In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Bacterial Strains
2.3. Pathogens
2.4. Inhibitory Activity of Isolates
2.5. Preliminary Identification
2.6. Inactivation Process
2.7. Broth Microdilution Assay
2.8. Coculture Challenge
2.9. Partial Sequencing of Gene 16s
2.10. Statistical Analysis
3. Results
3.1. Obtained Isolates and Inhibitory Activity against Pathogens
3.2. Preliminary Identification
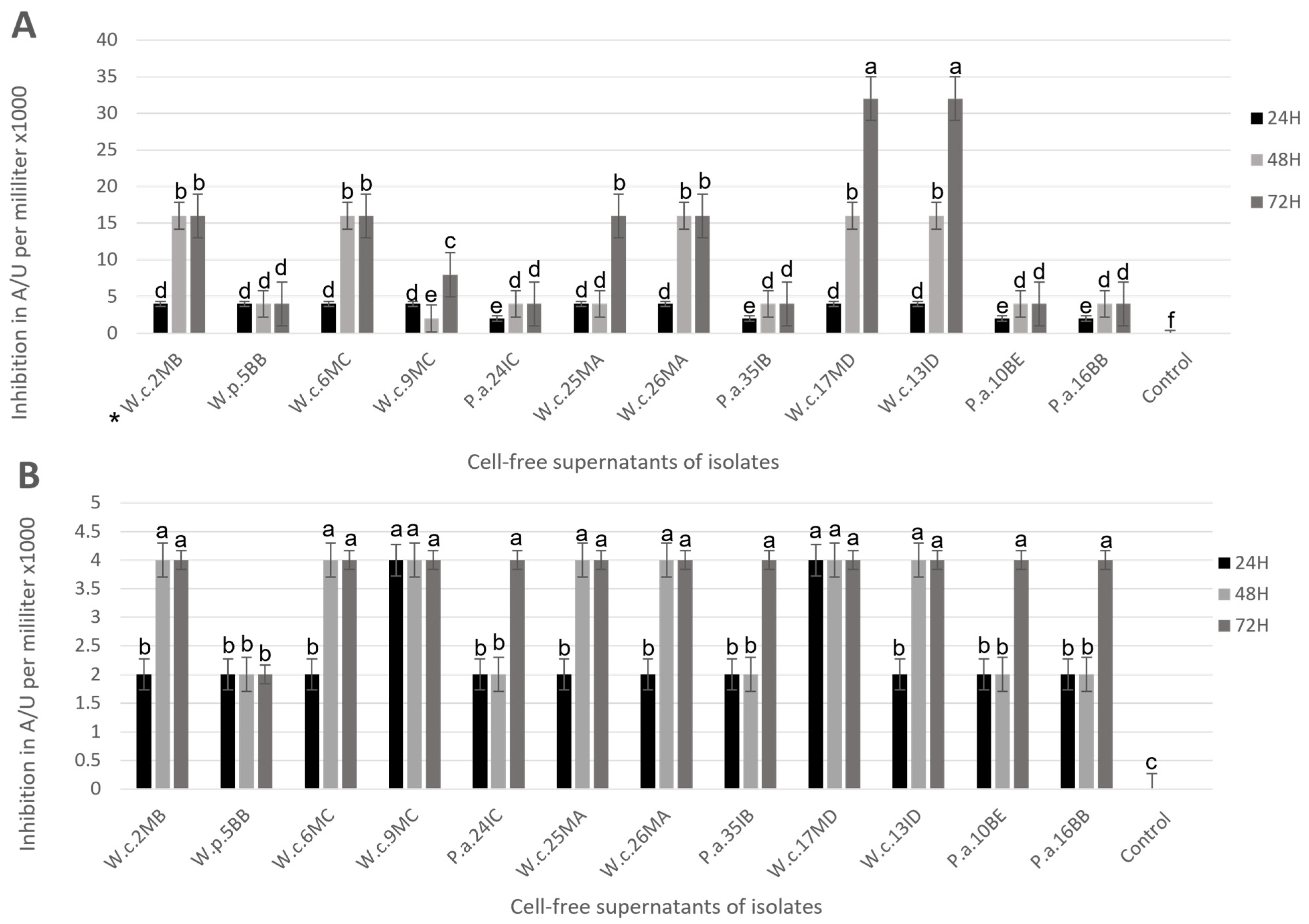
3.3. Postbiotic Production
3.4. Broth Microdilution Assay
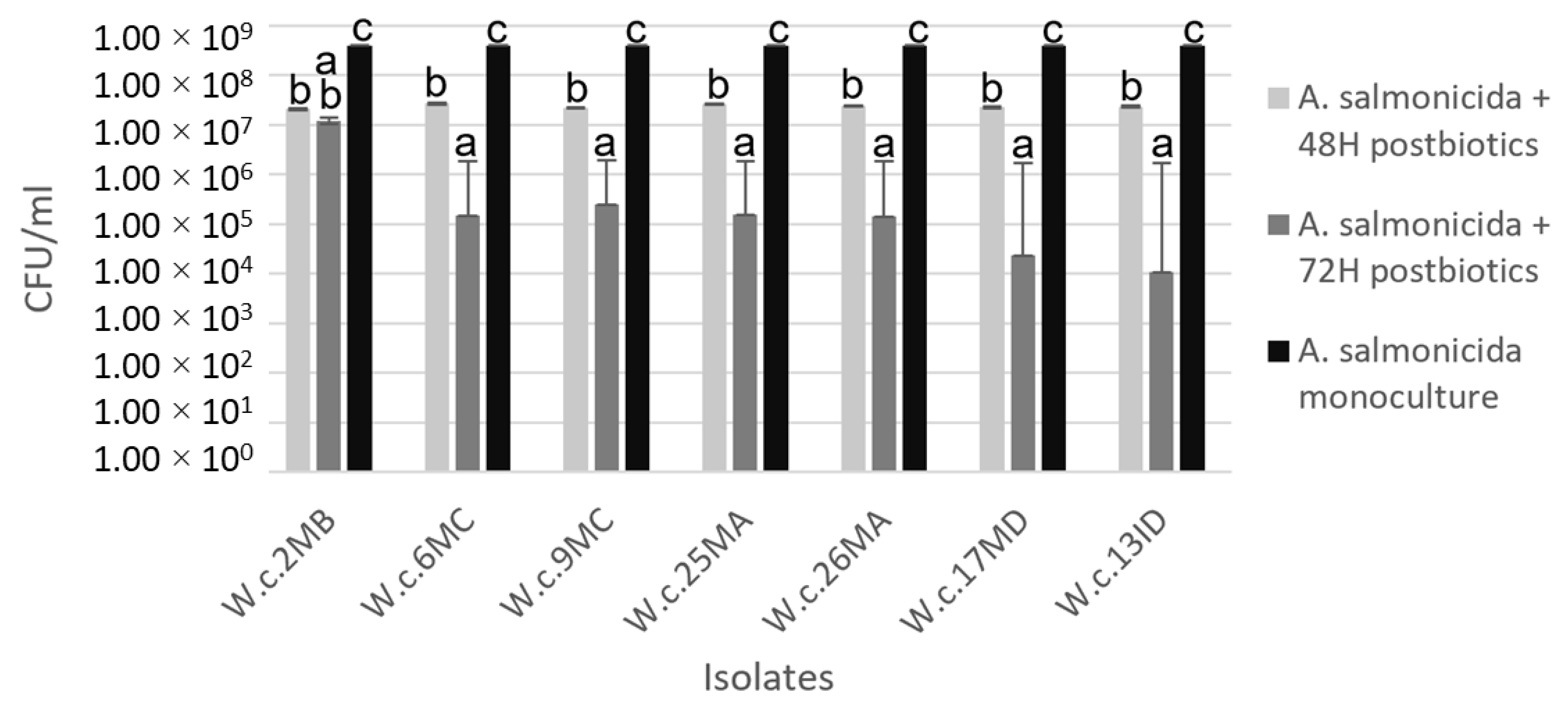
3.5. Coculture Challenge
3.6. Partial Sequencing of Gene 16s
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. SiA 2020, 54–55. [Google Scholar] [CrossRef]
- Medina, M.; Sotil, G.; Flores, V.; Fernández, C.; Sandoval, N. In vitro assessment of some probiotic properties and inhibitory activity against Yersinia ruckeri of bacteria isolated from rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Rep. 2020, 18, 100447. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Tobback, E.; Decostere, A.; Hermans, K.; Haesebrouck, F.; Chiers, K. Yersinia ruckeri infections in salmonid fish. J. Fish Dis. 2007, 30, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gudding, R.; van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Rømer Villumsen, K.; Koppang, E.O.; Raida, M.K. Adverse and long-term protective effects following oil-adjuvanted vaccination against Aeromonas salmonicida in rainbow trout. Fish Shellfish Immunol. 2015, 42, 193–203. [Google Scholar] [CrossRef]
- Salinas, I.; Díaz-Rosales, P.; Cuesta, A.; Meseguer, J.; Chabrillón, M.; Moriñigo, M.Á.; Esteban, M.Á. Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet. Immunol. Immunopathol. 2006, 111, 279–286. [Google Scholar] [CrossRef]
- Vatsos, I.N. Standardizing the microbiota of fish used in research. Lab. Anim. 2017, 51, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618, Wiley-Blackwell. [Google Scholar] [CrossRef]
- Ang, C.Y.; Sano, M.; Dan, S.; Leelakriangsak, M.; Lal, T.M. Postbiotics applications as infectious disease control agent in aquaculture. Biocontrol. Sci. 2020, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Touraki, M.; Frydas, I.; Karamanlidou, G.; Mamara, A. Partial purification and characterization of a bacteriocin produced by Bacillus subtilis NCIMB 3610 that exhibits antimicrobial activity against fish pathogens. J. Biol. Res. 2012, 18, 310–319. [Google Scholar]
