Abstract
Five cultivars of bread wheat and spelt and three of emmer were grown in replicate randomised field trials on two sites for two years with 100 and 200 kg nitrogen fertiliser per hectare, reflecting low input and intensive farming systems. Wholemeal flours were analysed for components that are suggested to contribute to a healthy diet. The ranges of all components overlapped between the three cereal types, reflecting the effects of both genotype and environment. Nevertheless, statistically significant differences in the contents of some components were observed. Notably, emmer and spelt had higher contents of protein, iron, zinc, magnesium, choline and glycine betaine, but also of asparagine (the precursor of acrylamide) and raffinose. By contrast, bread wheat had higher contents of the two major types of fibre, arabinoxylan (AX) and β-glucan, than emmer and a higher AX content than spelt. Although such differences in composition may be suggested to result in effects on metabolic parameters and health when studied in isolation, the final effects will depend on the quantity consumed and the composition of the overall diet.
Keywords:
bread wheat; emmer; spelt; fibre; metabolites; minerals; phenolics; fertilisation; health benefits 1. Introduction
Wheat is the major staple crop in temperate countries, with annual global yields exceeding 700 million tonnes. About 95% of the total production is hexaploid bread wheat (Triticum aestivum L. subsp. aestivum, genome constitution AABBDD) which originated about 10,000 years ago, with most of the remaining 5% production being tetraploid pasta wheat (Triticum turgidum L. subsp. durum) (AABB genomes). Bread and durum wheats are “free threshing” (the hulls being readily separated from the grain during harvest), which is regarded as an advanced trait. However, both bread and pasta wheats have been subjected to intensive breeding, focusing on improving their agronomic performance and increasing their yield and quality (for making bread and pasta, respectively). Hence, although there is wide genetic variation in both species, modern cultivars (those developed by scientific breeding during the last few decades) tend to be less genetically diverse than older cultivars and traditional types of wheat dating from before the application of breeding (called land races) [1].
Ancient wheats are diploid einkorn (T. monococcum, L., AA genome), tetraploid emmer (T. turgidum L. subsp. dicoccum Thell., AABB genomes) and hexaploid spelt (T. aestivum L. subsp. spelta Thell, AABBDD genomes) and are generally hulled as opposed to free threshing. Although the term “ancient” is taken to imply that the genotypes grown today are similar to those grown in antiquity, this is certainly not the case, at least for spelt and emmer. Einkorn is a distinct species, which includes cultivated and wild forms, while emmer and spelt are subspecies of T. turgidum and T. aestivum, respectively. Furthermore, modern commercial cultivars of spelt may have introgressions (transfer of genetic information) from bread wheat due to cross-breeding, while all types of wheat cultivated today have been grown and hence selected (either unconsciously or deliberately) over thousands of years [2].
The composition of wheat grain is determined by the genotype, the environment, the farming system and the interactions between the genotype and these factors. Environmental factors are particularly important when comparing ancient and modern wheats, as the former are often grown in organic or low input systems with low nitrogen application to avoid lodging (bending of the plant at or near ground level, making harvest difficult and often leading to premature germination of grains) while modern semi-dwarf wheats are more usually grown in intensive high input systems with high nitrogen fertilisation [3].
The breeding and selection of modern bread wheats have focused on increasing yield and improving breadmaking quality, which is largely determined by the content and composition of gluten proteins. It has therefore been suggested that this has led to modern wheats having lower contents of micronutrients (minerals, vitamins) and bioactive components (phytochemicals) and higher contents of proteins that may lead to adverse reactions and diseases such as coeliac disease, wheat allergy and non-coeliac wheat sensitivity [4,5]. Hence, there has been increased interest in ancient wheats, which are assumed to have more favourable compositions for health [2].
We have therefore carried out detailed analyses of grain samples of three commercial cultivars of emmer and five cultivars each of spelt and bread wheat. All of the cultivars are adapted to Northern Europe and corresponded, with one substitution due to unavailability, to those selected to compare the effects of breads on health as part of the “Well-on-Wheat?” research consortium programme (https://www.wellonwheat.org, accessed on 1 February 2023). These samples were grown in replicate field trials in two Northern European countries (the UK and the Netherlands) for two years with low (100 kg/Ha) and high (200 kg/ha) applications of nitrogen fertilisation to reflect the different inputs used for ancient and modern wheats. Wholemeal samples were analysed for the major types of dietary fibre in white flour (arabinoxylan and β-glucan), polar metabolites, protein (as nitrogen), phenolics and mineral micronutrients to identify differences in composition.
2. Materials and Methods
2.1. Grain Samples
Commercial samples of five cultivars each of bread wheat (RAGT Reform, Capo, Bernstein, Kometus, Akteur) and spelt (Comburger, Zollernspelz, Attergauer, Bauländer Spelz, Franckenkorn) and three cultivars of emmer (Ramses, Roter Heidfelder, Späths Albjuwel) were grown in two years (2017–2018 and 2018–2019) at two nitrogen levels (100 and 200 kg N/Ha) in Flevoland (WUR Field crops, Lelystad, Flevoland, Netherlands, 52°53′94.69″ N, 5°56′56.77″ E) in three randomised replicate 6 × 1.5 m plots and at Rothamsted Research (Harpenden, Hertfordshire, UK, 51°48′19.79″ N 0°21′11.39″ W) in three randomised replicate plots of 1 × 1 m. All trials were autumn sown, but sowing, fertiliser application and harvest dates varied between sites and years, according to local conditions. Standard agronomic practices for the two sites were used. Grain samples of emmer and spelt were mechanically dehulled. Whole grain samples at about 14% water content were milled in two stages: firstly, a Retsch ZM 200 Model Ultra-Centrifugal Mill (Retsch Gmbh, Dusselgorf, Germany) using a 0.5 mm ring sieve and then a Glen Creston Ball Mill Retsch Gmbh, Dusseldorf, Germany) using 5 ball bearings in a 5 cm diameter canister for 4 min for each sample.
2.2. Genotyping
The samples were genotyped using the Axiom 35k Wheat Breeders Genotyping Array (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using the Affymetrix GeneTitan (Thermo Fisher Scientific, Inc.) [6]. Alleles were identified using the Affymetrix proprietary software package Axiom Analysis Suite V4.0.3.3 (Thermo Fisher Scientific, Inc.) and prior model ‘Axiom_WhtBrd-1.r3’. A Dish QC threshold of 0.8 and call rate cut-off of 90% were used to adjust for hybridisation rates of spelt and emmer to the array. A distance matrix was generated from the scores using R package SNPRelate (Bioconductor Open Source, Harvard, MA, USA) [7]. The first two Principal Components accounting for over 25% of the variance (PC1:19.76%; PC2:5.53%) were plotted as a PCA plot.
2.3. Enzyme Fingerprinting of Arabinoxylan and β-Glucan
Three technical replicates of flour were digested with endoxylanase and lichenase (β-glucanase) to release arabinoxylan oligosaccharides (AXOS) from arabinoxylan (AX) and gluco-oligosaccharides (GOS) from β-glucan, respectively [8]. The oligosaccharides were separated using a 2 mm × 250 mm Carbopac PA-1 (Dionex) column [8] (dx.doi.org/10.17504/protocols.io.babriam6, accessed on 1 February 2023). The areas under the oligosaccharide peaks were combined to give total AX and total β-glucan (expressed in arbitrary units), respectively.
