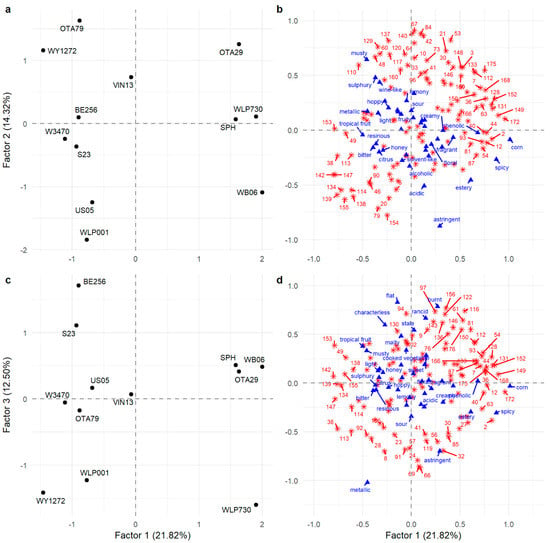
Figure 2.
Separation of twelve beer samples (a,c) with sensory attributes (blue triangles) and VOCs (red asterisks) (b,d) on the MFACT plot on Factors 1–3. Labelled VOC numbers correspond to important contributors; refer to Appendix A, Table A2 for VOC identification.
Factor 1 separated beers WB06, OTA29, SPH, and WLP730 from the other beers (Figure 2a). This placement was consistent with the co-occurrence similarity matrix where beer WLP730 was grouped 17 times with WB06 and 15 times with SPH, and WB06 and SPH were placed together 13 times (Table 3). The sensory attributes with the highest F1 positive loadings were spicy, corn, phenolic, and estery, while those with the highest F1 negative loadings were bitter, citrus, hoppy, and tropical fruit. This has been reflected in the contingency table (Appendix A, Table A1) where spicy had the highest frequency of use with WB06, WLP730, and SPH; corn and phenolic were associated more frequently with these four beers than any of the others, and estery was most frequently used with WB06. The FAST analysis also reflected this, where spicy was significantly associated with WLP730 and WB06, which was also significantly associated with estery (Table 2). In contrast, citrus and tropical fruit were never used to describe WB06, and although hoppy was used to describe all beers, it was used most frequently with, and was significantly associated to, W3470 while it was used least frequently with WB06.
The VOCs with the highest positive loadings on F1 were 4-vinylguaiacol (172; spicy, clove, phenolic), hexanoic acid (149; cheesy), dimethyl sulfide (2; sulfurous, onion, sweet corn, vegetable), 1-propanol (12; alcoholic, earthy, fermented), ethyl hexanoate (36; sweet, fruity, banana, estery), and propyl hexanoate (54; fruity) (Figure 2b). In WLP730, OTA29, SPH, and WB06 beers (Figure 3a), 4-vinylguaiacol was significantly higher. It is produced from malt-derived ferulic acid by heat and/or enzyme decarboxylation, with most conversion (60–90%) attributed to yeast activity. However, not all yeast strains expressed the POF+ (phenolic off-flavour) gene that allowed synthesis of 4-vinylguaiacol from ferulic acid [32]. Hence, the other eight beers only contained trace amounts of 4-vinylguaiacol, as the POF+ gene is not present in the majority of commercial ale and lager yeast strains. The volatile analysis showed that ethyl hexanoate (36) was significantly higher in beers OTA29 and WB06 (Figure 3b). The WB06 beer also showed the highest abundance of propyl hexanoate (54), which corresponds to the literature where WB06 is known to produce estery, fruity, and phenolic flavours [33,34,35]. On F1, there was good agreement between those VOCs with large positive loadings and the sensory attributes used to describe the beers. For example, spicy and phenolic attributes were associated with higher abundance of 4-vinylguaiacol, estery was associated with ethyl hexanoate and other esters, and higher levels of DMS (Figure 3c) were associated with a corn attribute.
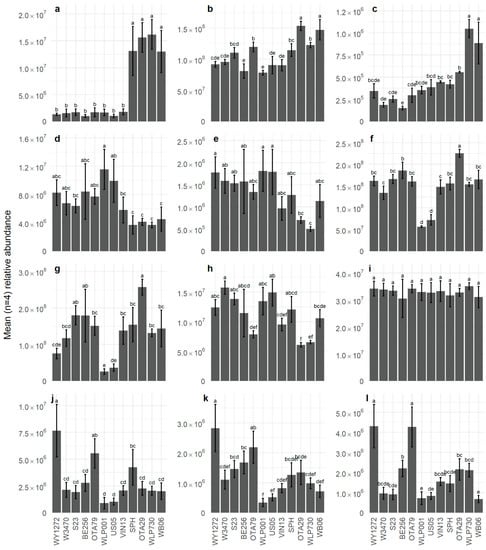
Figure 3.
Relative abundance (deconvoluted peak area) of VOC in twelve beer samples. (a) 4-vinylguaiacol, (b) ethyl hexanoate, (c) dimethyl sulfide, (d) geraniol, (e) nerol, (f) isoamyl acetate, (g) 2-phenylethyl acetate, (h) citronellol, (i) linalool, (j) citronellol acetate, (k) nerol acetate, and (l) geranyl acetate. Values are plotted as means ± standard deviation. In each graph, samples sharing the same letter are not significantly different at p < 0.05. Note the different y-axis values.
