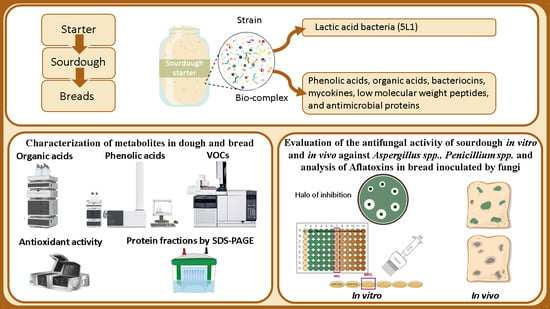
Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms and Culture Conditions
2.3. Sourdough Fermentation with LAB Lyophilized
2.4. Sourdough Bread Preparation
2.5. Effect of Sourdough on Dough and Bread Properties
2.5.1. pH and Titratable Acidity
2.5.2. Volume Increase
2.5.3. Specific Volume
2.5.4. Humidity
2.5.5. Color
2.5.6. aw
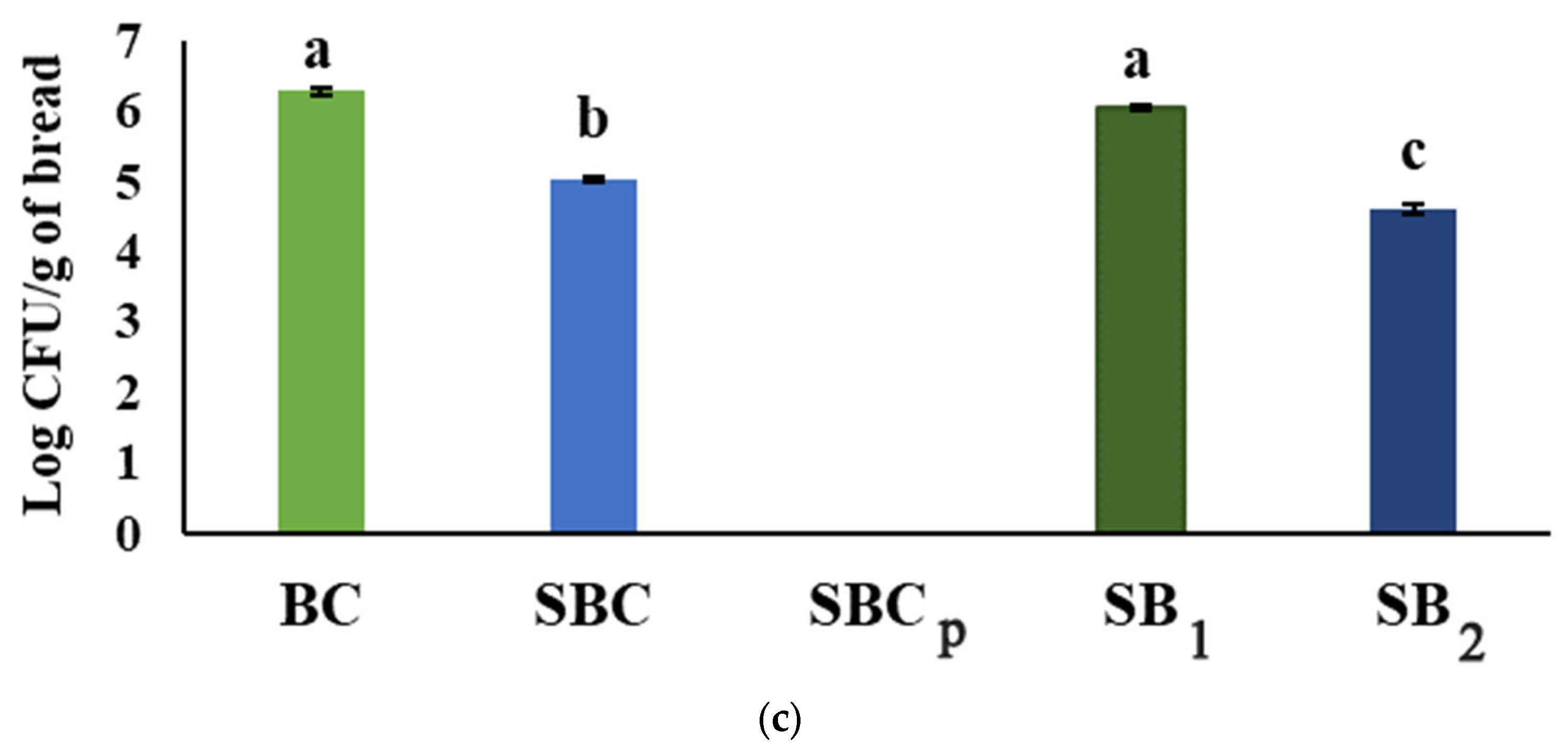
2.6. Microbiological Analysis
2.7. Analysis of Antioxidant Activity
2.8. Analysis of Total Phenolic Content
2.9. Analysis of Organic Acids
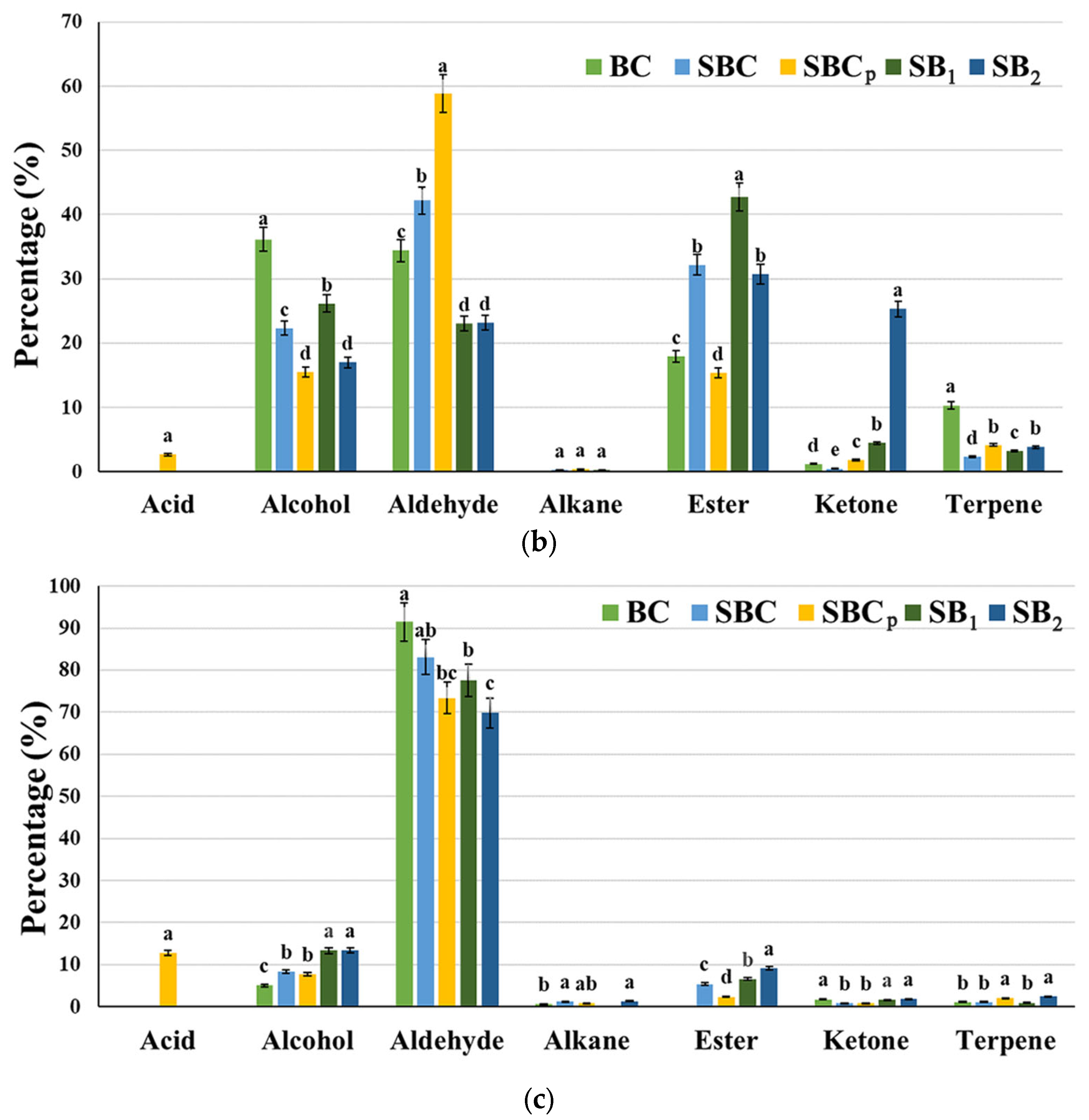
2.10. Analysis of Volatile Organic Compounds
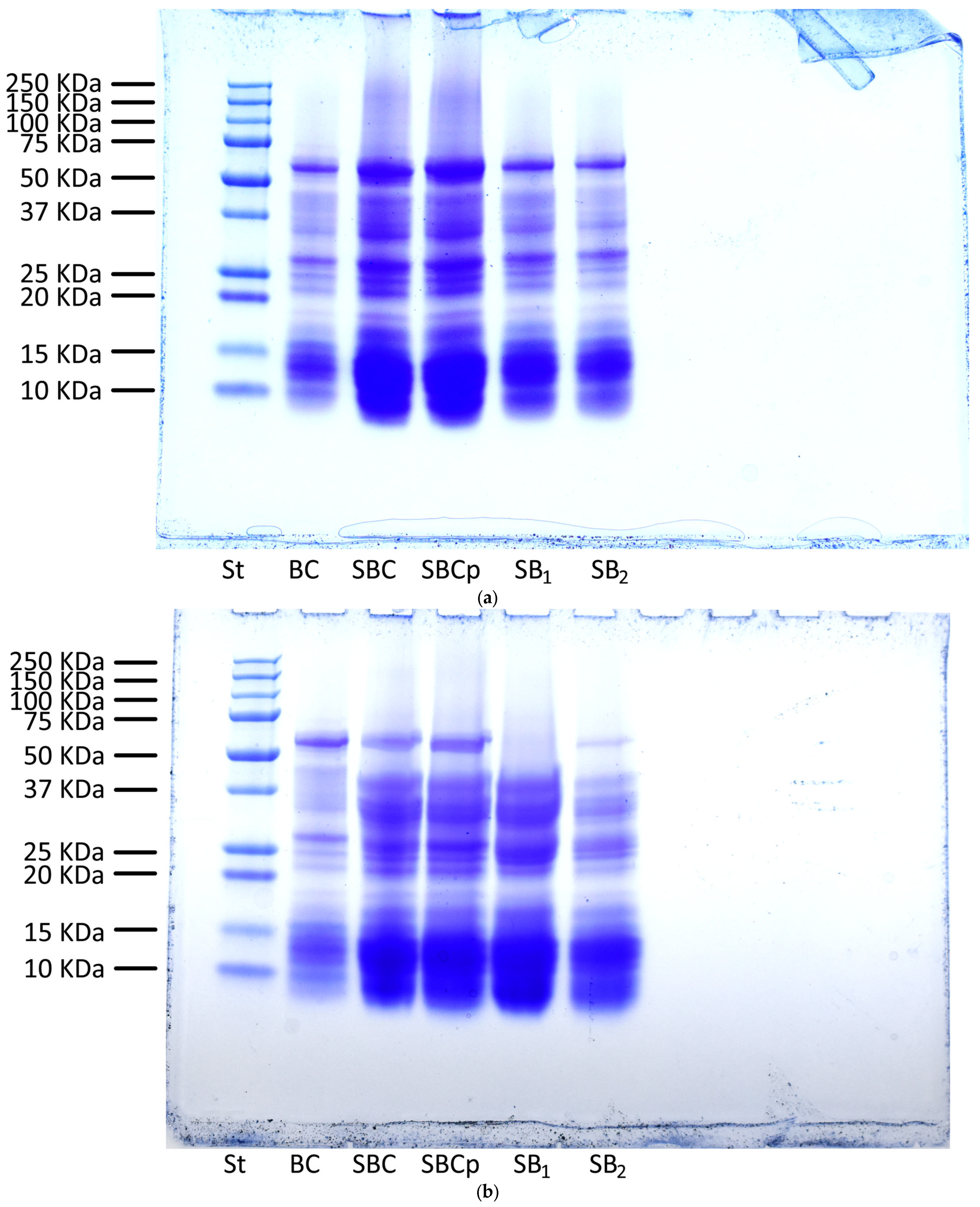
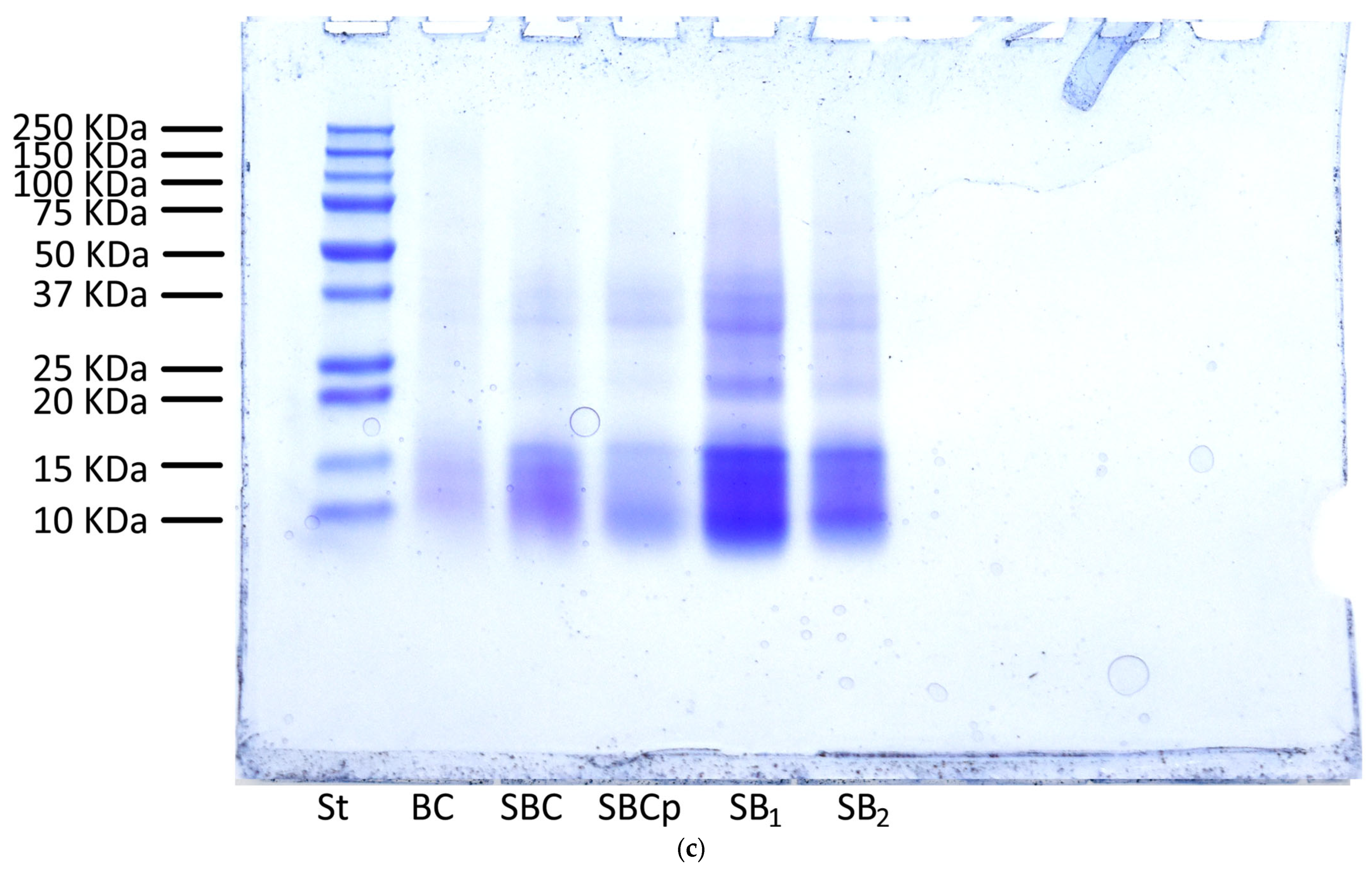
2.11. Analysis by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis of Bread and Dough
2.12. Biopreservation of Breads
2.13. Determination of the Fungal Population on Breads
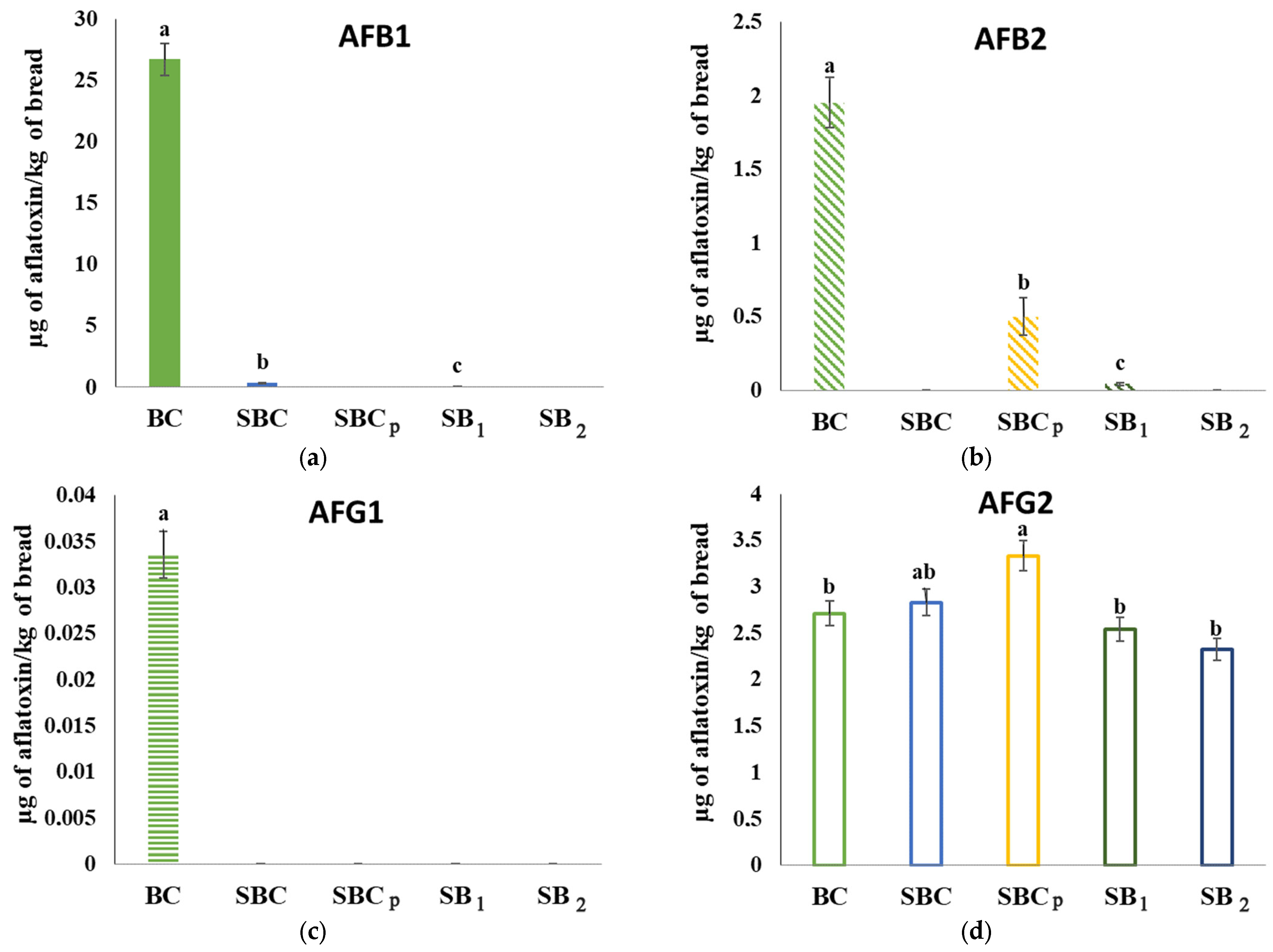
2.14. Analysis of Mycotoxins in Bread Inoculated by Fungi
2.15. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sourdough on the Properties of Dough and Bread
3.2. Results of Microbiological Analysis
3.3. Analysis of Antioxidant Activity
3.4. Analysis of Total Phenolic Content
3.5. Analysis of Organic Acids
3.6. Analysis of Volatile Organic Compounds
3.7. Analysis by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis of Bread and Dough
3.8. Biopreservation of Breads
3.9. Determination of the Fungal Population on Breads
3.10. Analysis of Mycotoxins in Bread Inoculated by Fungi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal. Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; pp. 169–337. [Google Scholar]
- García, P.; Rodríguez, L.; Rodríguez, A.; Martínez, B. Food biopreservation: Promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2019, 8, 64. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Katina, K.; Heiniö, R.L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; Benedito de Barber, C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Gantumur, M.A.; Sukhbaatar, N.; Qayum, A.; Bilawal, A.; Tsembeltsogt, B.; Oh, K.C.; Jiang, Z.; Hou, J. Characterization of major volatile compounds in whey spirits produced by different distillation stages of fermented lactose-supplemented whey. J. Dairy Sci. 2022, 105, 83–96. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef]