2.4. NMR Spectroscopy of Polar Metabolites
Sample preparation for 1H-NMR was carried out as described by [9]. Signal intensities for characteristic spectral regions for 29 major metabolites were compared with a library of spectra of standards analysed under the same conditions.
2.5. Mineral Analysis
Nitrogen was determined on each biological replicate by Dumas combustion, using a Leco combustion analyzer (Leco Corp., St. Paul, MN, USA). Iron and zinc were determined by Optima 7300 DV Inductively Coupled Plasma–Optical Emission Spectrometer (ICP–OES) (Perkin Elmer, Waltham, MA, USA) after digestion with nitric and perchloric acids. Certified external standards and in-house standards were used to monitor performance using Shewhart Control Charts.
2.6. Total Phenolics
Total phenolics were determined based on [10]. Triplicate 75 mg samples of each biological replicate were vortexed with 1.5 mL acidified methanol and then mixed at 850 rpm on an Eppendorf Thermomixer (Eppendorf Ltd., Stevenage, UK) for 2 h at 23 °C. After centrifugation (Eppendorf Ltd., Stevenage, UK) at 5000× g for 10 min, 1 mL of supernatant was removed into a fresh Eppendorf tube and 200 µL aliquots mixed with 1.5 mL of ×10 diluted Folin–Ciocalteau reagent (Sigma-Aldrich, St. Louis, MO, USA) and left to stand for 5 min. 1.5 mL of 6% (w/v) aqueous sodium carbonate solution was added, mixed and stood at room temperature for 90 min. The absorbance at 725 nm was then measured (Jenway 6715 UV/Vis spectrophotometer, Cole-Parmer, St Neots, UK), and the concentration of phenolics was calculated using ferulic acid as a standard (Sigma-Aldrich) and a standard curve from 20, 40, 100, 150 and 200 µg/mL with three technical replicates of each point.
2.7. Statistical Analysis
All data were analysed using analysis of variance (ANOVA) in Genstat 21 (VSN International, Hemel Hempstead, UK). The block structure was Trial/Block/Subblock, where Trial captures the location and year of each trial. There are 3 blocks within each trial and 2 sub-blocks within each block to which the Nlevel treatment was applied. Lines were considered to be applied to plots within each sub-block. The treatment structure was Nlevel*(Grain/(cultivarBreadwheat + cultivarEmmer + cultivarSpelt)) where the factor Grain tests for differences between the three grain types. The nested factors cultivarBreadwheat, cultivarEmmer and cultivarSpelt test for differences between the lines within each grain type. The Nlevel factor tests for differences between the two Nlevels and their interactions with grain type and lines are also included. Some variables were transformed in order to meet the normality and homoscedasticity assumptions of the analysis. Means and 95% confidence intervals are given in Tables 1 and 3 while the transformations used are indicated in Supplementary Tables of means and p values.
Principal component analysis (PCA) and orthogonal partial least squares-discrimination analysis (OPLS-DA) were carried out using SIMCA-P software (version 13, MKS Umetrics) (Sartorius UK Ltd., Epsom, UK).
3. Results
Five cultivars each of bread wheat (RAGT Reform, Capo, Bernstein, Kometus, Akteur) and spelt (Comburger, Zollernspelz, Attergauer, Bauländer Spelz, Franckenkorn) and three cultivars of emmer (Ramses, Roter Heidfelder, Späths Albjuwel) were selected, all of which have been grown commercially in Northern Europe. The genomic relationships between the cultivars were determined using the Axiom 35k Wheat Breeders Genotyping Array, comprising 35,143 single nucleotide polymorphism (SNP) markers. Principal component analysis (PCA) showed a clear separation of the three cereal types, confirming that the spelt lines used did not have recent introgressions from bread wheat (Figure 1).
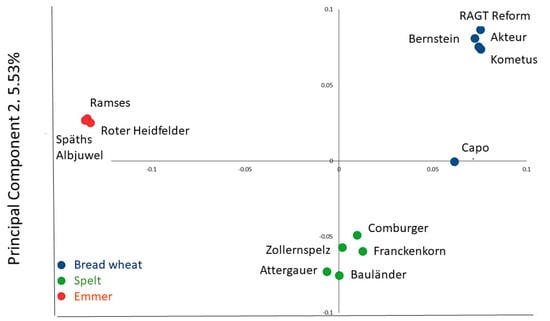
Figure 1.
Genomic relationships of the 13 genotypes, illustrated by Principal Component Analysis of markers determined using the Axiom HD Genotyping Array (comprising 819,571 SNP markers).
3.1. Grain Composition
In order to determine the variation in composition within and between bread wheat, spelt and emmer, the four environments (site × year combinations) were treated as blocks in the statistical analyses. In addition, to compare the effects of nitrogen fertilisation on grain composition, the data for the 100 kg/Ha and 200 kg/Ha treatments were analysed separately and compared formally by including nitrogen as a factor in the statistical analyses. The ranges of contents are illustrated in Figures 2, 3 and 5, while Table 1, Table 2, Table 3 and Table 4 and Supplementary Tables S1–S8 present means, 95% confidence intervals, SEMs and observed statistical significance determined by ANOVA.
The groups of components are discussed below.
3.2. Protein and Minerals
Grain protein content (determined as N × 5.7) (Figure 2A, Table 1) overlapped in range between bread wheat, spelt and emmer but was highest in spelt and lowest in bread wheat. It was also higher at 200 kg/Ha than at 100 kg/Ha. ANOVA (Table 2) showed statistically significant differences in protein content between bread wheat, emmer and spelt and statistically significant effects of nitrogen on their protein contents. By contrast, the three cereal types did not differ significantly in their response to nitrogen, and there was little variation in the effects of nitrogen between the cultivars within a single cereal type.
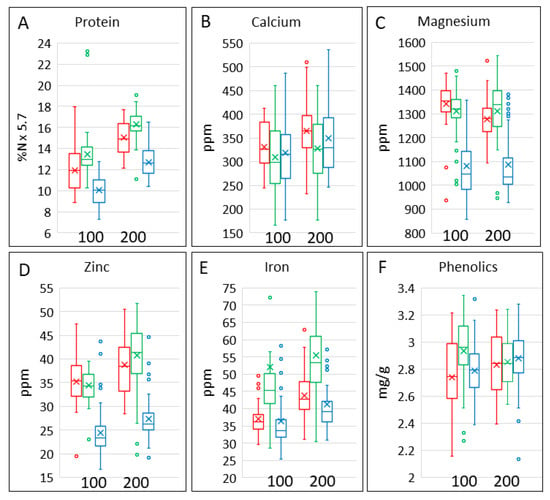
Figure 2.
Contents of protein, minerals and total phenolics in grains of the three cereal types grown in four environments. Colour code: red, emmer; green, spelt; blue, bread wheat. The bar shows the range of the whole dataset. The box shows the middle two quartiles, separated by the horizontal line, which is the median, and the vertical lines are the upper and lower quartiles, respectively. Outliers are shown as circles. The x is the mean average. All analyses are expressed on a dry weight basis.