The VOCs with the largest negative loadings on F1 were nerol (142; lemon, fruity), geraniol (147; floral, rose, fruity, citrus), ethyl dihydrocinnamate (153; rose, honey, fruity), β-ocimene (38; green, tropical, floral), citral (134; citrus, green, herbal), and citronellol (138; floral, fruity, citrus) (Figure 2b). This reflects the sensory attributes that were also negatively loaded on F1 (bitter, citrus, hoppy, and tropical fruit) and with the citrusy and resinous characteristics consistent with higher levels of the terpene compounds. For example, geraniol (147) was significantly higher in WLP001 and US05 compared to WB06, OTA29, SPH, and WLP730 (Figure 3d), and a similar pattern was found with nerol being lowest in WLP730 and OTA29 (142; Figure 3e).
The separation of beers on F2 was largely due to WLP001, US05, and WB06 with negative loadings and OTA29, OTA79, and WY1272 with positive loadings (Figure 2a). The main drivers in sensory characteristics were the significant association of astringency with WLP001 while OTA79 and WY1271 were significantly associated with sulfury (Table 2). Other negatively loaded sensory attributes included estery, acidic, and alcoholic while hoppy, musty, wine-like, and lemony were positively loaded. The most important positively loaded VOCs on F2 were 2-ethylhexyl acetate (67; earthy, herbal, dirty), octyl acetate (84; green, earthy, citrus), hexyl acetate (42; fruity, green apple), (E)-3-hexen-1-ol acetate (53; green, fruity, unripe banana, earthy), geranyl acetate (137; floral, green, citrus, winey), heptyl acetate (64; green, fruity, citrus), nerol acetate (129; floral, fruity, citrus, tropical), 2-phenylethyl acetate (146; floral, rose, honey), and 3-methylbutyl acetate (isoamyl acetate; 21; sweet, fruity, banana). Acetate esters are important flavour compounds in beer and are often present at relatively high concentrations [36]. The presence of these VOCs relates to the positively loaded sensory attributes and relative positioning of the WLP001 and OTA29 beers, which had the lowest and highest abundance of acetate esters, respectively, as illustrated by the abundance isoamyl acetate (Figure 3f) and 2-phenylethyl acetate (Figure 3g).
Factor 3 separated beer BE256 from beers WY1272 and WLP730 (Figure 2c). This separation was due to the sensory attributes metallic and astringent (negative loading) followed by flat, malty, and rancid (positive loadings) (Figure 2d). The FAST analysis identified that metallic was significantly associated with WY1272, which is driving this separation. The VOCs loaded negatively on F3 were 2-methyl-3-heptanone (24; fruity, green, leafy), 4-methyl-2-pentanone (8; solvent, green, fruity, dairy), 2-methylbutyl 2-methylpropanoate (32; fruity, tropical, banana), 2-nonanol (91; waxy, musty, fruity), and 6-methyl-5-hepten-2-one (57; green, musty fruity). In contrast, butyl 9-decenoate (156), 3-methylbutyl octanoate (116; sweet, fruity, green), 2-methylpropyl octanoate (97; fruity, green, floral), and ethyl nonanoate (94; fruity, waxy, tropical) had positive loadings.
3.5. Terpenoid Compounds Present in Beers
The primary aim of this study was to evaluate the impact of yeast strain on hop flavour characteristics in beer and to gain an understanding of what VOC in the beers may be responsible for these differences. The relative abundance of the monoterpene alcohols and monoterpene esters in the twelve beers was investigated to see whether any trends existed across the twelve yeast strains.
Hop terpenoids form part of the essential oil component found in hops that contributes aroma-active compounds to beer. Hop terpenoids in beer originate from hops added during the brewing process, which may be modified by biotransformation reactions by yeast during fermentation [5,37]. The monoterpene alcohols geraniol (147), nerol (142), citronellol (138), and linalool (95) are important aroma-active terpenoids found in beer. Geraniol was significantly higher in WLP001 and US05 compared to WB06, OTA29, SPH, and WLP730 beers (Figure 3d). The highest nerol concentration was found in the WLP001, WY1272, and US05 beers, and the lowest concentration occurred in the WLP730 and OTA29 beers (Figure 3e). Nerol contributed a rose-like flavour, and geraniol contributed a rose-like, floral, and citrusy flavour in beer [34]. Although the abundance of geraniol and nerol tended to be highest in the ale S. cerevisiae yeast strains, WB06 (S. cerevisiae yeast strain) had the fourth lowest abundance of geraniol and nerol, with two S. pastorianus strains producing beers with higher levels. King and Dickinson [5] suggested that the extent of monoterpene transformation is strain specific, rather than related to either S. cerevisiae or S. pastorianus strains.
Citronellol is a yeast biotransformation product of geraniol [5] typically found at low levels in hop essential oil, but at much greater abundance in beer. The abundance of citronellol followed a similar pattern to nerol (Figure 3h). Linalool, one of the most frequently occurring and abundant terpene alcohols in hops, is a product of the oxidation of myrcene, and it is found in hop essential oil. Linalool has been identified as an important odour-active compound in lager beer [38,39,40]. In the current study, the level of linalool (95; Figure 3i) did not significantly differ between the twelve beer samples. This means that although it may have contributed to a generic hop flavour, it did not appear to be an important compound in yeast-specific biotransformations, at least in beers late-hopped with New Zealand Motueka hops.
