Treatment with Supercritical CO2 Reduces Off-Flavour of White Alfalfa Protein Concentrate
Abstract
1. Introduction
2. Materials and Methods
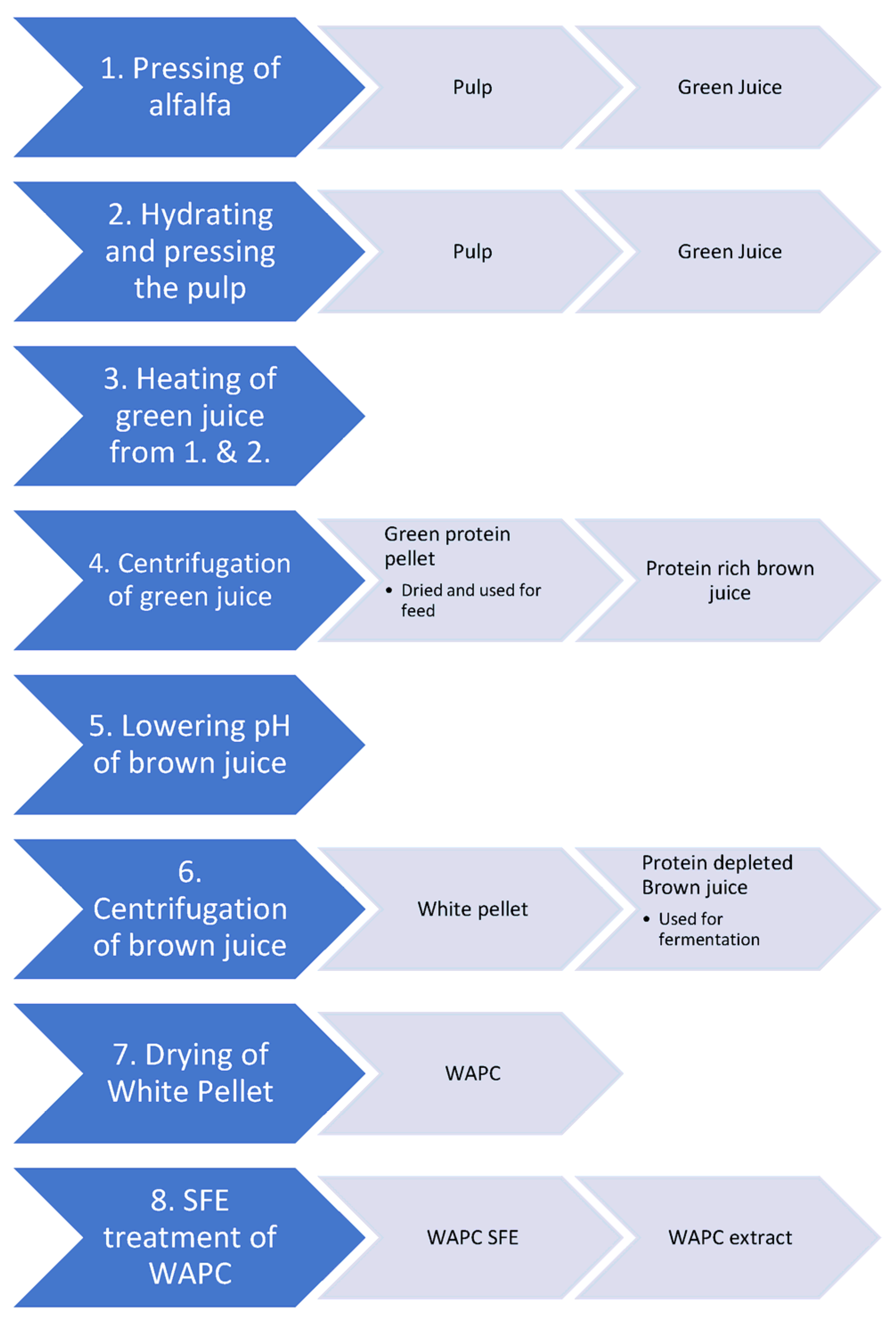
2.1. Production of White Protein Concentrate
2.2. SFE Treatment of WAPC
2.3. Moisture and Ash Content
2.4. L-a-b Colour Measurement
2.5. Total Fat Analysis
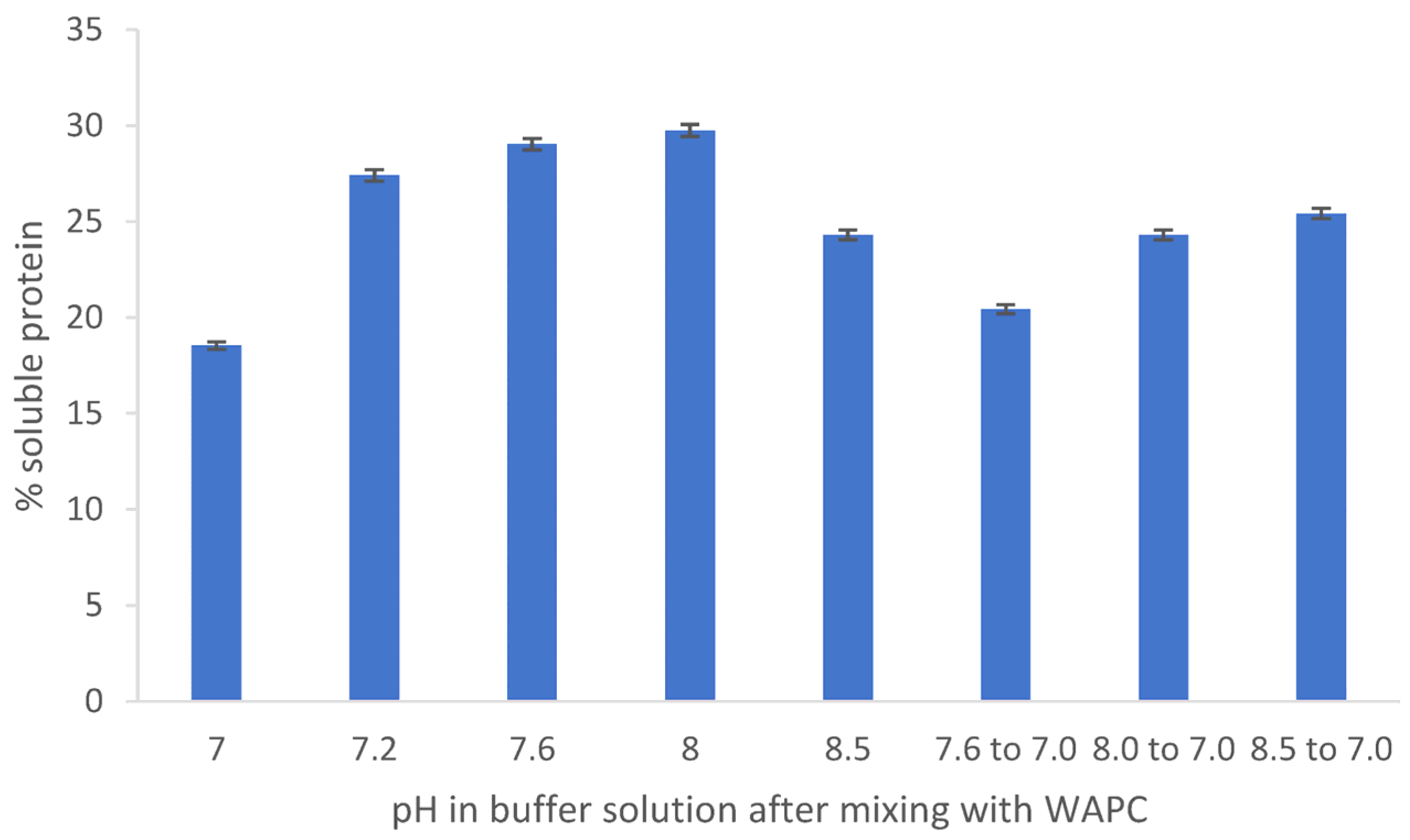
2.6. Effect of pH Change on Protein Solubility
2.7. SDS-PAGE
2.8. Protein Determination
2.9. Protein Digestibility
2.10. Foam Stability of WS and WSS Compared to Milk
2.11. Production of Meringues
2.12. Production of Chocolate Muffins
2.13. Texture Analysis of the Chocolate Muffins and Meringues
2.14. Sensory Analysis of the Meringues
2.15. Sensory Analysis of the Chocolate Muffins
2.16. Statiscal Analyses
3. Results
3.1. Yield and Protein Concentration of the WAPC Obtained from the Single and Twin Screw Presses
3.2. Solubility of the Proteins
3.3. Foam Stability of the Proteins