Table 1.
Means and 95% confidence intervals (in parentheses) of contents of selected minerals, metabolites and groups of metabolites in grain of the three types of wheat grown with 100 and 200 kg N/Ha.
Table 1.
Means and 95% confidence intervals (in parentheses) of contents of selected minerals, metabolites and groups of metabolites in grain of the three types of wheat grown with 100 and 200 kg N/Ha.
| 100 kg N/Ha | 200 kg N/Ha | |||||
|---|---|---|---|---|---|---|
| Bread Wheat | Emmer | Spelt | Bread Wheat | Emmer | Spelt | |
| %N | 1.764 (1.68, 1.847) | 2.095 (2.001, 2.189) | 2.354 (2.27, 2.437) | 2.23 (2.146, 2.313) | 2.636 (2.542, 2.73) | 2.857 (2.773, 2.94) |
| Ca | 311.6 (300.2, 323.5) | 327.7 (312.3, 343.8) | 301.8 (290.7, 313.3) | 341.6 (329.1, 354.6) | 358.5 (341.7, 376.1) | 320.2 (308.5, 332.4) |
| Fe | 35.33 (33.36, 37.42) | 36.65 (34.01, 39.5) | 47.65 (44.98, 50.46) | 40.04 (37.8, 42.4) | 43.22 (40.11, 46.58) | 54.01 (51, 57.21) |
| Mg | 1081 (1054, 1108) | 1341 (1309, 1374) | 1311 (1284, 1338) | 1087 (1060, 1114) | 1278 (1246, 1311) | 1312 (1285, 1339) |
| Zn | 24.41 (22.78, 26.04) | 35.22 (33.34, 37.11) | 34.37 (32.74, 36) | 27.27 (25.64, 28.9) | 38.76 (36.88, 40.65) | 40.78 (39.15, 42.41) |
| total phenolics | 2786 (2732, 2840) | 2742 (2677, 2807) | 2934 (2880, 2987) | 2879 (2826, 2933) | 2830 (2765, 2894) | 2854 (2800, 2908) |
| raffinose | 5.95 (5.819, 6.082) | 7.077 (6.908, 7.245) | 6.916 (6.785, 7.048) | 5.944 (5.813, 6.076) | 6.465 (6.296, 6.633) | 6.743 (6.611, 6.874) |
| asparagine | 0.4907 (0.4681, 0.5144) | 0.6588 (0.6195, 0.7007) | 0.6981 (0.666, 0.7318) | 0.5664 (0.5403, 0.5937) | 0.7803 (0.7337, 0.8298) | 0.8648 (0.825, 0.9065) |
| glycine betaine | 1.311 (1.262, 1.362) | 1.49 (1.432, 1.548) | 1.606 (1.551, 1.662) | 1.307 (1.257, 1.357) | 1.466 (1.41, 1.525) | 1.537 (1.483, 1.591) |
| choline | 0.1748 (0.1711, 0.1785) | 0.2148 (0.2101, 0.2195) | 0.2137 (0.21, 0.2174) | 0.1854 (0.1817, 0.1891) | 0.2207 (0.216, 0.2254) | 0.2229 (0.2192, 0.2266) |
| galactinol | 0.2224 (0.2122, 0.2328) | 0.3976 (0.3795, 0.4161) | 0.2692 (0.2579, 0.2806) | 0.2223 (0.2121, 0.2327) | 0.3657 (0.3484, 0.3834) | 0.2751 (0.2637, 0.2867) |
| inositol | 3.08 (3.024, 3.136) | 3.216 (3.141, 3.292) | 3.421 (3.365, 3.477) | 3.097 (3.041, 3.153) | 3.096 (3.021, 3.171) | 3.25 (3.194, 3.306) |
| total amino acids | 5.753 (5.618, 5.891) | 6.522 (6.331, 6.719) | 6.607 (6.452, 6.766) | 6.084 (5.941, 6.23) | 6.724 (6.528, 6.927) | 6.951 (6.788, 7.118) |
| total organic acids | 2.014 (1.934, 2.093) | 2.247 (2.156, 2.337) | 2.133 (2.053, 2.213) | 2.048 (1.968, 2.128) | 2.321 (2.231, 2.411) | 2.245 (2.166, 2.325) |
| total methyl donors | 1.482 (1.436, 1.529) | 1.702 (1.644, 1.761) | 1.814 (1.758, 1.871) | 1.486 (1.44, 1.533) | 1.685 (1.628, 1.744) | 1.758 (1.703, 1.813) |
| total sugars | 29.47 (28.78, 30.18) | 35.79 (34.71, 36.9) | 29.88 (29.18, 30.6) | 28.75 (28.07, 29.44) | 33.62 (32.61, 34.67) | 28.8 (28.13, 29.5) |
The box plots show that the contents of calcium (Figure 2B) and magnesium (Figure 2C) were not affected by nitrogen, but the content of magnesium was lower in bread wheat grain than in grains of spelt or emmer. The contents of iron and zinc were lowest in bread wheat grain (Figure 2D,E), while the content of iron was lower in emmer than in spelt grain. The contents of both minerals were also higher at 200 kg N/Ha than at 100 kg N/Ha. However, ANOVA showed significant differences between the contents of all minerals in the grain types and significant effects of nitrogen fertilisation on the contents of all minerals except magnesium, which showed a significant interaction due to a difference between nitrogen fertilisation in emmer only (Table 2). There were also significant differences between the contents of all minerals (except zinc in spelt and iron in all grain types) between the cultivars within each type, but no differences in the effects of nitrogen between the cultivars within the types (except for iron in spelt) (Table 2 and Table S1).
3.3. Total Phenolics
Phenolics are the most abundant phytochemicals in wheat grain [11]. The contents of total phenolics varied widely (Figure 2F), with significant differences between cereal types and cultivars within types (Table 2). There was an interaction between cereal type and nitrogen, with total phenolics being lower at 100 kg N/Ha for all cereal types apart from spelt where total phenolics were higher at 100 kg N/Ha than at 200 kg N/Ha.
3.4. Polar Metabolites
The contents of polar metabolites in the samples were determined by 1H NMR spectroscopy. This allowed the quantification of monosaccharide (glucose, fructose, galactose), disaccharide (maltose, sucrose) and trisaccharide (raffinose) sugars, organic acids (malic, acetic, fumaric), the sugar alcohols inositol and galactinol, the “methyl donors” choline and betaine and thirteen amino acids (alanine, aspartic acid, asparagine, glycine, glutamic acid, glutamine, γ-amino butyric acid (GABA), isoleucine, leucine, phenylalanine, tyrosine, tryptophan and valine). Data for all components are given in Supplementary Tables S2–S4, while selected components and groups of components are shown in Table 1 and Table 2 and Figure 3.
The contents of all metabolites and groups of metabolites overlapped between the cereal types, but differences between the ranges in the types are observed (Figure 3). Asparagine is of particular interest because it is the limiting factor for the formation of acrylamide during processing [12,13]. The contents of total amino acids (Figure 3A) and of asparagine (Figure 3B) were significantly lower in bread wheat and higher in spelt, with a significant effect of nitrogen. However, there were no differences in the effects of nitrogen between and within the three cereal types.