- Maldonado-Barragán, A.; Cárdenas, N.; Martínez, B.; Ruiz-Barba, J.L.; Fernández-Garayzábal, J.F.; Rodríguez, J.M.; Gibello, A. Garvicin a, a novel class IId bacteriocin from Lactococcus garvieae that inhibits septum formation in L. garvieae strains. AEM 2013, 79, 4336–4346. [Google Scholar] [CrossRef]
- Sequeiros, C.; Garcés, M.E.; Vallejo, M.; Marguet, E.R.; Olivera, N.L. Potential aquaculture probiont Lactococcus lactis TW34 produces nisin Z and inhibits the fish pathogen Lactococcus garvieae. Arch. Microbiol. 2015, 197, 449–458. [Google Scholar] [CrossRef]
- Mora-Sánchez, B.; Balcázar, J.L.; Pérez-Sánchez, T. Effect of a novel postbiotic containing lactic acid bacteria on the intestinal microbiota and disease resistance of rainbow trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus Plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef]
- Wu, X.; Teame, T.; Hao, Q.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Zhang, Y.; Zhou, Z.; Duan, M.; et al. Use of a paraprobiotic and postbiotic feed supplement (HWFTM) improves the growth performance, composition and function of gut microbiota in hybrid sturgeon (Acipenser baerii × Acipenser schrenckii). Fish Shellfish Immunol. 2020, 104, 36–45. [Google Scholar] [CrossRef]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Gironés, O.; Muzquiz, J.L.m. Sequencing of variable regions of the 16S rRNA gene for identification of lactic acid bacteria isolated from the intestinal microbiota of healthy salmonids. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Carmen Castro, M.; Berdasco, M.; de la Banda, I.G.; Moreno-Ventas, X.; de Rojas, A.H. Isolation and partial characterization of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiotics Antimicrob. Proteins 2019, 11, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Combes, T.; Martinez, F.O.; Cerrato, R.; Rey, J.; Garcia-Jimenez, W.; Fernandez-Llario, P.; Risco, D.; Gutierrez-Merino, J. Lactobacilli Isolated from wild Boar (Sus scrofa) antagonize Mycobacterium bovis Bacille Calmette-Guerin (BCG) in a species-dependent manner. Front. Microbiol. 2019, 10, 01663. [Google Scholar] [CrossRef]