- Galvao, A.M.; Zambelli, R.A.; Araújo, A.W.; Bastos, M.S. Edible coating based on modified corn starch/tomato powder: Effect on the quality of dough bread. LWT 2018, 89, 518–524. [Google Scholar] [CrossRef]
- Sánchez, L.; Vichi, J.; Llanes, M.; Castro, E.; Soler, D.M.; Espinosa, I.; Kociubinski, G.L.; Ferreira, C.L. In vitro isolation and characterization of Lactobacillus spp. strains as a probiotic candidate. Rev. Salud. Anim. 2011, 33, 154–160. [Google Scholar]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic content, Flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Dopazo, V.; Luz, C.; Mañes, J.; Quiles, J.; Carbonell, R.; Calpe, J.; Meca, G. Bio-Preservative Potential of Microorganisms Isolated from Red Grape against Food Contaminant Fungi. Toxins 2021, 13, 412. [Google Scholar] [CrossRef]
- Luz, C.; Quiles, J.M.; Romano, R.; Blaiotta, G.; Rodríguez, L.; Meca, G. Application of whey of Mozzarella di Bufala Campana fermented by lactic acid bacteria as a bread biopreservative agent. Int. J. Food Sci. Technol. 2021, 56, 4585–4593. [Google Scholar] [CrossRef]
- Ito, M.; Ito, T.; Aoki, H.; Nishioka, K.; Shiokawa, T.; Tada, H.; Takeuchi, Y.; Takeyasu, N.; Yamamoto, T.; Takashiba, S. Isolation and identification of the antimicrobial substance included in tempeh using Rhizopus stolonifer NBRC 30816 for fermentation. Int. J. Food Microbiol. 2020, 325, 108645. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce. Innov. Food Sci. Emerg. Technol. 2009, 10, 97–102. [Google Scholar] [CrossRef]
- Nazareth, T.D.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential application of lactic acid bacteria to reduce aflatoxin B1 and fumonisin B1 occurrence on corn kernels and corn ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Rutherfurf-Markwick, K.; Mutukumira, A.N. Identification of dominant lactic acid bacteria and yeast in rice sourdough produced in New Zealand. Curr. Res. Food Sci. 2021, 4, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto-ley 308/2019, de 26 de Abril, por el que se Aprueba la Norma de Calidad Para el Pan. BOE n.113. Available online: https://www.boe.es/eli/es/rd/2019/04/26/308 (accessed on 25 January 2022).