The contents of total sugars (mono-, di- and trisaccharides) and total organic acids varied widely (Figure 3C,D), but both were significantly higher in emmer. Total organic acids were also significantly lower in bread wheat (Table 2). The contents of sugars were significantly affected by nitrogen, with no differences between the effects of nitrogen on the different cereal types or cultivars within the types (Table 2). The concentration of glycine betaine was about 10-fold greater than that of choline, which is typical for wheat [14]. Although the ranges overlapped (Figure 3E,F), they were significantly higher in spelt and lower in bread wheat, with little effect of nitrogen or variation within the types (Table 2).
Raffinose (the trisaccharide galactose, glucose, fructose) is a non-digestible and fermentable carbohydrate (being part of the FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) fraction) while galactinol (1-alpha-D-Galactosyl-myo-inositol) and inositol ((1R,2S,3r,4R,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol) are precursors in raffinose synthesis [15]. Raffinose (Figure 3I) accounted for about a quarter of the total sugars (Figure 3C) and was significantly lower in bread wheat than in emmer or spelt. The contents of inositol were about half of those of raffinose and were higher in spelt (Figure 3G). Galactinol was present at much lower concentrations and was significantly higher in emmer and lower in bread wheat than in spelt (Figure 3H). The contents of raffinose and inositol were affected by nitrogen level and, with the exception of galactinol in spelt, varied significantly between cultivars of the three cereal types (Table 2).
PCA analysis of the metabolite dataset showed partial separation of the three cereal types, based on 48.9% of the total variance (Figure 4A). To improve the discrimination between cereal types, we repeated the analysis using supervised multivariate analysis (orthogonal partial least squares discrimination analysis, OPLS-DA), selecting for differences between the cereal types (Figure 4C). This gave clear separation between the three types with the loadings plot (Figure 4F) showing that emmer was characterised by high contents of maltose, galactinol and glucose and spelt by higher levels of amino acids (glycine, asparagine, leucine, isoleucine and valine) compared with bread wheat. OPLS-DA was also used to separate the samples based on nitrogen level (Figure 4B), the loadings plot (Figure 4E) showing higher contents of amino acids at 200 kg N/Ha. These differences are also illustrated by the difference plots in Supplementary Figure S1.

Table 2.
p values from ANOVA of minerals and selected metabolites in the three cereal types and cultivars.
Table 2.
p values from ANOVA of minerals and selected metabolites in the three cereal types and cultivars.
| NLevel | Grain | Nlevel. Grain | Grain. Cultivar. Bread Wheat | Grain. Cultivar. Emmer | Grain. Cultivar. Spelt | Nlevel. Grain. Cultivar Bread Wheat | Nlevel. Grain. Cultivar Emmer | Nlevel. Grain. Cultivar Spelt | |
|---|---|---|---|---|---|---|---|---|---|
| %N | <0.001 | <0.001 | 0.475 | <0.001 | 0.098 | <0.001 | 0.704 | 0.212 | 0.642 |
| log(Ca) | <0.001 | <0.001 | 0.639 | <0.001 | <0.001 | <0.001 | 0.672 | 0.195 | 0.495 |
| log(Fe) | <0.001 | <0.001 | 0.815 | 0.566 | 0.957 | 0.064 | 0.375 | 0.936 | 0.023 |
| Mg | 0.46 | <0.001 | 0.016 | <0.001 | 0.03 | <0.001 | 0.228 | 0.351 | 0.749 |
| Zn | <0.001 | <0.001 | 0.008 | 0.012 | <0.001 | 0.556 | 0.409 | 0.834 | 0.994 |
| Total phenolics | 0.407 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 0.253 | 0.516 | 0.153 |
| raffinose | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.988 | 0.271 | 0.709 |
| Log(asparagine) | <0.001 | <0.001 | 0.352 | <0.001 | 0.022 | <0.001 | 0.624 | 0.34 | 0.78 |
| Sqrt (glycine betaine) | 0.358 | <0.001 | 0.087 | <0.001 | <0.001 | <0.001 | 0.014 | 0.951 | 0.305 |
| choline | <0.001 | <0.001 | 0.535 | <0.001 | 0.005 | <0.001 | 0.852 | 0.109 | 1 |
| Sqrt (galactinol) | 0.399 | <0.001 | 0.061 | <0.001 | <0.001 | 0.605 | 0.935 | 0.146 | 0.951 |
| inositol | 0.002 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | 0.883 | 0.06 | 0.678 |
| loge(total amino acids) | 0.002 | <0.001 | 0.598 | 0.099 | <0.001 | 0.001 | 0.095 | 0.672 | 0.464 |
| total organic acids | 0.163 | <0.001 | 0.338 | <0.001 | <0.001 | <0.001 | 0.159 | 0.771 | 0.551 |
| loge (total methyl donors) | 0.529 | <0.001 | 0.141 | <0.001 | <0.001 | <0.001 | 0.011 | 0.905 | 0.395 |
| loge(total sugars) | 0.005 | <0.001 | 0.397 | <0.001 | <0.001 | <0.001 | 0.726 | 0.23 | 0.726 |
Statistically significant values (p < 0.05) are given in bold. Some variables required transformation, square root (Sqrt) or loge, to meet the assumptions of the analysis.
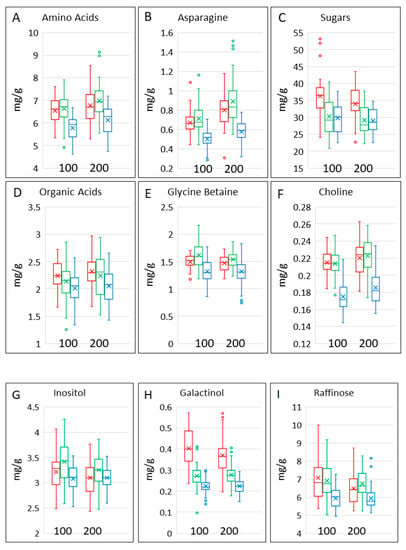
Figure 3.
Contents of selected polar metabolites and groups of metabolites in grains of the three cereal types grown in four environments. Colour code: red, emmer; green, spelt; blue, bread wheat. The bar shows the range of the whole data set. The box shows the middle two quartiles, separated by the horizontal line, which is the median, and the vertical lines are the upper and lower quartiles, respectively. Outliers are shown as circles. The x is the mean average. All analyses are expressed on a dry weight basis.
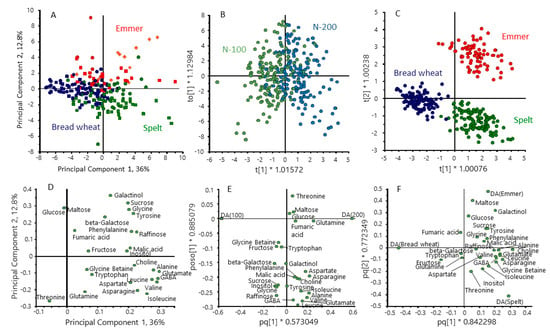
Figure 4.