- Shafipour Yordshahi, A.; Moradi, M.; Tajik, H.; Molaei, R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Yoon, T.-J. Retraction: Non-specific immune response of rainbow trout (Oncorhynchus mykiss) by dietary heat-inactivated potential probiotics. Immune Netw. 2011, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.; Combes, T.; Martinez, F.O.; Risco, D.; Gonçalves, P.; Garcia-Jimenez, W.L.; Cerrato, R.; Fernandez-Llario, P.; Gutierrez-Merino, J. Wildlife Symbiotic Bacteria Are Indicators of the Health Status of the Host and Its Ecosystem. AEM 2022, 88, e01385-21. [Google Scholar] [CrossRef]
- Casadei, G.; Grilli, E.; Piva, A. Pediocin A modulates intestinal microflora metabolism in swine in vitro intestinal fermentations. J. Anim. Sci. 2009, 87, 2020–2028. [Google Scholar] [CrossRef]
- Arahal, D.R.; Sánchez, E.; Macián, M.C.; Garay, E. Value of recN sequences for species identification and as a phylogenetic marker within the family “Leuconostocaceae”. Int. Microbiol. 2008, 11, 33–39. [Google Scholar] [CrossRef]
- Björkroth, K.J.; Schillinger, U.; Geisen, R.; Weiss, N.; Hoste, B.; Holzapfel, W.H.; Korkeala, H.J.; Vandamme, P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov. detected in food and clinical samples. IJSEM 2002, 52, 141–148. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R.; Kothari, D.; Shukla, R.; Goyal, A. Structural and biocompatibility properties of dextran from Weissella cibaria JAG8 as food additive. Int. J. Food Sci. Nutr. 2014, 65, 686–691. [Google Scholar] [CrossRef]
- Srionnual, S.; Yanagida, F.; Lin, L.H.; Hsiao, K.N.; Chen, Y.S. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. AEM 2007, 73, 2247–2250. [Google Scholar] [CrossRef]
- Kang, M.S.; Chung, J.; Kim, S.M.; Yang, K.H.; Oh, J.S. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006, 40, 418–425. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Nakase, T.; Oh, T.-K. Specific probiotic characterization of Weissella hellenica DS-12 isolated from flounder intestine. J. Gen. Appl. Microbiol. 