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0740002006001614 (accessed on 1 January 2022). [CrossRef] [PubMed]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Haros, M. Application of Bifidobacteria as Starter Culture in Whole Wheat Sourdough Breadmaking. Food Bioproc. Technol. 2012, 5, 2370–2380. [Google Scholar] [CrossRef]
- Zhang, L.; Taal, M.A.; Boom, R.M.; Chen, X.D.; Schutyser, M.A.I. Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 2018, 87, 318–325. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Hosseininezhad, M. Improving the quality of traditional Iranian bread by using sourdough and optimizing the fermentation conditions. SN Appl. Sci. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of Selected Sourdough Strains of Lactobacillus for Removing Gluten and Enhancing the Nutritional Properties of Gluten-Free Bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef]
- Abellana, M.; Sanchis, V.; Ramos, A.J. Effect of water activity and temperature on growth of three Penicillium species and Aspergillus flavus on a sponge cake analogue. Int. J. Food Microbiol. 2001, 71, 151–157. [Google Scholar] [CrossRef]
- Smith, B.M.; Ramsay, S.A.; Roe, A.; Ferrante, M.J.; Brooks, S. Reducing Visual Differences in Whole Grain Bread Prepared with Hard Red and Hard White Wheat: Application for Sensory Studies. J. Food Sci. 2019, 84, 2325–2329. [Google Scholar] [CrossRef]
- Lancetti, R.; Salvucci, E.; Moiraghi, M.; Pérez, G.T.; Sciarini, L.S. Gluten-free flour fermented with autochthonous starters for sourdough production: Effect of the fermentation process. Food Biosci. 2022, 47, 101723. [Google Scholar] [CrossRef]