Citronellol acetate (117) was found at the highest abundance in WY1272, significantly higher than all other beer samples other than OTA79 (Figure 3j), showing a different pattern compared to citronellol. Nerol acetate (129; Figure 3k) and geranyl acetate (137; Figure 3l) illustrated similar patterns, with the highest abundance in WY1272 and OTA79. Citronellol acetate was reported to contribute a floral, fruity, pear, and apple character, and nerol acetate contributed a floral and green flavour to beer [41]. Citronellol acetate is not naturally found in hops, which suggests the production of terpene esters during fermentation by yeast esterase activity. Citronellol acetate is formed either by acetylation of citronellol, after reduction from geraniol, or by reduction from geranyl acetate [42]. It is known that monoterpene acetate esters of terpenes are formed in beer during fermentation and are particularly expressed in high concentration in beers that are late-hopped. King and Dickinson [5] postulated that terpenoid ester formation occurs with lager yeast strains but not in ale strains, although only two strains for each of S. cerevisiae and S. bayanus were investigated for that study. In contrast, the current experiment, supported by a study conducted by Richter, Eyres, Silcock, and Bremer [3], showed that ale yeast strains can form higher amounts of citronellol acetate, geranyl acetate, and nerol acetate than the selected S. bayanus strains. It was also observed that the beers with high levels of the yeast-derived ethyl acetate and higher alcohol acetate esters (e.g., isoamyl acetate, 2-phenylethyl acetate) did not necessarily have the highest levels of terpene acetate esters. This is likely because the formation of the terpene acetate esters requires the presence of abundant monoterpene alcohols, in addition to alcohol acetyltransferase (AATase) activity and acetyl CoA. In contrast, US05 and WLP001, which had amongst the highest levels of geraniol and nerol but the lowest levels of the higher alcohol acetate esters, had the lowest levels of citronellol acetate and nerol acetate. This is probably due to reduced AATase activity rather than reduced acetyl-CoA availability, as yeast growth was not excessive [43].
4. Conclusions
The sensory characteristics and VOC profiles of beers fermented by twelve different yeast strains under the same conditions differed considerably, with links being evident between the presence of specific volatile compounds and perceived sensory attributes. Beers high in 4-vinylguaiacol were perceived to be spicy and clove-like, the abundance of dimethyl sulfide was associated with a corn character, and beers with higher concentrations of acetate and ethyl esters were perceived to be fruity and estery. Biotransformation of terpenes involved a complex series of reaction pathways, which led to distinct patterns of hop-derived terpenoid compounds in the twelve beers as a function of yeast strain. The levels of monoterpene alcohols (geraniol, nerol, and citronellol) varied across the twelve samples, although there were no differences in the abundance of linalool. The terpene acetate esters detected varied between the twelve beers, but their occurrence did not correlate with the abundance of fermentation esters or monoterpene alcohols.
To understand the generation of these biotransformation products in beer, further studies need to be conducted under model conditions where the precursors can be closely controlled, to further understand the biosynthetic reactions. Finally, understanding how yeast strain and fermentation factors influence hop aroma in beer will help the brewing industry to better understand how to control beer flavour to meet consumer demands.
Author Contributions
Conceptualisation, G.T.E., P.S., and P.J.B.; methodology, A.K., A.W., G.T.E., P.S., and P.J.B.; formal analysis, A.K., A.W., P.S., and G.T.E.; investigation, A.K.; resources, G.T.E., P.S., and P.J.B.; data curation, A.K. and A.W.; writing—original draft preparation, A.K. and A.W.; writing—review and editing, G.T.E., P.S., P.J.B., and A.W.; visualisation, A.K., G.T.E., P.S., and A.W.; supervision, G.T.E., P.S., and P.J.B.; project administration, G.T.E.; funding acquisition, G.T.E., P.S., and P.J.B. With the exception of A.K. All authors have read and agreed to the published version of the manuscript.
Funding