Multivariate analysis of the contents of polar metabolites in grains of the three cereal types grown in four environments. Principal component analysis PCA (A) and orthogonal partial least squares discrimination analysis (OPLS-DA) (B,C) selecting for differences between nitrogen treatments (B) and cereal types (C). (D–F) loading plots for (A–C), respectively. Colour code: red, emmer; green, spelt; blue, bread wheat.
3.5. Dietary Fibre
The contents of AX and β-glucan in the grain types are shown in Figure 5A,B and in Table 3. The contents of both AX and β-glucan were lower in emmer grain, with AX being highest in bread wheat grain and β-glucan highest in spelt grain. Hence, the ratio of AX: β-glucan was lower in spelt grain than in the other cereals (Figure 5D). The combined contents of these two components were also highest in bread wheat grain (Figure 5C), reflecting the fact that the content of AX was about three- to four-fold greater than that of β-glucan. There was significant variation in the contents of AXOS between the cultivars of the three types of wheat and of β-glucan between the cultivars of bread wheat and emmer, but no significant effects of nitrogen fertilisation (Table 4).
AX and β-glucan were determined by enzyme fingerprinting, which uses enzymes (endoxylanase and lichenase, respectively) to digest the polymers to release oligosaccharides separated and quantified by HP-AEC. The oligosaccharides released have defined structures, and their proportions, therefore, provide information on the structures of the polymers. In the case of AX, the oligosaccharides (AXOS) comprise chains of one to five xylose residues, one or more of which may be substituted with either one or two arabinose residues. The ratio of monosubstituted to disubstituted AX may affect the properties of the molecules and is generally higher in spelt and bread wheat than in emmer (Figure 6F). β-glucan comprises linear chains of glucose molecules linked predominantly by β(1-4) bonds. However, these β(1-4) bonds are interspersed with β(1-3) bonds that generally occur every three to four glucose residues, although some longer stretches of β(1-4) linked glucose residues (up to 14) have been reported. The distribution of β(1-3) bonds results in conformational changes in the linear glucan molecules, which affect their solubility and viscosity. Lichenase is a type of β-glucanase that releases mainly gluco-oligosaccharides (GOS) of three or four glucose residues (called G3 and G4), reflecting the relative abundances of β(1-3) and β(1-4) bonds. The ratio of G3:G4 GOS is higher in emmer and lower in spelt than in bread wheat (Figure 5E, Table 3).

Table 3.
Means and 95% confidence intervals (in parentheses) of contents and compositions of arabinoxylan and β-glucan in grain of the three types of wheat grown with 100 and 200 kg N/Ha.
Table 3.
Means and 95% confidence intervals (in parentheses) of contents and compositions of arabinoxylan and β-glucan in grain of the three types of wheat grown with 100 and 200 kg N/Ha.
| 100 kg N/Ha | 200 kg N/Ha | |||||
|---|---|---|---|---|---|---|
| Bread | Emmer | Spelt | Bread | Emmer | Spelt | |
| TOT-AX | 29.06 (28.18, 29.96) | 20.17 (19.43, 20.93) | 27.33 (26.51, 28.18) | 29.61 (28.72, 30.53) | 19.88 (19.16, 20.64) | 27.26 (26.44, 28.11) |
| TOT-BG | 8.146 (7.869, 8.432) | 5.617 (5.386, 5.859) | 9.256 (8.942, 9.581) | 7.63 (7.371, 7.898) | 5.142 (4.93, 5.363) | 8.521 (8.232, 8.82) |
| ratio G3:G4 GOS | 2.429 (2.395, 2.463) | 2.54 (2.497, 2.583) | 2.268 (2.234, 2.302) | 2.381 (2.347, 2.415) | 2.477 (2.434, 2.52) | 2.252 (2.218, 2.286) |
| ratio TOT-AXOS: TOT-BG | 3.596 (3.516, 3.677) | 3.619 (3.516, 3.723) | 2.968 (2.887, 3.048) | 3.915 (3.834, 3.996) | 3.895 (3.792, 3.999) | 3.212 (3.131, 3.293) |
| ratio M:D AXOS | 2.061 (2.035, 2.088) | 1.865 (1.834, 1.897) | 2.095 (2.068, 2.122) | 2.032 (2.005, 2.058) | 1.85 (1.818, 1.881) | 2.071 (2.044, 2.097) |
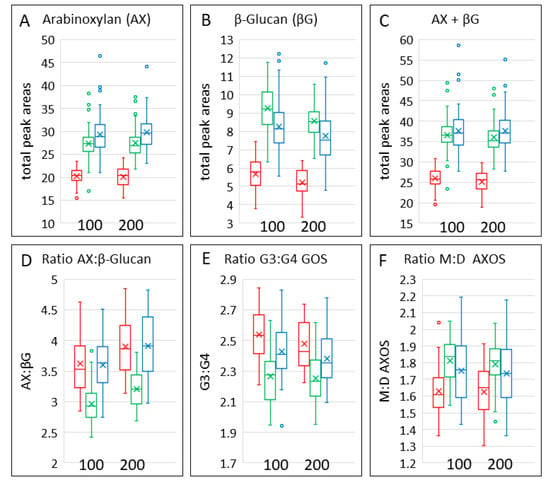
Figure 5.
Contents, ratios and structures of arabinoxylan and β-glucan in grains of the three cereal types grown in four environments. Colour code: red, emmer; green, spelt; blue, bread wheat. The box shows the middle two quartiles, separated by the horizontal line, which is the median, and the vertical lines are the upper and lower quartiles, respectively. Outliers are shown as circles. The x is the mean average.
Differences in the structures of AX and β-glucan in the cereal types are illustrated by the multivariate analysis in Figure 6. OPLS-DA confirmed that there were no effects of nitrogen on AX and β-glucan structure (Figure 6B) but gave clear separation of the cereal types (Figure 6C), with the loadings plot (Figure 6F) showing that spelt differed from emmer and bread wheat in having higher proportions of G3 and G4 GOS and bread wheat higher proportions of substituted AXOS These differences are also illustrated by the difference plots in Supplementary Figure S2

Table 4.
p-values from ANOVA of the proportions of AXOS and GOS in the three cereal types and cultivars.
Table 4.
p-values from ANOVA of the proportions of AXOS and GOS in the three cereal types and cultivars.
| Nlevel | Grain | Nlevel. Grain | Grain. Cultivar. Bread wheat | Grain. Cultivar. Emmer | Grain. Cultivar. Spelt | Nlevel. Grain. Cultivar. Bread wheat | Nlevel. Grain. Cultivar. Emmer | Nlevel. Grain. Cultivar. Spelt | |
|---|---|---|---|---|---|---|---|---|---|
| loge (TOT-AXOS) | 0.855 | <0.001 | 0.514 | <0.001 | <0.001 | <0.001 | 0.42 | 0.848 | 0.926 |
| loge (TOT-BG) | 0.002 | <0.001 | 0.758 | <0.001 | <0.001 | 0.085 | 0.659 | 0.501 | 0.76 |
| ratio G3:G4 GOS | 0.032 | <0.001 | 0.427 | 0.509 | 0.197 | <0.001 | 0.711 | 0.513 | 0.423 |
| ratio TOT -AXOS: TOT-BG | <0.001 | <0.001 | 0.655 | <0.001 | 0.121 | <0.001 | 0.715 | 0.218 | 0.564 |
| Sqrt (ratio M:D AXOS) | 0.093 | <0.001 | 0.899 | <0.001 | <0.001 | <0.001 | 0.249 | 0.775 | 0.902 |
Statistically significant values (p < 0.05) are given in bold. Some variables required transformation, square root (Sqrt) or loge, to meet the assumptions of the analysis.