3.4. Texture Analysis of the Meringues
3.5. Sensory Analysis of Merringues
3.6. Application of WAPC in Chocolate Muffins
3.7. Sensory Analysis of WAPC in Muffins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
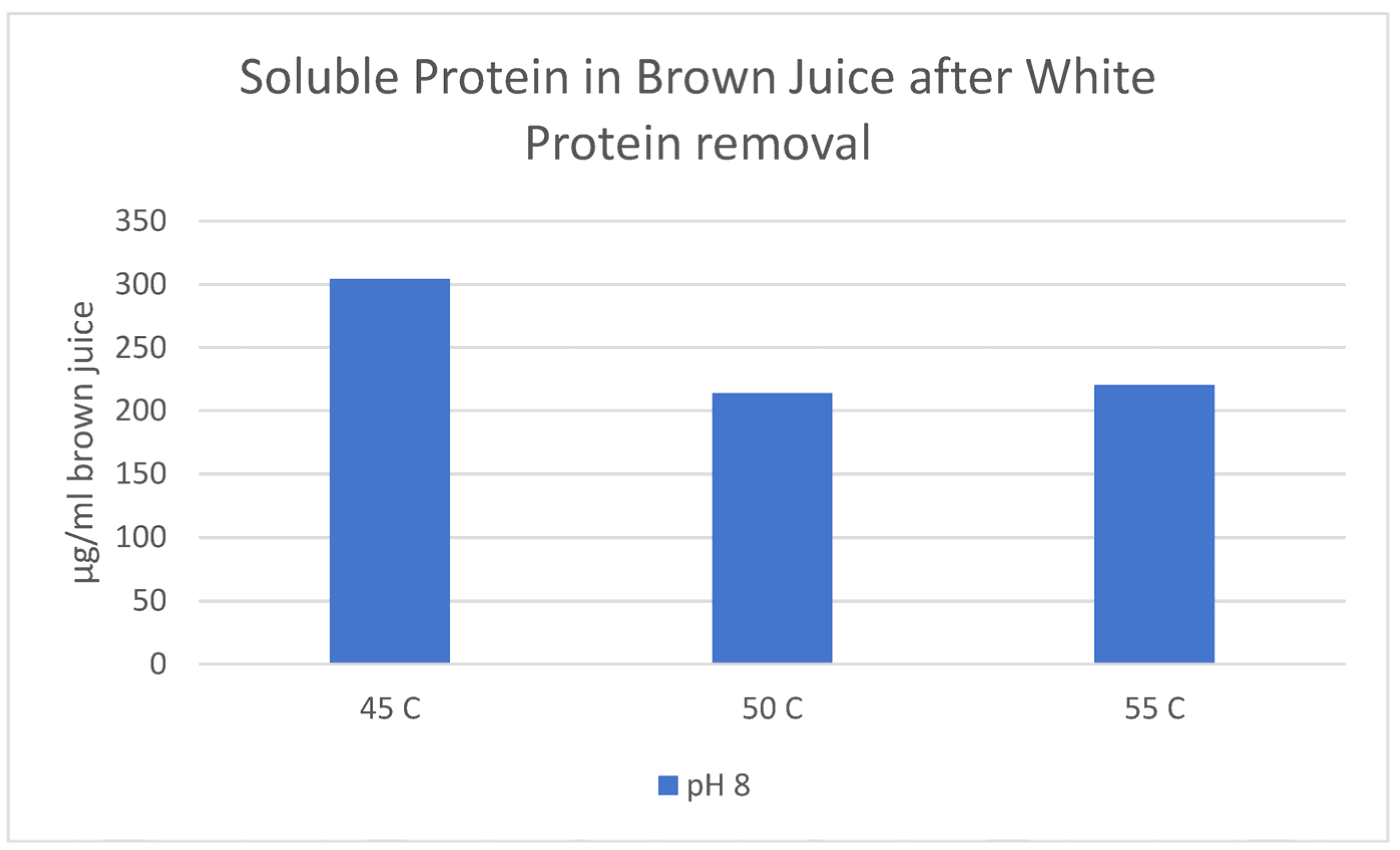
Appendix B
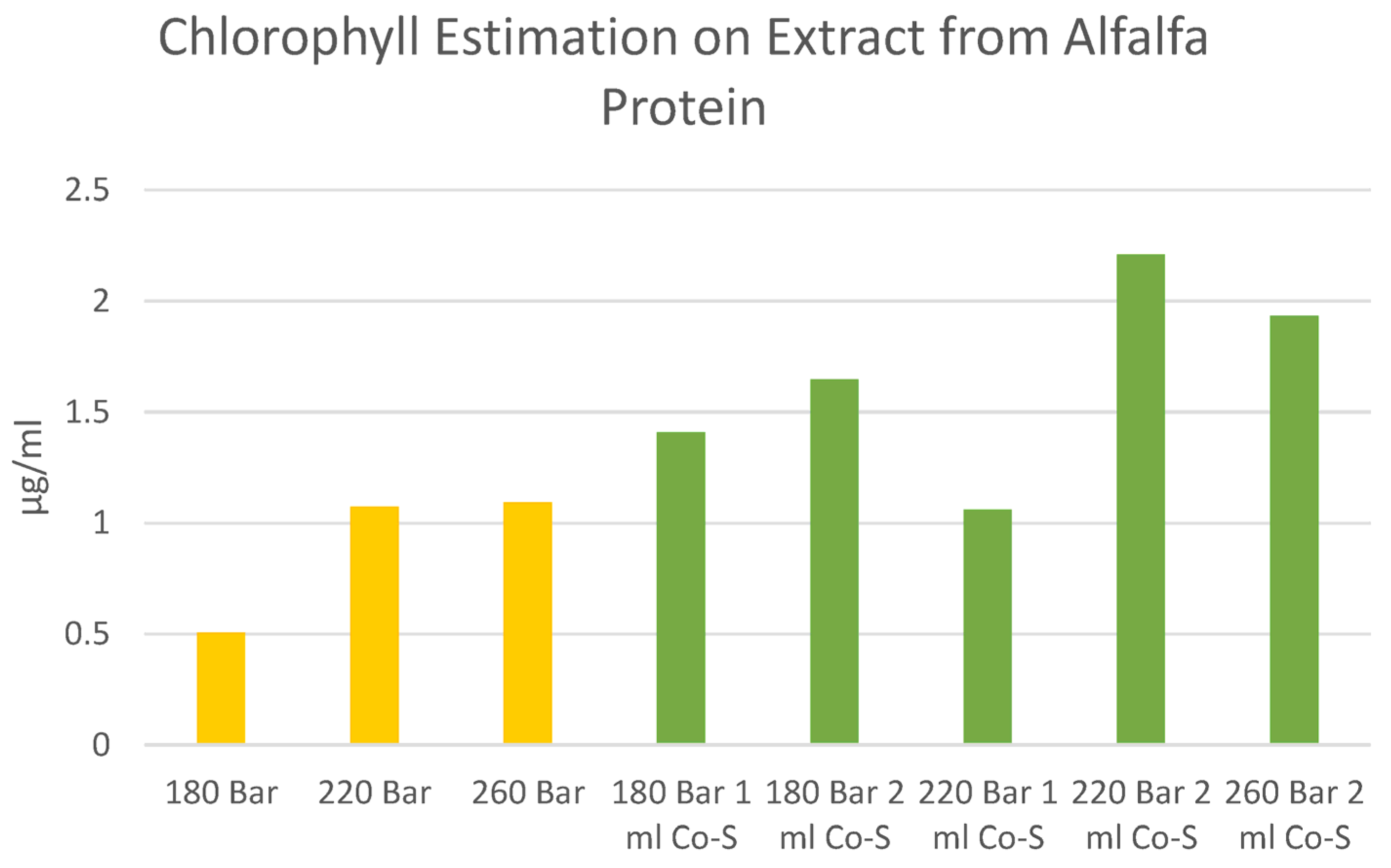
Appendix C
| Size (mm) | >1.25 | >1.00 | >0.50 | >0.25 | >0.125 | >0.00 |
| Percentage (%) | 0.00 | 6.62 | 12.79 | 46.04 | 21.25 | 13.30 |
References
- Commision Decision of 13 October 2009 Authorising the Placing on the Market of a Leaf Extract from Lucerne (Medicago sativa) as Novel Food or Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council; EFSA. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009D0826&from=EN (accessed on 2 April 2022).
- Ranganathan, J.; Vennard, D.; Waite, R.; Dumas, P.; Lipinski, B.; Searchinger, T. Shifting Diets toward a Sustainable Food Future; Creating a Sustainable Future; World Resources Institute: Washington, DC, USA, 2016; Volume 11. [Google Scholar]
- Santamaría-Fernández, M.; Schneider, R.; Lübeck, M.; Venus, J. Combining the production of L-lactic acid with the production of feed protein concentrates from alfalfa. J. Biotechnol. 2020, 323, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Colas, D.; Doumeng, C.; Pontalier, P.Y.; Rigal, L. Twin-screw extrusion technology, an original solution for the extraction of proteins from alfalfa (Medicago sativa). Food Bioprod. Process. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Heger, J. Standardized ileal digestibilities of amino acids and nitrogen in rye, barley, soybean meal, malt sprouts, sorghum, wheat germ and broken rice fed to growing pigs. Anim. Feed Sci. Technol. 2013, 186, 120–124. [Google Scholar] [CrossRef]
- Soy Meal Flour (Food ID: 154), The National Fooddatabase Version 4.1, 2022, The Natioanl Food Institute, The Technical University of Denmark. Available online: https://frida.fooddata.dk/food/154?lang=en (accessed on 27 April 2022).