A.K. received the JP Dufour Scholarship from Emerson’s Brewery to support this research.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was conducted as part of the MSc thesis of A.K. [44]. The authors thank Emerson’s Brewery for the JP Dufour Scholarship and Jamie Scrimgeour for supporting the research (2018–2019), as well as the Department of Food Science technicians, Michelle Leus, Michelle Petrie, and Ian Ross, for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Frequency of use of distinct descriptors with twelve beer samples.
Table A1.
Frequency of use of distinct descriptors with twelve beer samples.
| Attribute | US 05 | WLP 001 | W 3470 | S 23 | WB 06 | WLP 730 | OTA 29 | OTA 79 | WY 1272 | VIN 13 | BE 256 | SPH | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoppy | 7 | 6 | 12 | 3 | 2 | 3 | 8 | 11 | 7 | 6 | 6 | 4 | 75 |
| Fruity | 5 | 2 | 6 | 6 | 3 | 5 | 5 | 4 | 8 | 4 | 4 | 2 | 54 |
| Sulfury | 4 | 1 | 2 | 5 | 3 | 1 | 2 | 12 | 9 | 6 | 4 | 2 | 51 |
| Bitter | 3 | 8 | 7 | 7 | 2 | 3 | 1 | 5 | 5 | 1 | 5 | 2 | 49 |
| Floral | 6 | 5 | 2 | 3 | 5 | 3 | 6 | 2 | 3 | 4 | 1 | 4 | 44 |
| Citrus | 5 | 8 | 1 | 6 | 0 | 2 | 2 | 2 | 3 | 6 | 6 | 2 | 43 |
| Green/ Grassy | 2 | 3 | 4 | 5 | 2 | 2 | 7 | 0 | 6 | 0 | 2 | 6 | 39 |
| Spicy | 1 | 4 | 0 | 1 | 7 | 8 | 2 | 2 | 0 | 1 | 0 | 6 | 32 |
| Sweet | 5 | 2 | 0 | 3 | 5 | 1 | 3 | 2 | 1 | 4 | 3 | 2 | 31 |
| Honey | 4 | 3 | 2 | 6 | 2 | 1 | 1 | 1 | 3 | 4 | 2 | 1 | 30 |
| Sour | 0 | 2 | 4 | 0 | 3 | 3 | 0 | 2 | 4 | 6 | 1 | 2 | 27 |
| Phenolic | 2 | 1 | 1 | 2 | 4 | 5 | 4 | 1 | 0 | 2 | 1 | 4 | 27 |
| Woody | 2 | 3 | 1 | 2 | 1 | 2 | 4 | 1 | 2 | 2 | 1 | 2 | 23 |
| Estery | 3 | 3 | 0 | 1 | 6 | 4 | 2 | 0 | 2 | 0 | 0 | 2 | 23 |
| Malty | 1 | 0 | 3 | 4 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 3 | 19 |
| Lemony | 0 | 0 | 4 | 1 | 1 | 3 | 0 | 1 | 2 | 4 | 1 | 1 | 18 |
| Yeasty | 2 | 1 | 1 | 0 | 3 | 0 | 1 | 4 | 0 | 2 | 1 | 2 | 17 |
| Solvent-Like | 3 | 2 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 2 | 0 | 16 |
| Acidic | 2 | 2 | 3 | 1 | 3 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 16 |
| Fragrant | 1 | 1 | 2 | 0 | 2 | 3 | 2 | 1 | 0 | 0 | 2 | 1 | 15 |
| Creamy | 2 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 0 | 0 | 3 | 14 |
| Banana | 3 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 3 | 14 |
| Apple/Pear | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 14 |
| Caramel | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 3 | 2 | 1 | 14 |
| Corn | 0 | 1 | 0 | 0 | 3 | 3 | 2 | 0 | 0 | 1 | 1 | 3 | 14 |
| Tropical Fruit | 1 | 1 | 2 | 3 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 0 | 13 |
| Light | 1 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 3 | 0 | 13 |
| Alcoholic | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 12 |
| Wine-Like | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 0 | 12 |
| Resinous | 0 | 3 | 2 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 0 | 12 |
| Flat | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 10 |
| Grainy | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 2 | 2 | 10 |
| Cooked Vegetable | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 3 | 1 | 10 |
| Characterless | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 9 |
| Soapy | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 9 |
| Rancid | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 8 |
| Astringent | 0 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 7 |
| Musty | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 2 | 0 | 7 |
| Metallic | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 7 |
| Stale | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 4 |
| Burnt | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Total | 76 | 78 | 70 | 73 | 70 | 69 | 70 | 70 | 72 | 74 | 74 | 70 | 866 |

Table A2.
Volatile organic compounds identified in twelve beer samples.
Table A2.
Volatile organic compounds identified in twelve beer samples.
| No. | Volatile Compound | RI (calc) | RI (lit) | Match | CAS No. | p-Value |
|---|---|---|---|---|---|---|
| 1 | acetaldehyde | 713 | 702 | 852 | 75-07-0 | 0.000 |
| 2 | dimethyl sulfide | 760 | 754 | 948 | 75-18-3 | 0.000 |
| 3 | ethyl acetate | 896 | 888 | 944 | 141-78-6 | 0.000 |
| 4 | ethanol | 937 | 932 | 935 | 64-17-5 | 0.296 |
| 5 | ethyl propanoate | 963 | 953 | 793 | 105-37-3 | 0.000 |
| 6 | ethyl 2-methylpropanoate | 972 | 961 | 780 | 97-62-1 | 0.000 |
| 7 | n-propyl acetate | 981 | 973 | 942 | 109-60-4 | 0.000 |