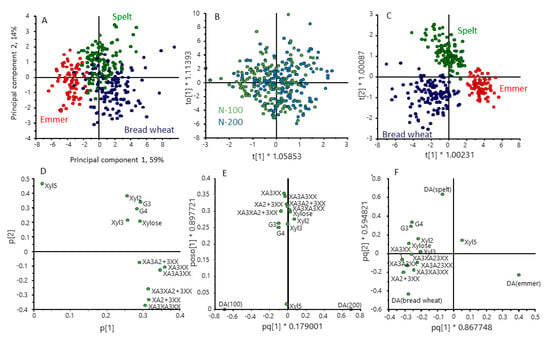
Figure 6.
Multivariate analysis of the contents of AXOS and GOS in grains of the three cereal types grown in four environments. Principal component analysis PCA (A) and orthogonal partial least squares discrimination analysis (OPLS-DA) (B,C) selecting for differences between nitrogen treatments (B) and cereal types (C). (D–F) loading plots for (A–C), respectively. Colour code: red, emmer; green, spelt; blue, bread wheat.
4. Discussion
We have carried out comparative analyses of the grain of five cultivars each of bread wheat and spelt and three cultivars of emmer, focusing on components that may contribute to health effects. The cultivars selected were all commercially available in Germany at the time of the study, and analyses of flours blended from commercial grain samples and of doughs and breads produced from the blended flours using sourdough and yeast-based systems have been reported elsewhere [16]. The cultivars were grown in replicated randomised field trials at sites in the UK and Netherlands for two years, giving four environments (sites and years). Furthermore, two levels of nitrogen fertiliser were applied to reflect the commercial use of low and high input systems for ancient wheats (emmer, spelt) and modern bread wheat, respectively.
Because only small numbers of cultivars were compared, the results cannot be taken to represent the full range of diversity within the three types of wheat. Nevertheless, wide variation within each type was observed, resulting from the effects of genotype, environment and nitrogen fertilisation. However, because only two sites and years were compared, it was not possible to calculate the separate contributions of genotype, environment and G x E interactions, and the year/site combinations were, therefore, treated as “environments”.
The variation in composition within emmer, spelt and bread wheat resulted in overlapping ranges in the contents of all components determined: protein, minerals, polar metabolites and AX and β-glucan fibre. Nevertheless, some statistically significant differences between the three cereal types were observed.
The lower protein content of modern bread wheats observed here is well-established and considered to result from “yield dilution”; the higher yields of modern wheats result from an increased accumulation of starch that dilutes other components [17]. Similarly, modern semi-dwarf bread wheats are known to have lower contents of Fe, Zn and Mg. This may result from the effects of the semi-dwarf phenotype, perhaps combined with some yield dilution [18,19]. The modern bread wheats were also significantly lower in asparagine, the precursor of acrylamide, which formed during processing [12] but also had lower contents of glycine betaine and choline, which have benefits for cardiovascular health by reducing the concentration of homocysteine in blood [20,21]. Hence, it is not possible to conclude that any of the three types of cereal is consistently “better” in terms of its content of polar metabolites.
Published values for the contents of total dietary fibre in whole grains of bread wheat range between 11.5–15.5% dry weight, of which about half (5.5–7.4% dry weight) is AX, with a lower content of β-glucan (0.51–0.96%) [22]. White flour contains significantly less fibre (due to the removal of the bran), about 4% dry weight, with AX and β-glucan accounting for about 70% and 20% of the total, respectively [23].
In the present study, bread wheat had higher contents of AX and β-glucan than emmer and higher AX content than spelt. Although spelt was higher in β-glucan, this component was present in lower concentrations than AX, and hence, the sum of the two types of fibre was highest in bread wheat. A meta-analysis of dietary fibre components in whole grain also showed slightly lower contents of AX in spelt than in bread wheat, with a wider range [24]. Some differences in fibre structure were also observed, with spelt having a lower ratio of G3:G4 GOS released from β-glucan while emmer had a lower ratio of monosubstituted:disubstituted AXOS released from AX. The significance of these differences for the behaviour of the AX and β-glucan fractions in foods and in the gastrointestinal tract is not known.
The bread wheats also had lower contents of raffinose, which is not absorbed in the human small intestine but rapidly fermented in the colon, forming part of the FODMAP fraction, which may contribute to discomfort due to gas production in individuals suffering from non-celiac wheat sensitivity and irritable bowel syndrome (IBS) [25]. However, the relevance of this to symptom control is limited as the major FODMAP fraction in wheat is fructans, which were not measured in the present work.
The effects of nitrogen fertilisation were also determined as it is usual to grown ancient and modern wheats under low input and intensive production systems, respectively. High nitrogen resulted in higher contents of minerals (iron, zinc and magnesium), as reported in a number of studies [26]. Similarly, a significant positive relationship between free asparagine content and total grain protein content has been reported [27,28]. Although only small effects of nitrogen on other components were observed, ANOVA showed that these varied between cereal types. ANOVA also showed interactions between N level and the proportions of AXOS and GOS released in all three types of wheat but no interactions with cultivars within the types.
A number of other studies have compared genotypes of modern and ancient wheats. For example, a series of studies compared the agronomic performance, yield, grain quality traits and contents of a range of “bioactive” components in 15 cultivars each of einkorn, emmer, spelt, bread wheat and durum wheat grown on four sites, with nitrogen fertilisation levels varying between wheat types to reflect commercial practice [29,30,31]. However, the relevance of such reported differences in composition to human health remains unclear.
This is, at least in part, due to the fact that the relevance of the parameters measured to human health has not been established. For example, although differences between the in vitro antioxidant capacity of cereal flours have been reported [32,33,34], these cannot be generalized to imply health benefits in humans in vivo [35]. Similarly, glucose released during the digestion of flour in vitro cannot be used to predict glycaemic response in vivo, which is determined by the competing effects of the appearance of glucose and the disappearance (cellular uptake) of glucose in blood [36,37,38]. As a result, although the glycaemic index (GI) calculated based on in vitro digestion may correlate with that determined in vivo, the absolute values may differ [39]. Such calculations based on in vitro digestion have been reported to result in over-estimation of the in vivo GI by 22% to 50% [40]. Differences in food structure resulting from processing will also affect oral mastication and gastrointestinal digestion. For example, the high density of pasta results in a significantly lower glycaemic response than those of flour and bread [41].
Finally, statistically significant differences in the compositions of cereal flours may not represent significant biological differences when considered in the context of processed food consumed as part of a mixed meal and with other foods consumed over a 24 h intake period.