- Edwards, R.H.; Miller, R.E.; de Fremery, D.; Knuckles, B.E.; Bickoff, E.M.; Kohler, G.O. Pilot Plant Production of an Edible White Fraction Leaf Protein Concentrate from Alfalfa. J. Agric. Food Chem. 1975, 23, 620–626. [Google Scholar] [CrossRef]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Weiss, W.P.; Conrad, H.R.; Shockey, W.L. Digestibility of Nitrogen in Heat-Damaged Alfalfa. J. Dairy Sci. 1986, 69, 2658–2670. [Google Scholar] [CrossRef]
- Saunders, R.M.; Connor, M.A.; Booth, A.N.; Bickoff, E.M.; Kohler, G.O. Measurement of digestibility of alfalfa protein concentrates by in vivo and in vitro methods. J. Nutr. 1973, 103, 530–535. [Google Scholar] [CrossRef]
- Mielmann, A. The utilisation of lucerne: A review. Br. Food J. 2013, 115, 590–600. [Google Scholar] [CrossRef]
- Atumo, T.T.; Kauffman, R.; Gemiyo Talore, D.; Abera, M.; Tesfaye, T.; Tunkala, B.Z.; Zeleke, M.; Kebede Kalsa, G. Adaptability, forage yield and nutritional quality of alfalfa (Medicago sativa) genotypes. Sustain. Environ. 2021, 7, 1895475. [Google Scholar] [CrossRef]
- Kavut, Y.T.; Avicioglu, R. Yield and Quality Performances of Various Alfalfa (Medicago sativa L.) Cultivars in Different Soil Textures in a Mediterranean Enviroment. Turk. J. Field Crops 2015, 20, 65–71. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Kovacs, S.; Domokos-Szabolcsy, É.; Bákonyi, N.; Fari, M.; Geilfus, C.M. Sustainable biorefinery of alfalfa (Medicago sativa L.): A review. Egypt. J. Bot. 2020, 60, 621–639. [Google Scholar] [CrossRef]
- Knuckles, B.E.; Bickoff, E.M.; Kohler, G.O. Pro-Xan Process: Methods for Increasing Protein Recovery from Alfalfa. J. Agric. Food Chem. 1972, 20, 1055–1057. [Google Scholar] [CrossRef]
- Zhou, F.; Hansen, M.; Hobley, T.J.; Jensen, P.R. Valorization of Green Biomass: Alfalfa Pulp as a Substrate for Oyster Mushroom Cultivation. Foods 2022, 11, 2519. [Google Scholar] [CrossRef]
- Hansen, M.; Andersen, C.A.; Jensen, P.R.; Hobley, T.J. Scale-Up of Alfalfa (Medicago sativa) Protein Recovery Using Screw Presses. Foods 2022, 11, 3229. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Singh, B.; Bobade, H. Cereal bar functionalised with non-conventional alfalfa and dhaincha protein isolates: Quality characteristics, nutritional composition and antioxidant activity. J. Food Sci. Technol. 2022, 59, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Rocchetti, G.; Sigolo, S.; Fortunati, P.; Lucini, L.; Gallo, A. Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: Nutritional, antioxidant and quality characteristics. Food Chem. 2018, 239, 679–687. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Stochmal, A.; Cieslak, A.; Kozłowska, M.; Kuznicki, D.; Kowalczyk, M.; Oleszek, W. Structural and quantitative changes of saponins in fresh alfalfa compared to alfalfa silage. J. Sci. Food Agric. 2019, 99, 2243–2250. [Google Scholar] [CrossRef]
- Kalač, P.; Price, K.R.; Fenwick, G.R. Changes in saponin content and composition during the ensilage of alfalfa (Medicago sativa L.). Food Chem. 1996, 56, 377–380. [Google Scholar] [CrossRef]
- Oleszek, W.; Jurzysta, M.; Ploszynski, M.; Colquhoun, I.J.; Price, K.R.; Fenwick, G.R. Zahnic Acid Tridesmoside and Other Dominant Saponins from Alfalfa (Medicago sativa L.) Aerial Parts. J. Agric. Food Chem. 1992, 40, 191–196. [Google Scholar] [CrossRef]
- Krakowska, A.; Rafińska, K.; Walczak, J.; Kowalkowski, T.; Buszewski, B. Comparison of Various Extraction Techniques of Medicago sativa: Yield, Antioxidant Activity, and Content of Phytochemical Constituents. J. AOAC Int. 2017, 100, 1681–1693. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Gong, X.F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT-Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Kerfai, S.; Fernández, A.; Mathé, S.; Alfenore, S.; Arlabosse, P. Production of green juice with an intensive thermo-mechanical fractionation process. Part II: Effect of processing conditions on the liquid fraction properties. Chem. Eng. J. 2011, 167, 132–139. [Google Scholar] [CrossRef]
- Tanambell, H.; Møller, A.H.; Corredig, M.; Dalsgaard, T.K. RuBisCO from alfalfa—Native subunits preservation through sodium sulfite addition and reduced solubility after acid precipitation followed by freeze-drying. LWT 2022, 154, 112682. [Google Scholar] [CrossRef]
- Firdaous, L.; Fertin, B.; Khelissa, O.; Dhainaut, M.; Nedjar, N.; Chataigné, G.; Ouhoud, L.; Lutin, F.; Dhulster, P. Adsorptive removal of polyphenols from an alfalfa white proteins concentrate: Adsorbent screening, adsorption kinetics and equilibrium study. Sep. Purif. Technol. 2017, 178, 29–39. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Coaquira-Quispe, J.J.; Angela, M.; Meireles, A. Supercritical fluid extraction of polyphenols from lees: Overall extraction curve, kinetic data and composition of the extracts. Bioresour. Bioprocess 2015, 2, 45. [Google Scholar] [CrossRef]
- Mouahid, A.; Seengeon, K.; Martino, M.; Crampon, C.; Kramer, A.; Badens, E. Selective extraction of neutral lipids and pigments from Nannochloropsis salina and Nannochloropsis maritima using supercritical CO2 extraction: Effects of process parameters and pre-treatment. J. Supercrit. Fluids 2020, 165, 104934. [Google Scholar] [CrossRef]
- Applied Biosystems Pierce Coomasie Plus Bradford Protocol. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0011344_CoomassiePlus_Bradford_Asy_Reag_UG.pdf (accessed on 28 August 2022).