1998, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Mortezaei, F.; Royan, M.; Allaf Noveirian, H.; Babakhani, A.; Alaie Kordghashlaghi, H.; Balcázar, J.L. In vitro assessment of potential probiotic characteristics of indigenous Lactococcus lactis and Weissella oryzae isolates from rainbow trout (Oncorhynchus mykiss Walbaum). J. Appl. Microbiol. 2020, 129, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-K.; Yang, B.-T.; Hao, Z.-P.; Li, H.-Z.; Cong, W.; Kang, Y.-H. Dietary supplementation with Weissella cibaria C-10 and Bacillus amyloliquefaciens T-5 enhance immunity against Aeromonas veronii infection in crucian carp (Carassius auratus). Microb. Pathog. 2022, 167, 105559. [Google Scholar] [CrossRef]
- Mouriño, J.L.P.; Pereira, G.D.V.; Vieira, F.D.N.; Jatobá, A.B.; Ushizima, T.T.; Silva BC da Seiffert, W.Q.; Jesus, G.F.A.; Martins, M.L. Isolation of probiotic bacteria from the hybrid South American catfish Pseudoplatystoma reticulatum × Pseudoplatystoma corruscans (Siluriformes: Pimelodidae): A haematological approach. Aquac. Rep. 2016, 3, 166–171. [Google Scholar] [CrossRef]
- Standen, B.T.; Rawling, M.D.; Davies, S.J.; Castex, M.; Foey, A.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Probiotic Pediococcus acidilactici modulates both localized intestinal- and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2013, 35, 1097–1104. [Google Scholar] [CrossRef]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of probiotic Pediococcus acidilactici on antioxidant defenses and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol. 2010, 28, 622–631. [Google Scholar] [CrossRef]
- Abriouel, H.; Lerma, L.L.; Casado Muñoz M del, C.; Montoro, B.P.; Kabisch, J.; Pichner, R.; Cho, G.S.; Neve, H.; Fusco, V.; Franz, C.M.A.P.; et al. The controversial nature of the Weissella genus: Technological and functional aspects versus whole genome analysis-based pathogenic potential for their application in food and health. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Maier, T.; Klepel, S.; Renner, U.; Kostrzewa, M. Fast and reliable MALDI-TOF MS-based microorganism identification. Nat. Methods 2006, 3, i–ii. [Google Scholar] [CrossRef]
- Stedman, A.; van Vliet AHM, A.; Chambers, M.; Gutierrez-Merino, J. Gut commensal bacteria show beneficial properties as wildlife probiotics. Ann. N. Y. Acad. Sci. 2020, 1467, 112–132. [Google Scholar] [CrossRef] [PubMed]