Comparisons are also often reported using analytical data from different studies and/or data from published food composition tables (for example, [42,43]). This is clearly not valid as the cultivars, growth environments, agronomic practices and methods used for sample preparation and analysis will affect the results obtained.
Taking these factors into account, it is not surprising that different conclusions have been drawn on the health benefits of ancient compared with modern wheats. Thus, whereas Shewry and Hey [24] concluded that based on a comparison of grain samples grown and analysed under the same conditions, there is little evidence that ancient wheats are more “healthy” than modern wheats, Serban et al. [43] suggested that ancient wheat species have health benefits in relation to their nutraceutical composition.
In the context of the data presented here, the small differences in mean compositions of bread wheat, emmer or spelt and their overlapping quantitative ranges are unlikely to result in significant differences in health outcomes, with a possible exception being mineral micronutrients (zinc and iron, which are subject to low intakes in many population groups, with wheat being a significant dietary source [44].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12040843/s1, Figure S1. Difference plots showing the metabolites contributing to the separations in Figure 4B (A) and Figure 4C (B, C, D). Figure S2. Difference plots showing the AXOS and GOS contributing to the separations in Figure 6A (A) and Figure 6B (B, C). Table S1. Means and SEMs of the contents of minerals and metabolites in grains of individual cultivars of the three cereal types grown in four environments. Table S2. Means and SEMs of minerals and metabolites in cultivars of the three cereal types grown at 100 and 200 kg N/Ha. Table S3. Means and SEMs of contents of polar metabolites in grains of the three cereal types grown in four environments at two Nlevels. Table S4. p values from ANOVA of the contents of polar metabolites in grains of the three cereal types grown in four environments at two Nlevels. Table S5. Means and SEMs for AXOS and GOS in grains of the three cereal types grown at 100 and 200 kg N/Ha. Table S6. p values from ANOVA of AXOS and GOS in the three types and cultivars of wheat. Statistically significant values (p < 0.05) are highlighted. Table S7. Means and SEMs of AXOS in the cultivars of the three cereals grown in four environments. Some variables required transformation, square root (Sqrt) or loge (log), to meet the assumptions of the analysis. Table S8. Means and SEMs of AXOS in the cultivars of the three cereals grown in four environments with 100 and 200 kg N/Ha.
Author Contributions
Conceptualization, F.B., P.R.S., J.P.v.S., L.G., A.L. and D.J.; methodology, A.L., J.D. and J.L.W.; formal analysis, J.D., J.L.W., A.J.B. and J.H.; investigation, J.P.v.S., J.L.W., T.K.P., J.L.W., A.H.P.A., R.T. and Z.A.M.P.-H.; resources, P.R.S. and J.P.v.S.; data curation, J.D., J.H., J.L.W. and Z.A.M.P.-H.; writing—original draft preparation, F.B. and P.R.S.; writing—review and dditing, T.K.P., J.P.v.S., J.L.W., A.L., L.G., A.H.P.A. and D.J.; visualization, supervision, P.R.S., A.L., J.L.W. and D.J.; project administration, F.B. and D.J.; funding acquisition, F.B., P.R.S. and J.P.v.S. All authors have read and agreed to the published version of the manuscript.
Funding
“Well on Wheat?” is financed by a grant of the Dutch Government—“TKI- Top Knowledge Institute” and a wide range of additional partners* from the Agri-Food chain who donated unrestricted research grants. *These partners are AB-Mauri bakery Ingredients, Made, Netherlands; Borgesius Holding BV-Albert Heijn, Stadskanaal Netherlands; CSM innovation Bakery Center, Bingen, Germany; CYMMIT, Texcoco, Mexico; DSM Food Specialties, Delft, Netherlands; Fazer Bakeries Oy, Helsinki, Finland; Health Grain Forum, Vienna, Austria; ICC- Intl. Vienna, Austria; IWGA, Kansas 66210, USA; Lantmännen EK, Stockholm, Sweden; Mondelez, Saclay, France; Nederlands Bakkerij Centrum, Wageningen, Netherlands; Baking Industry Research Trust, Wellington, New Zealand; Nutrition et Sante, Revel, France; Puratos BV, Groot Bijgaarden, Belgium; Rademaker BV-Bakery equipments, Culemborg, Netherlands; Sonneveld Group BV, Papendrecht, Netherlands; Zeelandia-HJ Doeleman BV, Zierikzee, Netherlands. The project is coordinated and executed by an academic research consortium team (ARCT). Research specialists from funding partners, represented in a Research Steering Team (RST), are entitled to give scientific inputs to ARCT, which on its sole decision, may or may not take inputs into consideration. Decisions on studies set-up, execution and data interpretation of data are exclusively taken by ARCT. Scientific output communications are exclusively organized by ARCT without the involvement of funding partners. Rothamsted Research receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and the work forms part of the Designing Future Wheat strategic programme (BB/P016855/1).
Data Availability Statement
Full datasets are available from the corresponding author on request.
Acknowledgments
We thank Friedrich Longin (University of Hohenheim, Germany) for helping with the provision of grain samples and for dehulling emmer and spelt samples prior to analysis.
Conflicts of Interest
Part of the work of DJ outside the submitted work has been financed by public-private partnership grants of Top Knowledge Institute (TKI) Agri&Food and Health Holland, the NWO Carbohydrate Competence Center, by Organic A2BV/Mothersfinest BV, EU/FP7 SysmedIBD/305564, BIOM/305479 and Character/305676 and H2020 DISCOvERIE/848228. The authors declare no other conflict of interest.