- DTU Food Frida—Fødevare ID: 1664. Available online: https://frida.fooddata.dk/food/1664? (accessed on 1 August 2022).
- Wrona, O.; Rafińska, K.; Walczak-Skierska, J.; Mozeński, C.; Buszewski, B. Extraction and Determination of Polar Bioactive Compounds from Alfalfa (Medicago sativa L.) Using Supercritical Techniques. Molecules 2019, 24, 4608. [Google Scholar] [CrossRef]
- Murador, D.C.; Salafia, F.; Zoccali, M.; Martins, P.L.G.; Ferreira, A.G.; Dugo, P.; Mondello, L.; de Rosso, V.V.; Giuffrida, D. Green extraction approaches for carotenoids and esters: Characterization of native composition from orange peel. Antioxidants 2019, 8, 613. [Google Scholar] [CrossRef]
- Emilia Nagybákay, N.; Syrpas, M.; Vilimait, V.; Tamkut, L.; Pukalskas, A.; Rimantas Venskutonis, P.; Kitryt, V.; González-Álvarez, J. Optimized Supercritical CO2 Extraction Enhances the Recovery of Valuable Lipophilic Antioxidants and Other Constituents from Dual-Purpose Hop (Humulus lupulus L.) Variety Ella. Antioxidants 2021, 10, 918. [Google Scholar] [CrossRef]
- Palmer, L.S. Xanthophyll, the principal natural yellow pigment of the egg yolk, body fat, and blood serum of the hen. The physiological relation of the pigment to the xanthophyll of plants. J. Biol. Chem. 1915, 23, 261–279. [Google Scholar] [CrossRef]