| 8 | 4-methyl-2-pentanone | 1014 | 1010 | 891 | 108-10-1 | 0.016 |
| 9 | 2-methylpropyl acetate | 1019 | 1012 | 952 | 110-19-0 | 0.000 |
| 10 | 3-methyl-2-pentanone | 1025 | 1019 | 731 | 565-61-7 | 0.000 |
| 11 | α-pinene | 1031 | 1028 | 840 | 80-56-8 | 0.251 |
| 12 | 1-propanol | 1038 | 1036 | 938 | 71-23-8 | 0.000 |
| 13 | ethyl butanoate | 1042 | 1035 | 941 | 105-54-4 | 0.000 |
| 14 | 5-methyl-3-hexanone | 1082 | 1036 | 746 | 623-56-3 | 0.334 |
| 15 | dimethyl disulfide | 1083 | 1077 | 738 | 624-92-0 | 0.000 |
| 16 | 2-methylpropyl propanoate | 1085 | 1079 | 708 | 540-42-1 | 0.002 |
| 17 | 2-methyl-1-propanol | 1088 | 1092 | 949 | 78-83-1 | 0.000 |
| 18 | 2-methylpropyl 2-methylpropanoate | 1094 | 1090 | 940 | 97-85-8 | 0.000 |
| 19 | 1-(1-ethoxyethoxy)-pentane | 1107 | 1098 | 924 | 13442-89-2 | 0.206 |
| 20 | β-pinene | 1115 | 1112 | 741 | 127-91-3 | 0.020 |
| 21 | 3-methyl-1-butyl acetate | 1126 | 1122 | 951 | 123-92-2 | 0.000 |
| 22 | ethyl pentanoate | 1138 | 1134 | 877 | 539-82-2 | 0.050 |
| 23 | 5-methyl-2-hexanone | 1145 | 1156 | 873 | 110-12-3 | 0.000 |
| 24 | 2-methyl-3-heptanone | 1154 | 1179 | 844 | 13019-20-0 | 0.001 |
| 25 | β-myrcene | 1167 | 1161 | 959 | 123-35-3 | 0.079 |
| 26 | α-phellandrene | 1167 | 1167 | 863 | 99-83-2 | 0.105 |
| 27 | 2-methylpropyl 2-methylbutanoate | 1178 | 1179 | 827 | 2445-67-2 | 0.026 |
| 28 | 2-heptanone | 1186 | 1182 | 871 | 110-43-0 | 0.000 |
| 29 | α-terpinene | 1188 | 1180 | 897 | 99-86-5 | 0.346 |
| 30 | 2-methylbutyl propanoate | 1191 | 1197 | 907 | 2438-20-2 | 0.000 |
| 31 | ethyl 4-methylpentanoate | 1192 | 1190 | 755 | 25415-67-2 | 0.000 |
| 32 | 2-methylbutyl 2-methylpropanoate | 1197 | 1199 | 976 | 2445-69-4 | 0.006 |
| 33 | 2-methyl-1-butanol | 1201 | 1208 | 945 | 137-32-6 | 0.000 |
| 34 | 3-methyl-1-butanol | 1203 | 1209 | 960 | 123-51-3 | 0.254 |
| 35 | β-phellandrene | 1218 | 1211 | 920 | 555-10-2 | 0.940 |
| 36 | ethyl hexanoate | 1236 | 1233 | 978 | 123-66-0 | 0.000 |
| 37 | (2R,5R)-2-methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 1248 | 1243 | 779 | 54750-70-8 | 0.164 |
| 38 | β-ocimene | 1255 | 1250 | 883 | 13877-91-3 | 0.001 |
| 39 | unknown | 1259 | - | - | - | 0.000 |
| 40 | styrene | 1267 | 1261 | 962 | 100-42-5 | 0.000 |
| 41 | 4-pentenyl butanoate | 1271 | - | 726 | 30563-31-6 | 0.004 |
| 42 | hexyl acetate | 1274 | 1272 | 923 | 142-92-7 | 0.000 |
| 43 | bicyclo [4.2.0]oct-1-ene, 7-exo-ethenyl- | 1276 | - | 836 | - | 0.579 |
| 44 | ethyl 5-hexenoate | 1280 | 1271 | 761 | - | 0.000 |
| 45 | 2-methylbutyl 2-methylbutanoate | 1282 | 1284 | 935 | 2445-78-5 | 0.752 |
| 46 | ethyl 5-methylhexanoate | 1288 | - | 922 | 10236-10-9 | 0.000 |
| 47 | α-terpinolene | 1292 | 1283 | 873 | 586-62-9 | 0.137 |
| 48 | 1,2,4-trimethyl-benzene | 1293 | 1282 | 709 | 95-63-6 | 0.000 |
| 49 | ethyl (Z)-3-hexenoate | 1293 | 1292 | 752 | 64187-83-3 | 0.000 |
| 50 | 2-methylbutyl 3-methylbutanoate | 1297 | 1299 | 825 | 2445-77-4 | 0.285 |
| 51 | ethyl (E)-3-hexenoate | 1302 | 1289 | 912 | 26553-46-8 | 0.018 |
| 52 | unknown ester | 1316 | - | - | - | 0.198 |
| 53 | (E)-3-hexenyl acetate | 1319 | 1306 | 769 | 3681-82-1 | 0.000 |
| 54 | propyl hexanoate | 1320 | 1316 | 762 | 626-77-7 | 0.000 |
| 55 | ethyl heptanoate | 1335 | 1331 | 943 | 106-30-9 | 0.000 |
| 56 | methyl 4-methylenehexanoate | 1340 | 1345 | 817 | 73805-48-8 | 0.000 |
| 57 | 6-methyl-5-hepten-2-one | 1342 | 1338 | 916 | 110-93-0 | 0.001 |
| 58 | 1-hexanol | 1346 | 1355 | 909 | 111-27-3 | 0.355 |
| 59 | ethyl 2-hexenoate | 1349 | 1340 | 821 | 1552-67-6 | 0.000 |
| 60 | unknown | 1351 | - | - | - | 0.000 |
| 61 | 2-methylpropyl hexanoate | 1354 | 1350 | 754 | 105-79-3 | 0.000 |
| 62 | rose oxide | 1360 | 1350 | 895 | 16409-43-1 | 0.000 |
| 63 | 3-ethoxy-1-propanol | 1373 | 1373 | 853 | 111-35-3 | 0.000 |
| 64 | heptyl acetate | 1374 | 1377 | 905 | 112-06-1 | 0.000 |
| 65 | verbenyl ethyl ether | 1376 | 1377 | 722 | 80581-06-2 | 0.992 |
| 66 | hop ether | 1380 | 1360 | 900 | 344294-72-0 | 0.032 |
| 67 | 2-ethylhexyl acetate | 1384 | 1420 | 895 | 103-09-3 | 0.000 |
| 68 | unknown | 1387 | - | - | - | 0.001 |
| 69 | unknown | 1391 | - | 784 | - | 0.006 |
| 70 | 2-nonanone | 1394 | 1390 | 943 | 821-55-6 | 0.000 |
| 71 | 2-isobutenyl-4-vinyl-tetrahydrofuran | 1400 | - | 732 | - | 0.146 |
| 72 | (E)-4-hexenyl butanoate | 1404 | 1478 | 749 | - | 0.000 |
| 73 | 1,3-dimethyl-1-cyclohexene | 1405 | - | 787 | 2808-76-6 | 0.000 |