References
- Pont, C.; Leroy, T.; Seidel, M.; Tondelli, A.; Duchemin, W.; Armisen, D.; Lang, D.; Bustos-Korts, D.; Goué, N.; Balfourier, F. Tracing the ancestry of modern bread wheats. Nat. Genet. 2019, 51, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Gilissen, L.J.W.J.; Smulders, M.J.M. Gluten Quality and Quantity in Wheat and in Wheat-Derived Products. In Biotechnological Strategies for the Treatment of Gluten Intolerances; Rossi, M., Ed.; Academic Press-Elsevier: London, UK, 2021; pp. 79–129. [Google Scholar]
- Shewry, P.R. What is gluten—Why is it special. Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Sands, D.C. The breeder’s dilemma–yield or nutrition? Nat. Biotechnol. 2003, 24, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; Morris, C.E.; Dratz, E.A.; Pilgeram, A.L. Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods. Plant Sci. 2009, 177, 377–389. [Google Scholar] [CrossRef]
- Burridge, A.J.; Winfield, M.O.; Allen, A.M.; Wilkinson, P.A.; Barker, G.L.A.; Coghill, J.; Waterfall, C.; Edwards, K.J. High-density SNP genotyping array for hexaploidy wheat and its relatives. In Wheat Biotechnology: Methods and Protocols; Bhalla, P.L., Singh, M.B., Eds.; Humana Press: New York, NY, USA, 2017; pp. 293–306. [Google Scholar] [CrossRef]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef]
- Lovegrove, A.; Wilkinson, M.D.; Freeman, J.; Pellny, T.; Tosi, P.; Saulnier, L.; Shewry, P.R.; Mitchell, R.A.C. RNA interference suppression of genes in glycosyl transferase families 43 and 47 in wheat starchy endosperm causes large decreases in arabinoxylan content. Plant Physiol. 2013, 163, 95–107. [Google Scholar] [CrossRef]
- Shewry, P.R.; Corol, D.; Jones, H.D.; Beale, M.H.; Ward, J.L. (2017) Defining genetic and chemical diversity in wheat grain by 1H-NMR spectroscopy of polar metabolites. Mol. Nutr. Food Res. 2017, 61, 1600807. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Oomah, B.D.; Mazza, G. Wheat quality: Antioxidant activity of wheat millstreams. In Wheat Quality Elucidation; Ng, P., Wrigley, C.W., Eds.; AACC International: St. Paul, MN, USA, 2002; pp. 219–233. [Google Scholar]
- Ward, J.L.; Poutanen, K.; Gebruers, K.; Piironen, V.; Lampi, A.-M.; Nyström, L.; Andersson, A.A.M.; Åman, P.; Boros, D.; Rakszegi, M.; et al. The HEALTHGRAIN cereal diversity screen: Concept, results and prospects. J. Agric. Food Chem. 2008, 56, 9699–9709. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Muttucumaru, N.; Elmore, J.S.; Curtis, T.; Mottram, D.S.; Parry, M.A.J.; Halford, N.G. Reducing acrylamide precursors in raw materials derived from wheat and potato. J. Agric. Food Chem. 2008, 56, 6167–6172. [Google Scholar] [CrossRef]
- Corol, D.I.; Ravel, C.; Raksegi, M.; Bedo, Z.; Charmet, G.; Beale, M.H.; Shewry, P.R.; Ward, J.L. Effects of genotype and environment on the contents of betaine, choline and trigonelline in cereal grains. J. Agric. Food Chem. 2012, 60, 5471–5481. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; America, A.H.P.; Lovegrove, A.; Wood, A.J.; Plummer, A.; Evans, J.; van den Broeck, H.C.; Gilissen, L.; Mumm, R.; Ward, J.L.; et al. Comparative compositions of metabolites and dietary fibre components in doughs and breads produced from bread wheat, emmer and spelt and using yeast and sourdough processes. Food Chem. 2022, 374, 131710. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Pellny, T.K.; Hassall, K.; Plummer, A.; Wood, A.; Bellisai, A.; Przewieslik-Allen, A.; Burridge, A.L.; Ward, J.L.; Shewry, P.R.; et al. Historical changes in the contents and compositions of fibre components and polar metabolites in white flour. Sci. Rep. 2020, 10, 5920. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2018, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Pellny, T.K.; Lovegrove, A. Is modern wheat bad for health? Nat. Plants 2016, 2, 16097. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Andersson, R.; Piironen, V.; Lampi, A.-M.; Nyström, L.; Boros, D.; Fraś, A.; Gebruers, K.; Courtin, C.M.; Delcour, J.; et al. Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem. 2013, 136, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Stone, B.A. Studies on wheat endosperm. I. Chemical composition and ultrastructure of the cell walls. Aus. J. Biol. Sci. 1973, 26, 793–812. [Google Scholar]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gibson, P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals. Ther. Adv. Gastroenterol. 2012, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zebarth, B.J.; Warren, C.J.; Sheard, R.W. Influence of the rate of nitrogen fertilisation on the mineral content of winter wheat in Ontario. J. Agric. Food Chem. 1992, 40, 1526–1530. [Google Scholar] [CrossRef]
- Shewry, P.R. Effects of nitrogen and sulfur nutrition on grain composition and properties of wheat and related cereals. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 103–120. [Google Scholar]
- Oddy, J.; Raffan, S.; Wilkinson, M.D.; Elmore, J.S.; Halford, N.G. Understanding the Relationships between Free Asparagine in Grain and Other Traits to Breed Low-Asparagine Wheat. Plants 2022, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Longin, C.F.H.; Ziegler, J.U.; Schweiggert, R.M.; Koehler, P.; Carle, R.; Würschum, T. Comparative study of hulled (einkorn, emmer and spelt) and naked wheats (durum and bread wheat): Agronomic performance and quality traits. Crop Sci. 2016, 56, 302–311. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Schweiggert, R.M.; Würschum, T.; Longin, C.F.H.; Carle, R. Lipophilic antioxidants in wheat (Triticum spp.): A target for breeding new varieties for future functional cereal products. J. Funct. Foods 2016, 20, 594–605. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steiner, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the irritable bowel syndrome–FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Leváková, L.; Lacko-Bartošová, M. Phenolic acids and antioxidant activity of wheat species: A review. Agriculture 2017, 63, 92–101. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Joniec, J. The antioxidant potential of grains in selected cereals grown in an organic and conventional system. Agriculture 2022, 12, 1485. [Google Scholar] [CrossRef]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Borczak, B.; Marek, M.; Sikora, E.; Dobosz, A.; Kapusta-Duch, J. Glycaemic index of white bread. Starch 2018, 70, 17022. [Google Scholar] [CrossRef]
- Schenk, S.; Davidson, C.J.; Zderic, T.W.; Byerley, L.O.; Coyle, E.F. Different glycemic indexes of breakfast cereals are not due to glucose entry into blood but to glucose removal by tissue. Am. J. Clin. Nutr. 2003, 78, 742–748. [Google Scholar] [CrossRef]
- Eelderink, C.; Moerdijk-Poortvliet, T.C.; Wang, H.; Schepers, M.; Preston, T.; Boer, T.; Vonk, R.J.; Schierbeek, H.; Priebe, M.G. The glycemic response does not reflect the in vivo starch digestibility of fiber-rich wheat products in healthy men. J. Nutr. 2012, 142, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Bonzi, P.; Vangsøe, C.T.; Nielsen, K.L.; Lærke, H.N.; Hedemann, M.S.; Knudsen, K.E.B. The relationship between In vitro and In vivo starch digestion kinetics of breads varying in dietary fibre. Foods 2020, 9, 1337. [Google Scholar] [CrossRef]
- Dodd, H.; Williams, S.; Brown, R.; Venn, B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index. Am. J. Clin. Nutr. 2011, 94, 992–996. [Google Scholar] [CrossRef]
- Vanhatalo, S.; Dall’Asta, M.; Cossu, M.; Chiavaroli, L.; Francinelli, V.; Pede, G.D.; Dodi, R.; Närväinen, J.; Antonini, M.; Goldoni, M.; et al. Pasta structure affects mastication, bolus properties, and postprandial glucose and insulin metabolism in healthy adults. J. Nutr. 2022, 152, 994–1005. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Serban, L.R.; Paucean, A.; Man, S.M.; Chis, M.S.; Muresan, V. Ancient wheat species: Biochemical profile and impact on sourdough bread characteristics—A review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Balk, J.; Connorton, J.M.; Wan, Y.; Lovegrove, A.; Moore, K.L.; Uauy, C.; Sharp, P.; Shewry, P.R. Improving wheat as a source of iron and zinc for global nutrition. Nutr. Bull. 2018, 44, 53–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).