- Howarth, R.E.; Sarkar, S.K.; Fesser, A.C.; Schnarr, G.W. Some Properties of Soluble Proteins from Alfalfa (Medicago sativa) Herbage and Their Possible Relationship to Ruminant Bloat. J. Agric. Food Chem. 1977, 25, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Sheen, S.J. Comparison of Chemical and Functional Properties of Soluble Leaf Proteins from Four Plant Speciest. J. Agric. Food Chem. 1991, 39, 681–685. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, composition, and functional properties of dried alfalfa (Medicago sativa L.) leaf protein. J. Sci. Food Agric. 2017, 97, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Livingston, A.L.; Knuckles, B.E.; Edwards, R.H.; de Fremery, D.; Miller, R.E.; Kohler, G.O. Distribution of Saponin in Alfalfa Protein Recovery Systems. J. Agric. Food Chem. 1979, 27, 362–365. [Google Scholar] [CrossRef]
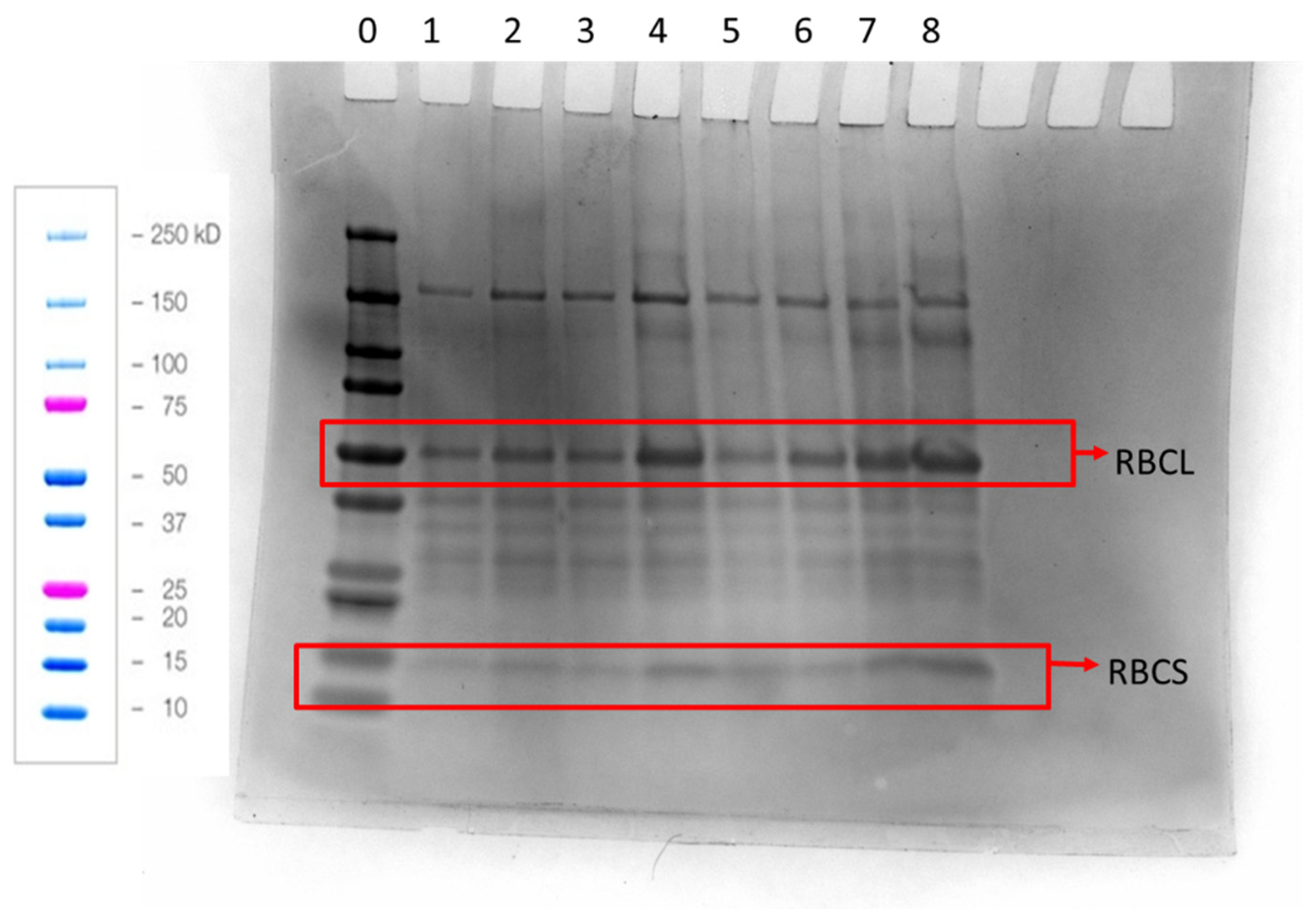
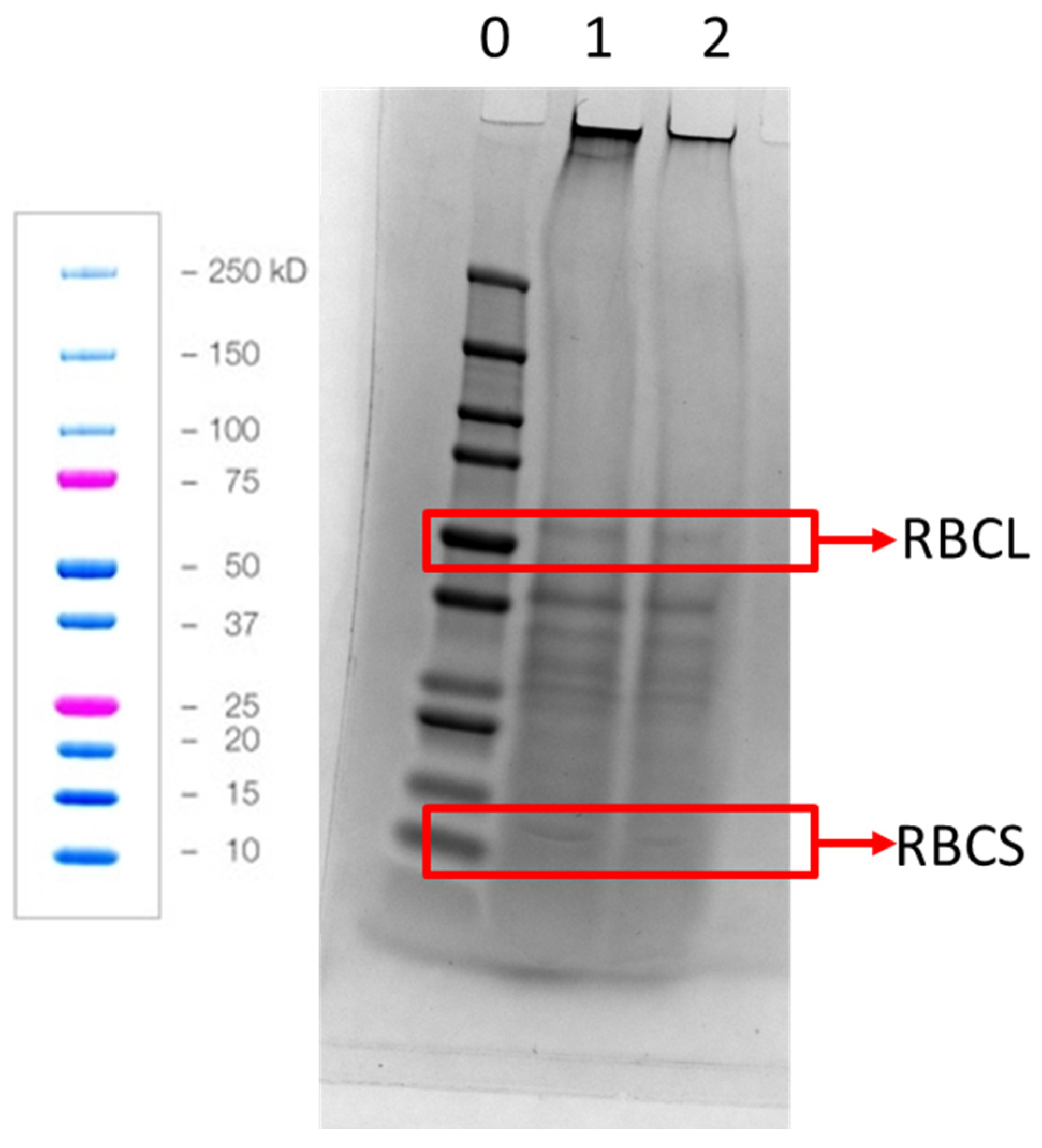
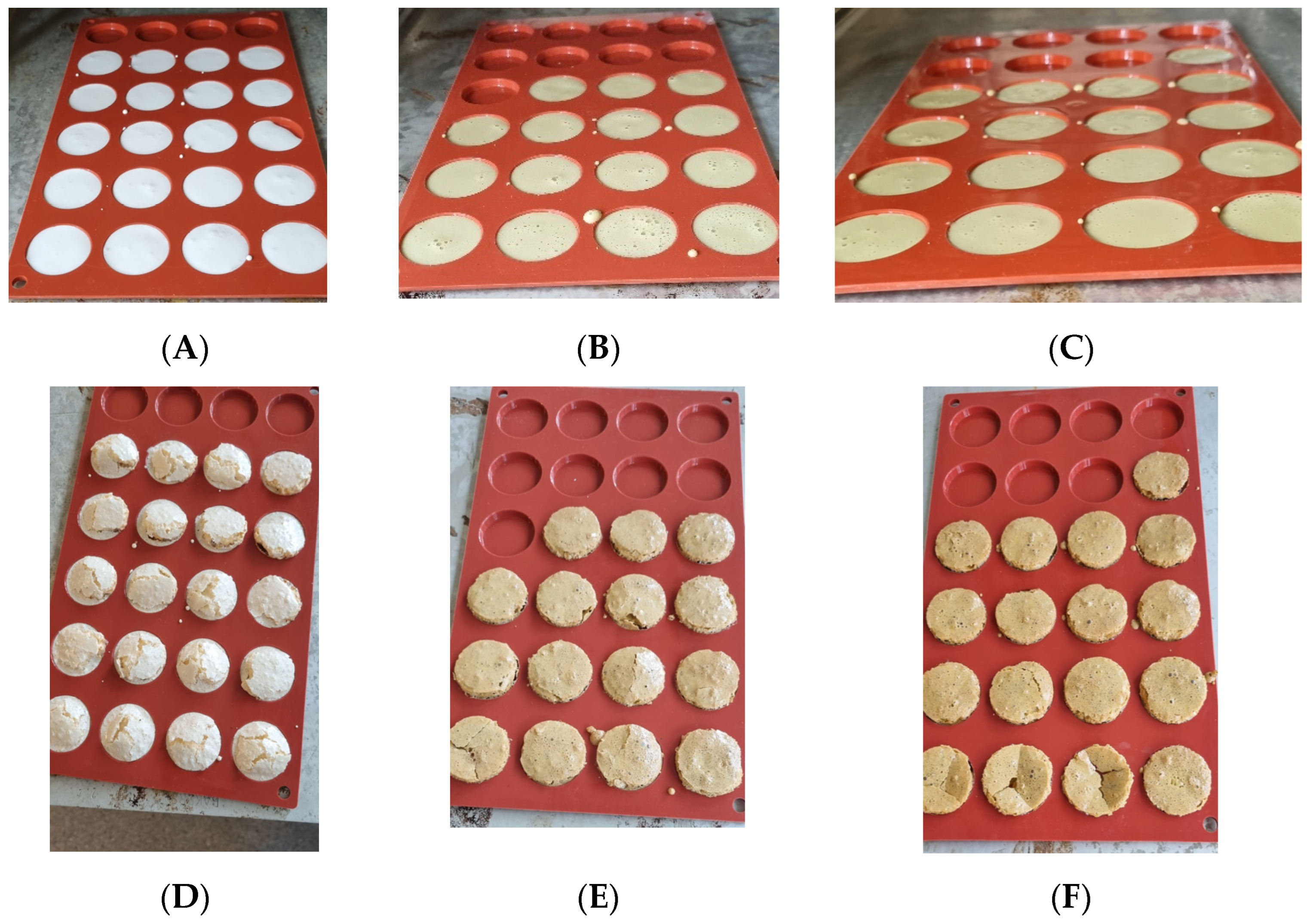
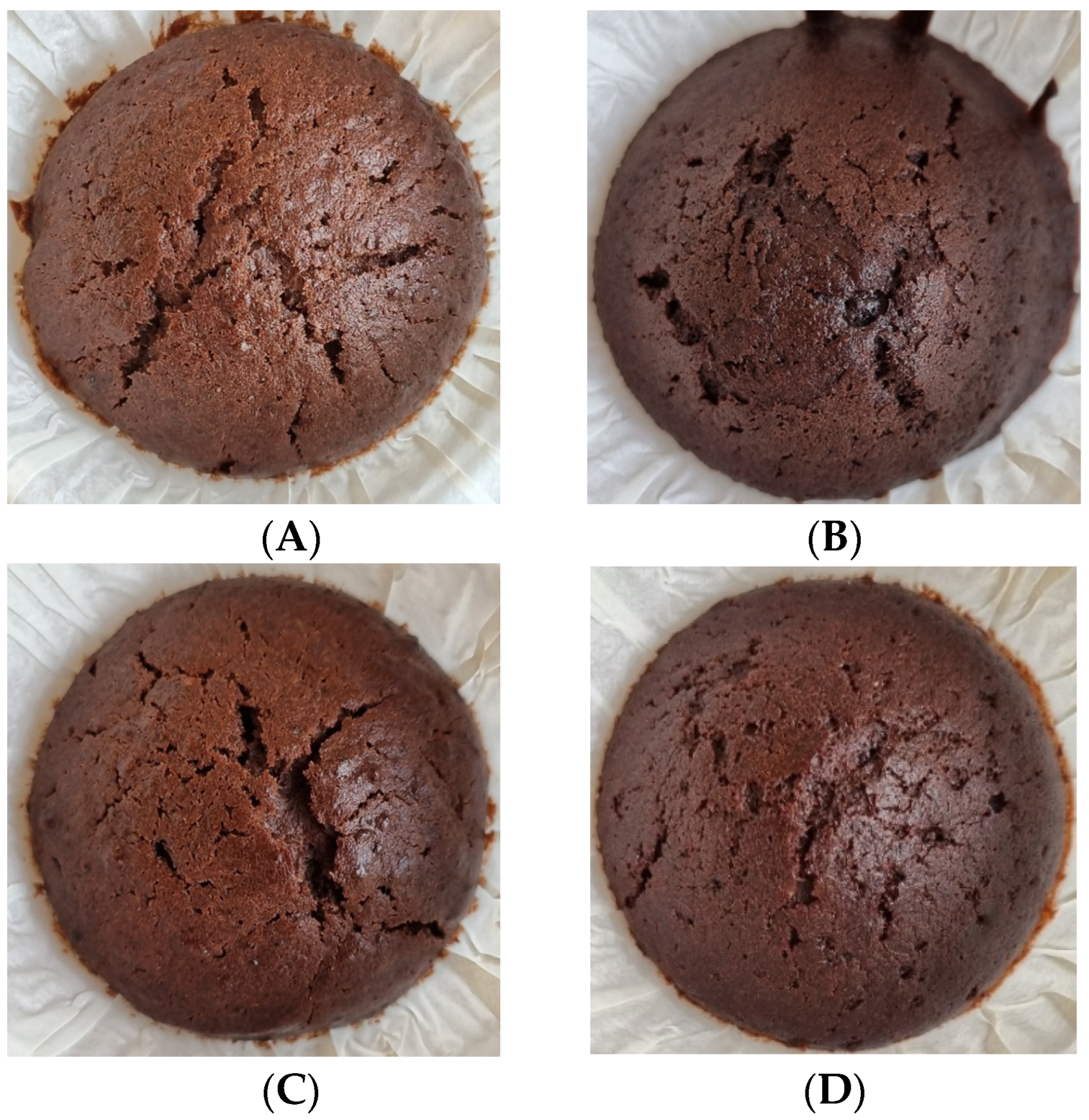
| Component [Reference] | APC [1] | Welpro (WAPC) [7] | SMF [5,6] | SPI [8] |
|---|---|---|---|---|
| Protein (% DM) | 45–60 | 88.7 | 40.7 b–49 a | 90.5–92.2 |
| Protein digestibility (%) | 63.6 [9] | 91.7 [10] | 86 a | 94.4–97.8 |
| Fat (% DM) | 9–11 | <0.5 | 22.2 b | 0.2–1.2 |
| Fibre (% DM) | 11–15 | <0.5 | 10.4 b | - |
| Treated with Supercritical CO2 | Type of Press | |
|---|---|---|
| Single Screw | Twin Screw | |