| Species | n | Location | Organ | |||||
|---|---|---|---|---|---|---|---|---|
| Gut | Gill | Skin | ||||||
| Obtained | Selected | Obtained | Selected | Obtained | Selected | |||
| O. mykiss | 20 | 43°04′38.4″ N 1°55′08.9″ W | 43 | 11 | 97 | 3 | 95 | 11 |
| O. niloticus | 11 | 10°20′51.5″ N 85°08′13.8″ W | 31 | 9 | 11 | 4 | 18 | 6 |
| 11 | 10°12′41.6″ N 83°46′21.9″ W | 30 | 7 | 13 | 7 | 31 | 11 | |
| Total | 42 | 3 | 104 | 27 | 121 | 14 | 144 | 28 |
| Isolate * | Y. ruckeri | A. salmonicida subsp. salmonicida |
|---|---|---|
| 7MB | 17.68 ± 0.28 a | 18.97 ± 0.66 c |
| 10BE | 16.48 ± 0.54 ab | 17.46 ± 0.84 efg |
| 11MH | 16.75 ± 0.71 ab | 17.16 ± 0.68 def |
| 13ID | 16.03 ± 0.65 bc | 21.51 ± 0.97 b |
| 16BB | 16.37 ± 0.77 bc | 18.13 ± 1.01 de |
| 17MD | 17.17 ± 0.61 a | 17.26 ± 0.79 efg |
| 18ME | 17.19 ± 0.89 a | 17.75 ± 1.03 de |
| 18IC | 16.05 ± 0.84 bc | 17.68 ± 0.72 defg |
| 2MB | 13.61 ± 0.21 fg | 23.45 ± 0.90 a |
| 5BB | 13.67 ± 0.70 fg | 21.36 ± 0.06 b |
| 6MC | 12.79 ± 0.91 g | 24.45 ± 0.31 a |
| 9MC | 13.09 ± 0.30 g | 18.18 ± 0.21 cde |
| 24IC | 14.33 ± 0.82 ef | 15.76 ± 0.67 h |
| 25MA | 14.45 ± 0.48 ef | 18.80 ± 0.40 cd |
| 26MA | 13.78 ± 0.34 fg | 16.73 ± 1.10 fgh |
| 35IB | 15.05 ± 0.42 de | 19.05 ± 0.91 c |
| 35IH | 14.48 ± 0.44 cd | 16.17 ± 0.38 gh |
| Isolate Code * | Bacterial Identification | Log Score | Database Resemblance |
|---|---|---|---|
| 7MB | Lactococcus garvieae | 2.16 | 20684T |
| P.a.10BE | Pediococcus acidilactici | 2.08 | 146 RTL |
| 11MH | Lactococcus garvieae | 2.20 | 20684T |
| W.c.13ID | Weissella cibaria | 2.37 | DSM 15878T |
| P.a.16BB | Pediococcus acidilactici | 2.38 | 146 RTL |
| W.c.17MD | Weissella cibaria | 2.41 | DSM 15878T |
| 18ME | Lactococcus garvieae | 2.19 | 20684T |
| 18IC | Lactococcus garvieae | 2.13 | 20684T |
| W.c.2MB | Weissella cibaria | 2.34 | DSM 15878T |
| W.c.5BB | Weissella paramesenteroides | 1.96 | DSM 20288T |
| W.c.6MC | Weissella cibaria | 2.31 | DSM 15878T |
| W.c.9MC | Weissella cibaria | 2.35 | DSM 15878T |
| P.a.24IC | Pediococcus acidilactici | 2.01 | 146 RTL |
| W.c.25MA | Weissella cibaria | 2.32 | DSM 15878T |
| W.c.26MA | Weissella cibaria | 2.37 | DSM 15878T |
| P.a.35IB | Pediococcus acidilactici | 2.14 | 146 RTL |