| 74 | 2-octanol | 1410 | 1412 | 777 | 123-96-6 | 0.000 |
| 75 | 3-(4-methyl-3-pentenyl)-furan | 1425 | 1429 | 911 | 539-52-6 | 0.225 |
| 76 | ethyl octanoate | 1439 | 1435 | 941 | 106-32-1 | 0.000 |
| 77 | 1-octen-3-ol | 1442 | 1450 | 768 | 3391-86-4 | 0.976 |
| 78 | (Z)-linalool oxide | 1446 | 1444 | 800 | 5989-33-3 | 0.928 |
| 79 | 1-heptanol | 1448 | 1453 | 878 | 111-70-6 | 0.000 |
| 80 | 6-methyl-5-hepten-2-ol | 1455 | 1465 | 774 | 1569-60-4 | 0.064 |
| 81 | 3-methylbutyl hexanoate | 1461 | 1451 | 884 | 2198-61-0 | 0.000 |
| 82 | acetic acid | 1464 | 1449 | 938 | 64-19-7 | 0.000 |
| 83 | 3-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-propenal | 1465 | - | 744 | 4951-40-0 | 0.000 |
| 84 | octyl acetate | 1476 | 1474 | 903 | 112-14-1 | 0.000 |
| 85 | 4-tert-pentylcyclohexene | 1477 | - | 717 | 51874-62-5 | 0.000 |
| 86 | nerol oxide | 1479 | 1469 | 827 | 1786-08-9 | 0.556 |
| 87 | ethyl 7-octenoate | 1487 | 1478 | 925 | 35194-38-8 | 0.000 |
| 88 | 2-decanone | 1499 | 1494 | 706 | 693-54-9 | 0.000 |
| 89 | unknown | 1502 | - | 704 | - | 0.001 |
| 90 | decanal | 1505 | 1498 | 748 | 112-31-2 | 0.000 |
| 91 | 2-nonanol | 1510 | 1521 | 822 | 628-99-9 | 0.000 |
| 92 | 2-acetylfuran | 1517 | 1499 | 755 | 1192-62-7 | 0.008 |
| 93 | propyl octanoate | 1521 | 1510 | 935 | 624-13-5 | 0.000 |
| 94 | ethyl nonanoate | 1537 | 1531 | 914 | 123-29-5 | 0.009 |
| 95 | linalool | 1540 | 1547 | 975 | 78-70-6 | 0.820 |
| 96 | 1-octanol | 1550 | 1557 | 932 | 111-87-5 | 0.000 |
| 97 | 2-methylpropyl octanoate | 1553 | 1548 | 944 | 5461-06-3 | 0.000 |
| 98 | (E)-p-2-menthen-1-ol | 1567 | 1571 | 902 | 29803-81-4 | 0.213 |
| 99 | ethyl 3-nonenoate | 1570 | - | 832 | 91213-30-8 | 0.068 |
| 100 | methyl 4,4-dimethyl-3-oxopentanoate | 1577 | - | 744 | - | 0.828 |
| 101 | isopulegol | 1578 | 1571 | 805 | 89-79-2 | 0.669 |
| 102 | 2-methylpropanoic acid | 1582 | 1570 | 910 | 79-31-2 | 0.000 |
| 103 | fenchol | 1589 | 1582 | 846 | 1632-73-1 | 0.776 |
| 104 | myrcenol | 1603 | 1585 | 867 | 543-39-5 | 0.758 |
| 105 | 2-undecanone | 1604 | 1598 | 781 | 112-12-9 | 0.000 |
| 106 | 2-decanol | 1609 | 1601 | 764 | 1120-06-5 | 0.016 |
| 107 | terpinen-4-ol | 1611 | 1602 | 911 | 562-74-3 | 0.953 |
| 108 | cis-verbenol | 1613 | 1663 | 781 | 1845-30-3 | 0.501 |
| 109 | caryophyllene | 1624 | 1595 | 827 | 87-44-5 | 0.155 |
| 110 | citronellol formate | 1627 | 1660 | 732 | 105-85-1 | 0.000 |
| 111 | (Z)-p-2-menthen-1-ol | 1631 | 1638 | 901 | 29803-82-5 | 0.440 |
| 112 | ethyl decanoate | 1641 | 1638 | 946 | 110-38-3 | 0.000 |
| 113 | 2,6-dimethyl-5-hepten-1-ol | 1647 | 1654 | 730 | 4234-93-9 | 0.022 |
| 114 | 4-(1-methylethyl)-cyclohexanol | 1649 | 1667 | 879 | 4621-04-9 | 0.003 |
| 115 | 1-nonanol | 1652 | 1660 | 875 | 143-08-8 | 0.000 |
| 116 | 3-methylbutyl octanoate | 1660 | 1658 | 920 | 2035-99-6 | 0.001 |
| 117 | citronellol acetate | 1663 | 1660 | 948 | 150-84-5 | 0.000 |
| 118 | ethyl trans-4-decenoate | 1668 | 1676 | 891 | 76649-16-6 | 0.062 |
| 119 | ipsdienol | 1674 | 1631 | 876 | 35628-00-3 | 0.025 |
| 120 | decyl acetate | 1681 | 1680 | 868 | 112-17-4 | 0.000 |
| 121 | 3-methylbutanoic acid | 1683 | 1666 | 766 | 503-74-2 | 0.874 |
| 122 | ethyl 9-decenoate | 1694 | 1694 | 917 | 67233-91-4 | 0.000 |
| 123 | humulene | 1698 | 1667 | 905 | 6753-98-6 | 0.411 |
| 124 | methyl geranate | 1703 | 1686 | 892 | 2349-14-6 | 0.403 |
| 125 | 2-undecanol | 1709 | 1717 | 861 | 1653-30-1 | 0.431 |
| 126 | endo-borneol | 1713 | 1702 | 917 | 507-70-0 | 0.986 |
| 127 | 3-(methylthio)-1-propanol | 1722 | 1719 | 903 | 505-10-2 | 0.000 |
| 128 | propyl decanoate | 1724 | 1724 | 817 | 30673-60-0 | 0.000 |
| 129 | nerol acetate | 1728 | 1724 | 898 | 141-12-8 | 0.000 |
| 130 | unknown | 1734 | - | - | - | 0.001 |
| 131 | unknown | 1737 | - | - | - | 0.000 |
| 132 | 2,6-dimethyl-1,5,7-octatrien-3-ol | 1740 | - | 886 | 29414-56-0 | 0.067 |
| 133 | ethyl undecanoate | 1741 | 1739 | 746 | 627-90-7 | 0.003 |
| 134 | citral | 1743 | 1718 | 764 | 5392-40-5 | 0.000 |
| 135 | cis-piperitol | 1750 | 1758 | 780 | 16721-38-3 | 0.557 |
| 136 | 1-decanol | 1755 | 1760 | 879 | 112-30-1 | 0.000 |
| 137 | geranyl acetate | 1758 | 1752 | 911 | 105-87-3 | 0.000 |
| 138 | citronellol | 1760 | 1765 | 944 | 106-22-9 | 0.000 |
| 139 | 7-methyl-3-methylene-6-octen-1-ol | 1784 | 1800 | 827 | 13066-51-8 | 0.000 |
| 140 | methyl perillate | 1789 | - | 772 | 26460-67-3 | 0.000 |