- Ataç, F.; Ertekin Filiz, B.; Guzel-Seydim, Z.B. The use of yeast-rich kefir grain as a starter culture in bread making. J. Food Process. Preserv. 2022, 46, e15242. [Google Scholar] [CrossRef]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of Lactic Fermentation on Antioxidant Capacity of Rye Sourdough and Bread. Food Sci. Technol. Res. 2010, 16, 571–576. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Izzo, L.; Luz, C.; Ritieni, A.; MaÃ, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Settanni, L.; Ventimiglia, G.; Alfonzo, A.; Corona, O.; Miceli, A.; Moschetti, G. An integrated technological approach to the selection of lactic acid bacteria of flour origin for sourdough production. Food Res. Int. 2013, 54, 1569–1578. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Fang, L.; Wang, W.; Dou, Z.; Chen, J.; Meng, Y.; Cai, L.; Li, Y. Effects of mixed fermentation of different lactic acid bacteria and yeast on phytic acid degradation and flavor compounds in sourdough. LWT 2023, 174, 114438. [Google Scholar] [CrossRef]
- Loviso, C.L.; Libkind, D. Synthesis and regulation of flavor compounds derived from brewing yeast: Esters. Rev. Argent. Microbiol. 2018, 50, 436–446. [Google Scholar] [PubMed]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of volatile compounds of lupin protein isolate-enriched wheat flour bread. Food Res. Int. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Hansen, B.; Hansen, Å. Volatile compounds in wheat sourdoughs produced by lactic acid bacteria and sourdough yeasts. Z. Leb. Unters. Forsch. 1994, 198, 202–209. [Google Scholar] [CrossRef]
- Abdelaleem, M.A.; Al-Azab, K.F. Evaluation of flour protein for different bread wheat genotypes. Braz. J. Biol. 2021, 81, 719–727. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.A.; Cunsolo, V.; Giuliani, M.M.; Di Francesco, A.; Saletti, R.; Foti, S.; Flagella, Z. Gluten proteome comparison among durum wheat genotypes with different release date. J. Cereal. Sci. 2020, 96, 103092. [Google Scholar] [CrossRef]
- De Santis, M.A.; Cunsolo, V.; Giuliani, M.M.; Di Francesco, A.; Saletti, R.; Foti, S.; Flagella, Z. Sourdough Bread Made from Wheat and Nontoxic Flours and Started with Selected Lactobacilli Is Tolerated in Celiac Sprue Patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar]