| 35IH | Lactococcus garvieae | 2.28 | 20684T |
| Isolate * | 24 h | 48 h | 72 h | MRS-B | |||
|---|---|---|---|---|---|---|---|
| Counts | pH | Counts | pH | Counts | pH | pH | |
| W.c.2MB | 9.31 ± 0.02 a | 4.42 ± 0.01 a | 9.19 ± 0.03 b | 4.44 ± 0.01 a | 6.84 ± 0.03 c | 4.44 ± 0.01 a | 6.40 ± 0.00 |
| W.p.5BB | 9.33 ± 0.01 a | 4.41 ± 0.00 a | 8.69 ± 0.09 b | 4.43 ± 0.00 b | 7.42 ± 0.01 c | 4.41 ± 0.00 a | 6.40 ± 0.00 |
| W.c.6MC | 9.45 ± 0.02 a | 4.41 ± 0.04 a | 9.17 ± 0.03 b | 4.41 ± 0.02 a | 0.00 ± 0.00 c | 4.47 ± 0.00 b | 6.40 ± 0.00 |
| W.c.9MC | 9.30 ± 0.02 a | 4.44 ± 0.03 a | 8.93 ± 0.03 b | 4.43 ± 0.00 a | 0.00 ± 0.00 c | 4.46 ± 0.00 a | 6.40 ± 0.00 |
| P.a.24IC | 9.34 ± 0.02 c | 4.62 ± 0.02 c | 9.49 ± 0.01 b | 4.24 ± 0.00 b | 9.57 ± 0.01 a | 4.07 ± 0.01 a | 6.40 ± 0.00 |
| W.c.25MA | 9.31 ± 0.01 a | 4.45 ± 0.02 a | 9.29 ± 0.02 a | 4.48 ± 0.02 b | 4.00 ± 0.04 b | 4.49 ± 0.00 b | 6.40 ± 0.00 |
| W.c.26MA | 9.50 ± 0.01 a | 4.45 ± 0.03 a | 9.19 ± 0.01 b | 4.42 ± 0.00 ab | 0.00 ± 0.00 c | 4.46 ± 0.00 b | 6.40 ± 0.00 |
| P.a.35IB | 9.08 ± 0.01 c | 4.58 ± 0.01 c | 9.45 ± 0.01 a | 4.22 ± 0.00 b | 9.41 ± 0.01 b | 4.05 ± 0.01 a | 6.40 ± 0.00 |
| W.c.17MD | 9.42 ± 0.02 a | 4.44 ± 0.01 b | 9.24 ± 0.02 b | 4.43 ± 0.00 a | 0.00 ± 0.00 c | 4.49 ± 0.00 c | 6.40 ± 0.00 |
| W.c.13ID | 9.32 ± 0.01 a | 4.42 ± 0.01 a | 9.07 ± 0.02 b | 4.42 ± 0.00 a | 0.00 ± 0.00 c | 4.47 ± 0.06 a | 6.40 ± 0.00 |
| P.a.10BE | 9.32 ± 0.01 b | 4.57 ± 0.03 c | 9.38 ± 0.01 b | 4.24 ± 0.02 b | 9.45 ± 0.01 a | 4.08 ± 0.00 a | 6.40 ± 0.00 |
| P.a.16BB | 9.47 ± 0.02 b | 4.61 ± 0.02 c | 9.53 ± 0.01 a | 4.20 ± 0.01 b | 9.4 ± 0.01 a | 4.05 ± 0.03 a | 6.40 ± 0.00 |
| A. salmonicida subsp. salmonicida | Y. ruckeri | |||||
|---|---|---|---|---|---|---|
| Isolate | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
| W.c.2MB | 0.13 ± 0.13 a | 1.14 ± 0.02 a | 1.94 ± 1.02 c | 0.18 ± 0.09 a | 0.18 ± 0.04 a | 0.38 ± 0.10 a |
| W.p.5BB | 0.02 ± 0.07 abc | 0.02 ± 0.07 cd | −0.17 ± 0.04 d | 0.02 ± 0.07 cde | 0.19 ± 0.04 a | 0.39 ± 0.13 a |
| W.c.6MC | −0.01 ± 0.08 abc | 1.03 ± 0.06 b | 3.32 ± 0.17 b | 0.03 ± 0.00 bcde | 0.19 ± 0.08 a | 0.24 ± 0.11 a |
| W.c.9MC | 0.00 ± 0.09 abc | 1.11 ± 0.03 ab | 3.07 ± 0.10 b | 0.11 ± 0.04 abc | 0.25 ± 0.07 a | 0.37 ± 0.07 a |