| 141 | ethyl 10-undecenoate | 1795 | - | 869 | 692-86-4 | 0.000 |
| 142 | nerol | 1798 | 1797 | 940 | 106-25-2 | 0.000 |
| 143 | ethyl benzeneacetate | 1799 | 1783 | 735 | 101-97-3 | 0.046 |
| 144 | myrtenol | 1802 | 1797 | 753 | 515-00-4 | 0.216 |
| 145 | 17-octadecynoic acid | 1810 | - | 792 | 34450-18-5 | 0.000 |
| 146 | 2-phenylethyl acetate | 1831 | 1813 | 787 | 103-45-7 | 0.000 |
| 147 | geraniol | 1842 | 1847 | 962 | 106-24-1 | 0.000 |
| 148 | ethyl dodecanoate | 1844 | 1841 | 951 | 106-33-2 | 0.000 |
| 149 | hexanoic acid | 1858 | 1846 | 977 | 142-62-1 | 0.001 |
| 150 | 3-methylbutyl pentadecanoate | 1863 | 1863 | 827 | 2306-91-4 | 0.000 |
| 151 | benzyl alcohol | 1886 | 1870 | 754 | 100-51-6 | 0.014 |
| 152 | (Z)-ethyl pentadec-9-enoate | 1898 | - | 799 | 56219-09-1 | 0.000 |
| 153 | ethyl dihydrocinnamate | 1901 | 1893 | 884 | 2021-28-5 | 0.000 |
| 154 | bicyclo [2.1.1]hexane-1-carboxylic acid, 5,5-dimethyl- | 1910 | - | 756 | 3753-38-6 | 0.000 |
| 155 | p-menth-1(7)-en-9-ol | 1914 | 1889 | 836 | 29548-16-1 | 0.000 |
| 156 | butyl 9-decenoate | 1918 | 1874 | 731 | 0-00-0 | 0.001 |
| 157 | phenylethyl alcohol | 1924 | 1906 | 944 | 60-12-8 | 0.136 |
| 158 | 2-ethyl-hexanoic acid | 1961 | 1960 | 852 | 149-57-5 | 0.923 |
| 159 | heptanoic acid | 1969 | 1950 | 722 | 111-14-8 | 0.255 |
| 160 | β-phenylethyl butanoate | 1980 | 1958 | 785 | 103-52-6 | 0.000 |
| 161 | 2-acetylpyrrole | 1985 | 1973 | 728 | 1072-83-9 | 0.484 |
| 162 | cis-1,3,5-trimethyl-cyclohexane | 1996 | - | 664 | 1795-27-3 | 0.473 |
| 163 | p-mentha-1,8-dien-7-ol | 2016 | 2016 | 756 | 536-59-4 | 0.439 |
| 164 | unknown acid | 2028 | - | - | - | 0.328 |
| 165 | (E)-nerolidol | 2034 | 2042 | 922 | 40716-66-3 | 0.066 |
| 166 | ethyl tetradecanoate | 2048 | 2049 | 866 | 124-06-1 | 0.036 |
| 167 | γ-nonalactone | 2056 | 2024 | 885 | 104-61-0 | 0.972 |
| 168 | octanoic acid | 2068 | 2060 | 930 | 124-07-2 | 0.000 |
| 169 | nonanoic acid | 2183 | 2171 | 746 | 112-05-0 | 0.000 |
| 170 | 2-phenylethyl hexanoate | 2188 | 2162 | 716 | 6290-37-5 | 0.000 |
| 171 | epicubebol | 2190 | 2169 | 752 | 38230-60-3 | 0.307 |
| 172 | 4-vinylguaiacol | 2214 | 2188 | 916 | 7786-61-0 | 0.000 |
| 173 | ethyl hexadecanoate | 2253 | 2251 | 790 | 628-97-7 | 0.062 |
| 174 | ethyl 9-hexadecenoate | 2283 | 2281 | 873 | 54546-22-4 | 0.218 |
| 175 | n-decanoic acid | 2287 | 2276 | 953 | 334-48-5 | 0.000 |
| 176 | 9-decenoic acid | 2353 | 2341 | 931 | 14436-32-9 | 0.000 |
| 177 | neric acid | 2363 | 2366 | 751 | 4613-38-1 | 0.989 |
References
- Bamforth, C.W. Current perspectives on the role of enzymes in brewing. J. Cereal Sci. 2009, 50, 353–357. [Google Scholar] [CrossRef]
- Liu, S.Q.; Quek, A.Y. Evaluation of beer fermentation with a novel yeast Williopsis saturnus. Food Technol. Biotechnol. 2016, 54, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P. Beer flavor. In Beer: A Quality Perspective; Bamforth, C.W., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 61–83. [Google Scholar]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Dietz, C.; Cook, D.; Wilson, C.; Oliveira, P.; Ford, R. Exploring the multisensory perception of terpene alcohol and sesquiterpene rich hop extracts in lager style beer. Food Res. Int. 2021, 148, 110598. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.-P. Hop essential oil: Analysis, chemical composition and odor characteristics. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 239–254. [Google Scholar]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar−sorptive extraction method with gc-ms. J. Agric. Food Chem. 2005, 53, 4701–4707. [Google Scholar] [CrossRef]
- Yuan, T.-T.; Chen, Q.-Q.; Zhao, P.-J.; Zeng, Y.; Liu, X.-Z.; Lu, S. Identification of enzymes responsible for the reduction of geraniol to citronellol. Nat. Prod. Bioprospect. 2011, 1, 108–111. [Google Scholar] [CrossRef]
- Chollet, S.; Lelièvre, M.; Abdi, H.; Valentin, D. Sort and beer: Everything you wanted to know about the sorting task but did not dare to ask. Food Qual. Prefer. 2011, 22, 507–520. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Pfeil, J.; Frohme, M.; Schulze, K. Mobile microscopy and automated image analysis. Opt. Photon. 2018, 13, 36–39. [Google Scholar] [CrossRef]
- Russell, I.; Kellershohn, J. Advances in technology and new product development in the beer, wine, and spirit industry. In Innovations in Technologies for Fermented Food and Beverage Industries; Panda, S.K., Shetty, P.H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 89–104. [Google Scholar]