- Diowksz, A.; Malik, A.; Jasniewska, A.; Leszczyńska, J. The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread. Foods 2020, 9, 656. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control. 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Mañes, J.; Meca, G. Antifungal activity and shelf life extension of loaf bread produced with sourdough fermented by Lactobacillus strains. J. Food Process. Preserv. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- Luz, C.; Rodriguez, L.; Romano, R.; Mañes, J.; Meca, G. A natural strategy to improve the shelf life of the loaf bread against toxigenic fungi: The employment of fermented whey powder. Int. J. Dairy Technol. 2020, 73, 88–97. [Google Scholar] [CrossRef]
- Belz, M.C.E.; Mairinger, R.; Zannini, E.; Ryan, L.A.M.; Cashman, K.D.; Arendt, E.K. The effect of sourdough and calcium propionate on the microbial shelf-life of salt reduced bread. Appl. Microbiol. Biotechnol. 2012, 96, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; De Melo Nazareth, T.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an Antifungal and Antimycotoxigenic Device Containing Allyl Isothiocyanate for Silo Fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Raeisi, M.; Nematollahi, Z. Biological control of foodborne pathogens and aflatoxins by selected probiotic LAB isolated from rice bran sourdough. Biol. Control 2019, 130, 70–79. [Google Scholar] [CrossRef]
- Illueca, F.; Vila-Donat, P.; Calpe, J.; Luz, C.; Meca, G.; Quiles, J.M. Antifungal activity of biocontrol agents in vitro and potential application to reduce mycotoxins (Aflatoxin B1 and ochratoxin A). Toxins 2021, 13, 752. [Google Scholar] [CrossRef]
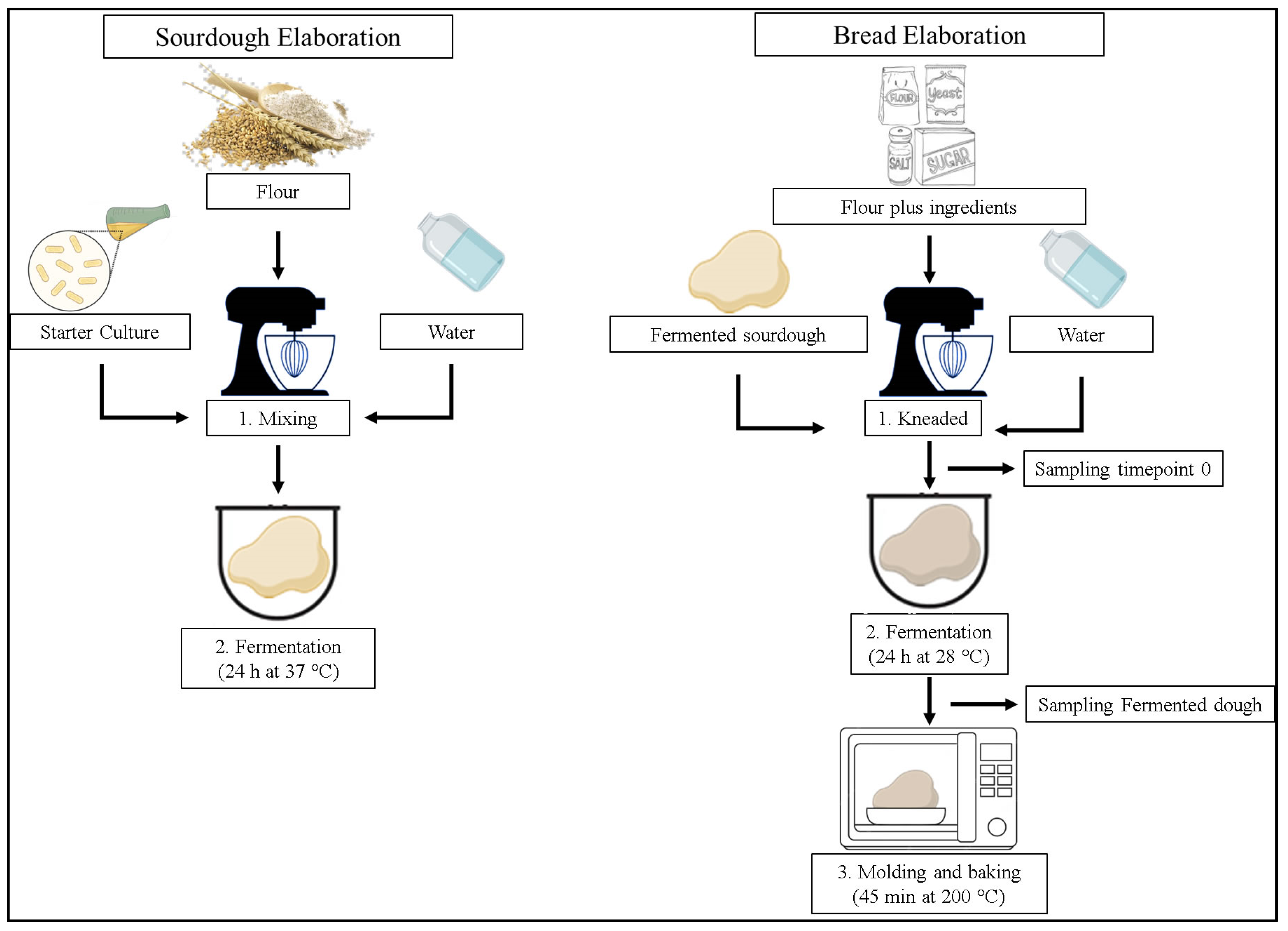




| Sourdough | Ingredients (g) | ||
|---|---|---|---|
| Wheat Flour | Mineral Water | LAB | |
| SC | 33.50 | 66.50 | 0.00 |
| S1 | 33.00 | 66.50 | 0.50 |
| S2 | 28.50 | 66.50 | 5.00 |
| Ingredients (g) | BC | SBC | SBCp | SB1 | SB2 |
|---|---|---|---|---|---|
| Wheat flour | 60.00 | 54.25 | 54.2 | 54.25 | 54.25 |
| Mineral water | 32.99 | 17.32 | 17.1 | 17.32 | 17.32 |