| Not treated | WS | WT |
| Treated | WSS | WTS |
| Component | WT | WTS |
|---|---|---|
| Protein(% DM) | 57.00 ± 0.44 | 64.56 ± 0.43 |
| Dry matter (%) | 93.83 ± 0.69 | 90.85 ± 1.26 |
| Fat (% DM) | 0.12 ± 0.16 | 0.00 ± 0.00 |
| L-a-b colour | 82.0, −6.9, 17.5 | 80.6, −6.9, 18.3 |
| Component | Alfalfa | WS | WSS |
|---|---|---|---|
| Protein (% DM) | 20.90 ± 2.42 | 57.73 ± 0.84 | 63.61 ± 0.07 |
| Protein Digestibility (% DM) | NA | 93.28 ± 0.89 | 92.65 ± 0.64 |
| Soluble Protein (% of protein) | NA | 14.98 ± 0.24 | 15.80 ± 0.42 |
| Dry matter (%) | 20.621 | 100 ± 0.00 | 99.8 ± 0.00 |
| Ash (% DM) | 8.61 ± 1.02 | 0.91 ± 0.14 | Not determined |
| Fat (% DM) | 1.22 ± 0.00 | 0.23 ± 0.25 | 0.00 ± 0.00 |
| L-a-b colour | NA | 63.6, 0.7, 26.5 | 61.3, 3.0, 22.0 |
| Sample | Force (N) | Foam Height (mm) |
|---|---|---|
| Milk | 0.061 | 20.49 |
| WS | 0.256 | 38.766 |
| WSS | 0.251 | 34.117 |
| Protein Source | Height (mm) | Hardness (N) |
|---|---|---|
| Egg white | 15.6 ± 0.6 | 27.3 ± 0.2 |
| WT | 13.0 ± 0.6 | 8.1 ± 0.3 |
| WTS | 12.8 ± 0.6 | 26.5 ± 3.56 |
| Type of Meringue | No Grass | Mild Grass | Strong Grass |
|---|---|---|---|
| Egg white | 19 | 0 | 0 |
| WT | 0 | 0 | 19 |
| WTS | 0 | 18 | 1 |
| Type of Muffin | Hardness (N) | Chewiness (N) | Springiness (%) | Height (mm) |
|---|---|---|---|---|