- Saerens, S.M.G.; Verbelen, P.J.; Vanbeneden, N.; Thevelein, J.M.; Delvaux, F.R. Monitoring the influence of high-gravity brewing and fermentation temperature on flavour formation by analysis of gene expression levels in brewing yeast. Appl. Microbiol. Biotechnol. 2008, 80, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, F.; Cuvelier, G.; Sieffermann, J.M. Consumer texture descriptions of a set of processed cheese. Food Qual. Prefer. 2015, 40, 316–325. [Google Scholar] [CrossRef]
- Symoneaux, R.; Galmarini, M.V.; Mehinagic, E. Comment analysis of consumer’s likes and dislikes as an alternative tool to preference mapping. A case study on apples. Food Qual. Prefer. 2012, 24, 59–66. [Google Scholar] [CrossRef]
- Cadoret, M.; Lê, S.; Pagès, J. A factorial approach for sorting task data (FAST). Food Qual. Prefer. 2009, 20, 410–417. [Google Scholar] [CrossRef]
- Bécue-Bertaut, M.; Pagès, J. A principal axes method for comparing contingency tables: Mfact. Comput. Stat. Data Anal. 2004, 45, 481–503. [Google Scholar] [CrossRef]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef]
- Warburton, A.; Silcock, P.; Eyres, G.T. Impact of sourdough culture on the volatile compounds in wholemeal sourdough bread. Food Res. Int. 2022, 161, 111885. [Google Scholar] [CrossRef]
- Halang, W.A.; Langlais, R.; Kugler, E. Cubic spline interpolation for the calculation of retention indices in temperature-programmed gas-liquid chromatography. Anal. Chem. 1978, 50, 1829–1832. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple factor analysis: Principal component analysis for multitable and multiblock data sets. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Kostov, B.; Bécue-Bertaut, M.; Husson, F. Multiple factor analysis for contingency tables in the FactoMineR package. R J. 2013, 5, 29–38. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- RStudio Team. Rstudio: Integrated Development for R; RStudio Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.2-8. 2017. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 13 March 2020).
- Husson, F.; Lê, S.; Cadoret, M. Sensominer: Sensory Data Analysis; R Package Version 1.26. 2020. Available online: https://CRAN.R-project.org/package=SensoMineR (accessed on 13 March 2020).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Tuszyński, T. The effect of wort aeration on fermentation, maturation and volatile components of beer produced on an industrial scale. J. Inst. Brew. 2017, 123, 31–38. [Google Scholar] [CrossRef]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef]
- Barth, R. The chemistry of flavor. In The Chemistry of Beer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 194–209. [Google Scholar]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef]
- Sharp, D.C.; Qian, Y.; Shellhammer, G.; Shellhammer, T.H. Contributions of select hopping regimes to the terpenoid content and hop aroma profile of ale and lager beers. J. Am. Soc. Brew. Chem. 2017, 75, 93–100. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Unveiling the lager beer volatile terpenic compounds. Food Res. Int. 2018, 114, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, D.; Forster, C.; Flieher, M.; Nielsen, T. The influence of dry hop-ping on three different beer styles. Brauwelt Int. 2013, 6, 355–359. [Google Scholar]
- Kuo, C.-H.; Chen, G.-J.; Chen, C.-I.; Liu, Y.-C.; Shieh, C.-J. Kinetics and optimization of lipase-catalyzed synthesis of rose fragrance 2-phenylethyl acetate through transesterification. Process Biochem. 2014, 49, 437–444. [Google Scholar] [CrossRef]
- Praet, T.; Van Opstaele, F.; Jaskula-Goiris, B.; Aerts, G.; De Cooman, L. Biotransformations of hop-derived aroma compounds by Saccharomyces cerevisiae upon fermentation. Cerevisia 2012, 36, 125–132. [Google Scholar] [CrossRef]
- Dufour, J.P.; Malcorps, P.; Silcock, P. Control of ester synthesis during brewery fermentation. In Brewing Yeast Fermentation Performance, 2nd ed.; Smart, K.A., Ed.; Blackwell Science: Oxford, UK, 2003; pp. 213–233. [Google Scholar]
- Kumar, A. Impact of Yeast Strain and Fermentation on Perceived Hop Flavour in Beer. Master’s Thesis, University of Otago, Dunedin, New Zealand, 13 March 2020. Available online: http://hdl.handle.net/10523/9968 (accessed on 13 March 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).