| Baker’s yeast | 3.70 | 0.12 | 0.12 | 0.12 | 0.12 |
| Salt | 1.31 | 1.31 | 1.31 | 1.31 | 1.31 |
| Sugar | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Calcium propionate | 0.00 | 0.00 | 0.30 | 0.00 | 0.00 |
| Sourdough | 0.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Properties | Dough T0 | ||||
|---|---|---|---|---|---|
| BC | SBC | SBCp | SB1 | SB2 | |
| (a) pH | 5.83 ± 0.04 c | 6.02 ± 0.10 b | 6.16 ± 0.09 a | 5.05 ± 0.02 d | 4.77 ± 0.08 e |
| (b) Titratable acidity (mL NaOH) | 14.40 ± 0.36 a | 2.50 ± 0.10 d | 3.00 ± 0.10 d | 4.13 ± 0.06 c | 6.33 ± 0.47 b |
| Properties | Fermented dough | ||||
| BC | SBC | SBCp | SB1 | SB2 | |
| (a) pH | 5.40 ± 0.02 a | 4.30 ± 0.62 c | 5.13 ± 0.62 b | 3.69 ± 0.02 d | 3.68 ± 0.45 d |
| (b) Titratable acidity (mL NaOH) | 11.17 ± 0.40 a | 9.27 ± 0.85 b | 9.57 ± 0.15 b | 8.07 ± 0.06 b | 8.73 ± 0.31 b |
| (c) Volume increase (cm) | 1.40 ± 0.10 a | 1.23 ± 0.06 ab | 0.97 ± 0.15 b | 0.97 ± 0.06 b | 1.07 ± 0.15 ab |
| Properties | Bread | ||||
| BC | SBC | SBCp | SB1 | SB2 | |
| (a) pH | 5.83 ± 0.04 c | 6.02 ± 0.10 b | 6.16 ± 0.09 a | 5.05 ± 0.02 b | 4.77 ± 0.08 e |
| (b) Titratable acidity (mL NaOH) | 14.40 ± 0.36 a | 2.50 ± 0.10 d | 3.00 ± 0.10 d | 4.13 ± 0.06 c | 6.33 ± 0.47 b |
| (c) Specific volume (mL/g) | 2.20 ± 0.10 a | 2.10 ± 0.10 a | 2.20 ± 0.10 a | 2.50 ± 0.10 b | 2.60 ± 0.11 b |
| (d) aw | 0.67 ± 0.05 a | 0.68 ± 0.03 a | 0.69 ± 0.01 a | 0.70 ± 0.03 a | 0.72 ± 0.01 a |
| (e) Humidity (%) | 22.30 ± 2.70 a | 17.40 ± 0.20 a | 18.20 ± 1.70 a | 17.40 ± 0.80 a | 19.80 ± 0.80 a |
| (f) Color | |||||
| L* | 85.00 ± 0.40 a | 86.30 ± 0.70 bc | 86.00 ± 0.40 bc | 86.40 ± 0.20 c | 85.40 ± 0.30 ab |
| a* | 0.50 ± 0.20 a | −0.60 ± 0.01 c | −0.10 ± 0.30 b | −0.50 ± 0.20 bc | −0.10 ± 0.20 b |
| b* | 1.50 ± 0.40 a | 1.50 ± 0.50 a | 1.80 ± 0.30 a | 1.70 ± 0.40 a | 1.50 ± 0.20 a |
| Samples | log CFU/g Sample | |||||
|---|---|---|---|---|---|---|
| Dough T0 | Fermented Dough | Bread | ||||
| LAB | Yeast | LAB | Yeast | LAB | Yeast | |
| BC | 7.42 ± 0.07 b | 7.41 ± 0.02 b | 9.30 ± 0.09 a | 9.44 ± 0.01 b | n.d | n.d |