| P.a.24IC | −0.06 ± 0.05 c | 0.00 ± 0.04 de | 0.19 ± 0.06 d | 0.07 ± 0.05 bcd | 0.14 ± 0.04 ab | 0.39 ± 0.06 a |
| W.c.25MA | −0.03 ± 0.10 bc | 1.04 ± 0.03 b | 3.27 ± 0.07 b | 0.04 ± 0.02 bcde | 0.24 ± 0.10 a | 0.29 ± 0.13 a |
| W.c.26MA | 0.04 ± 0.09 abc | 1.08 ± 0.06 ab | 3.34 ± 0.16 b | 0.08 ± 0.02 abcd | 0.15 ± 0.10 ab | 0.26 ± 0.10 a |
| P.a.35IB | 0.13 ± 0.05 ab | 0.09 ± 0.05 c | −0.01 ± 0.09 d | 0.13 ± 0.00 ab | 0.20 ± 0.07 a | 0.38 ± 0.10 a |
| W.c.17MD | 0.03 ± 0.04 abc | 1.10 ± 0.04 ab | 4.09 ± 0.05 a | 0.06 ± 0.07 bcd | 0.20 ± 0.08 a | 0.30 ± 0.12 a |
| W.c.13ID | 0.01 ± 0.11 abc | 1.09 ± 0.06 ab | 4.49 ± 0.26 a | −0.06 ± 0.11 e | 0.20 ± 0.11 a | 0.26 ± 0.05 a |
| P.a.10BE | 0.02 ± 0.02 abc | 0.01 ± 0.03 cd | 0.04 ± 0.09 d | 0.06 ± 0.09 bcd | 0.21 ± 0.05 a | 0.30 ± 0.04 a |
| P.a.16BB | −0.08 ± 0.04 c | −0.09 ± 0.02 e | 0.10 ± 0.07 d | 0.00 ± 0.03 cde | 0.21 ± 0.07 a | 0.30 ± 0.07 a |
| Control | 0.00 ± 0.00 abc | 0.00 ± 0.00 de | 0.00 ± 0.00 d | 0.00 ± 0.00 de | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla-Pineda, M.; Achou, C.G.; Díaz, J.; Gutiérrez-Falcon, A.; Bravo, M.; Herrera-Muñoz, J.I.; Peña-Navarro, N.; Alvarado, C.; Ibañez, F.C.; Marzo, F. In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida. Foods 2023, 12, 861. https://doi.org/10.3390/foods12040861
Quintanilla-Pineda M, Achou CG, Díaz J, Gutiérrez-Falcon A, Bravo M, Herrera-Muñoz JI, Peña-Navarro N, Alvarado C, Ibañez FC, Marzo F. In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida. Foods. 2023; 12(4):861. https://doi.org/10.3390/foods12040861
Chicago/Turabian StyleQuintanilla-Pineda, Mario, Chajira Garrote Achou, Jesús Díaz, Ana Gutiérrez-Falcon, María Bravo, Juan Ignacio Herrera-Muñoz, Nelson Peña-Navarro, Carlos Alvarado, Francisco C. Ibañez, and Florencio Marzo. 2023. "In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida" Foods 12, no. 4: 861. https://doi.org/10.3390/foods12040861
APA StyleQuintanilla-Pineda, M., Achou, C. G., Díaz, J., Gutiérrez-Falcon, A., Bravo, M., Herrera-Muñoz, J. I., Peña-Navarro, N., Alvarado, C., Ibañez, F. C., & Marzo, F. (2023). In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida. Foods, 12(4), 861. https://doi.org/10.3390/foods12040861