| Muffin control | 229.18 ± 34.48 | 41.83 ± 9.35 | 36.09 ± 2.91 | 23.76 ± 0.92 |
| Std. Muffin w. Egg | 135.42 ± 32.16 | 38.04 ± 12.47 | 58.57 ± 3.46 | 29.43 ± 2.43 |
| Muffin w. WS | 217.56 ± 65.34 | 34.05 ± 15.54 | 36.45 ± 5.13 | 28.13 ± 3.16 |
| Muffin w. WSS | 239.89 ± 47.53 | 36.03 ± 10.55 | 35.80 ± 2.49 | 27.02 ± 3.14 |
| Type of Muffin/Taste | No Grass | Mild Grass | Strong Grass |
|---|---|---|---|
| Muffin control | 11 | 0 | 0 |
| Std. Muffin w. Egg | 11 | 0 | 0 |
| Muffin w. WS | 0 | 0 | 11 |
| Muffin w. WSS | 0 | 11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, M.; Hobley, T.J.; Jensen, P.R. Treatment with Supercritical CO2 Reduces Off-Flavour of White Alfalfa Protein Concentrate. Foods 2023, 12, 845. https://doi.org/10.3390/foods12040845
Hansen M, Hobley TJ, Jensen PR. Treatment with Supercritical CO2 Reduces Off-Flavour of White Alfalfa Protein Concentrate. Foods. 2023; 12(4):845. https://doi.org/10.3390/foods12040845
Chicago/Turabian StyleHansen, Mikkel, Timothy John Hobley, and Peter Ruhdal Jensen. 2023. "Treatment with Supercritical CO2 Reduces Off-Flavour of White Alfalfa Protein Concentrate" Foods 12, no. 4: 845. https://doi.org/10.3390/foods12040845
APA StyleHansen, M., Hobley, T. J., & Jensen, P. R. (2023). Treatment with Supercritical CO2 Reduces Off-Flavour of White Alfalfa Protein Concentrate. Foods, 12(4), 845. https://doi.org/10.3390/foods12040845