| SBC | 6.42 ± 0.08 c | 5.92 ± 0.10 c | 9.29 ± 0.23 a | 10.81 ± 0.11 a | n.d | n.d |
| SBCp | 8.22 ± 0.13 a | 8.16 ± 0.03 a | 8.00 ± 0.11 b | 8.10 ± 0.02 d | n.d | n.d |
| SB1 | 7.47 ± 0.03 b | 7.47 ± 0.03 b | 7.95 ± 0.13 b | 8.90 ± 0.08 c | n.d | n.d |
| SB2 | 7.69 ± 0.01 b | 8.19 ± 0.09 a | 7.80 ± 0.01 b | 8.78 ± 0.04 c | n.d | n.d |
| Relative antioxidant activity (%) | ||||||
| Dough T0 | Fermented dough | Bread | ||||
| BC | 60.07 ± 10.16 a | 54.22 ± 9.72 ab | 67.63 ± 7.29 a | |||
| SBC | 55.00 ± 8.32 a | 47.15 ± 7.09 ab | 62.96 ± 3.57 a | |||
| SBCp | 53.19 ± 21.14 a | 45.85 ± 0.61 b | 54.96 ± 7.55 a | |||
| SB1 | 63.96 ± 0.55 a | 65.59 ± 0.39 a | 66.22 ± 0.39 a | |||
| SB2 | 51.89 ± 14.38 a | 47.63 ± 0.31 ab | 67.52 ± 0.71 a | |||
| Total phenolic content (mg of gallic acid equivalents/kg) | ||||||
| Dough T0 | Fermented dough | Bread | ||||
| BC | 93.99 ± 4.27 a | 80.66 ± 8.26 a | 69.45 ± 2.52 ab | |||
| SBC | 72.05 ± 5.10 b | 78.96 ± 7.64 a | 67.02 ± 8.50 b | |||
| SBCp | 69.33 ± 2.41 b | 92.30 ± 2.12 a | 72.05 ± 7.03 ab | |||
| SB1 | 75.27 ± 8.80 b | 79.57 ± 7.94 a | 76.05 ± 4.64 ab | |||
| SB2 | 72.24 ± 1.11 b | 86.84 ± 8.77 a | 89.08 ± 5.86 a | |||
| Lactic acid (g/kg) | ||||||
| Dough T0 | Fermented dough | Bread | ||||
| BC | 0.49 ± 0.02 c | 0.43 ± 0.01 d | 0.76 ± 0.01 d | |||
| SBC | 0.36 ± 0.01 d | 1.18 ± 0.05 c | 0.70 ± 0.03 e | |||
| SBCp | 0.32 ± 0.01 d | 1.66 ± 0.03 a | 1.79 ± 0.01 b | |||
| SB1 | 0.58 ± 0.01 b | 1.16 ± 0.02 c | 2.03 ± 0.02 a | |||
| SB2 | 0.86 ± 0.03 a | 1.31 ± 0.01 b | 1.27 ± 0.01 c | |||
| Breads | Fungi | Percentage of Grown Spots (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| BC | A. flavus | 0 ± 0 a | 38 ± 14 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | - | - | - |
| SBC | 0 ± 0 a | 0 ± 0 b | 19 ± 13 b | 31 ± 13 c | 75 ± 0 b | 88 ± 14 a | 100 ± 0 a | - | - | - | |
| SBCp | 0 ± 0 a | 0 ± 0 b | 0 ± 0 c | 0 ± 0 d | 0 ± 0 d | 0 ± 0 c | 0 ± 0 c | - | - | - | |
| SB1 | 0 ± 0 a | 0 ± 0 b | 38 ± 14 b | 69 ± 13 b | 94 ± 13 a | 94 ± 13 a | 100 ± 0 a | - | - | - | |
| SB2 | 0 ± 0 a | 0 ± 0 b | 0 ± 0 c | 0 ± 0 d | 19 ± 13 c | 56 ± 13 b | 75 ± 0 b | - | - | - | |
| BC | P. verrucosum | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 31 ± 13 a | 56 ± 13 a | 88 ± 14 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 d |
| SBC | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 13 ± 14 b | 19 ± 13 b | 38 ± 14 b | 56 ± 12 c | |
| SBCp | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 c | 0 ± 0 c | 0 ± 0 a | |
| SB1 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 63 ± 14 a | 94 ± 13 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 d | |
| SB2 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 6 ± 13 bc | 13 ± 14 bc | 19 ± 13 b | |
| BC | P. commune | 0 ± 0 a | 0 ± 0 a | 94 ± 13 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| SBC | 0 ± 0 a | 0 ± 0 a | 69 ± 13 a | 88 ± 14 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | |
| SBCp | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 c | 0 ± 0 c | |
| SB1 | 0 ± 0 a | 0 ± 0 a | 69 ± 13 a | 88 ± 14 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | |
| SB2 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 6 ± 13 b | 6 ± 13 b | 6 ± 13 b | 19 ± 13 b | 31 ± 13 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illueca, F.; Moreno, A.; Calpe, J.; Nazareth, T.d.M.; Dopazo, V.; Meca, G.; Quiles, J.M.; Luz, C. Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough. Foods 2023, 12, 864. https://doi.org/10.3390/foods12040864
Illueca F, Moreno A, Calpe J, Nazareth TdM, Dopazo V, Meca G, Quiles JM, Luz C. Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough. Foods. 2023; 12(4):864. https://doi.org/10.3390/foods12040864
Chicago/Turabian StyleIllueca, Francisco, Ana Moreno, Jorge Calpe, Tiago de Melo Nazareth, Victor Dopazo, Giuseppe Meca, Juan Manuel Quiles, and Carlos Luz. 2023. "Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough" Foods 12, no. 4: 864. https://doi.org/10.3390/foods12040864
APA StyleIllueca, F., Moreno, A., Calpe, J., Nazareth, T. d. M., Dopazo, V., Meca, G., Quiles, J. M., & Luz, C. (2023). Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough. Foods, 12(4), 864. https://doi.org/10.3390/foods12040864