Abstract
Leisure dried tofu (LD-tofu) was prepared using two different marinating processes: the repeated heating method (RHM) and the vacuum pulse method (VPM). The quality characteristics and bacterial community succession of LD-tofu and the marinade were evaluated. The results showed that the nutrients in LD-tofu were easily dissolved into the marinade during the marinating process, while the protein and moisture content of RHM LD-tofu changed most dramatically. With the increase in marinade recycling times, the springiness, chewiness and hardness of VPM LD-tofu increased significantly. The total viable count (TVC) of the VPM LD-tofu decreased from the initial value of 4.41 lg cfu/g to 2.51–2.67 lg cfu/g as a result of the marinating process, which had a significant inhibitory effect. Additionally, 26, 167 and 356 communities in the LD-tofu and marinade were detected at the phylum, family and genus levels, respectively. Pearson correlation analysis showed that Pseudomonadaceae, Thermaceae and Lactobacillaceae were closely related to the quality characteristics of LD-tofu, whereas Caulobacteriaceae, Bacillaceae and Enterobacteriae were closely related to the marinade. The present work provides a theoretical basis for the screening of functional strains and quality control in LD-tofu and marinade.
1. Introduction
Leisure dried tofu (LD-tofu) is a reprocessed soybean product made from tofu through drying, marinating, sterilisation and other processes [1,2,3]. The LD-tofu retains the original nutritional composition of tofu and is popular with consumers due to its unique flavour. Hence, LD-tofu has become a typical representative of leisure food, which has promoted the rapid expansion of the LD-tofu market [1,4].
The quality characteristics of LD-tofu are the core element of its grading, and monitoring the changes in LD-tofu quality is one of the key aspects for the healthy growth of the industry [1,4]. The marinating process involves the repeated heating of the food in the pre-cooked marinade (cooked from spices, rapeseed oil, pig bones and other materials), which is the critical control point affecting the quality of LD-tofu [3]. The texture and quality of tofu and its products could change as a result of nonstandard processes or inaccurate parameters, leading to serious financial losses [5,6,7]. In order to reduce production costs, producers often use deteriorated raw materials or recycled marinade (even hundreds of times) to make LD-tofu, which can readily cause microbial contamination [8,9]. Given the drawbacks of traditional marinating processes, our team developed a vacuum pulse marinating process and supporting equipment, which could shorten the marinating time and improve the flavour as well as colour of LD-tofu [1]. However, the changes in the quality and microbial diversity of the LD-tofu during this marinating process are unclear.
Controlling the microbial community is a key factor in food quality and safety control [10,11]. The complex process in tofu and tofu product production often utilizes fermented soybean whey as a coagulant, resulting in complex and diverse microbial communities [12,13]. In addition, LD-tofu has rich nutrition and high water activity, which is suitable for the reproduction and metabolism of microorganisms [4]. Even though the microbial characteristics and specific spoilage microorganisms of fresh soybean products have been studied [14,15], it is still unclear how the bacterial communities in LD-tofu and marinade progress under marinade recycling. Therefore, clarifying the correlation between the succession of bacterial communities and the quality changes in LD-tofu during the marinating process, as well as identifying the key bacterial communities, is a new strategy to avoid quality deterioration and achieve safety control in LD-tofu production.
To evaluate the effect of the marinating process on the quality characteristics and bacterial community of LD-tofu, LD-tofu was prepared by using repeatedly recycled marinade (0, 30, 60, 90, 120, 150 cycles) via the repeated heating method (RHM) and vacuum pulse method (VPM) in this study. The changes in the nutritional composition, texture characteristics and total viable count (TVC) of the LD-tofu and marinade were determined by conventional physicochemical methods and a texture analyser. The bacterial community succession in the LD-tofu and marinade was analysed by high-throughput sequencing. The correlation between the target microorganisms and the quality characteristics was determined. The purpose of this work was to provide a theoretical basis for the sterilisation and quality control of LD-tofu while supporting the safe and efficient production of marinated leisure food.
2. Materials and Methods
2.1. Sample Preparation and Collection
The LD-tofu (8 × 3 × 1.5 cm, 25.00 ± 0.50 g) were obtained from different marinating processes according to Figure 1 [1]. Six groups—0 (control), 30, 60, 90, 120 and 150—were created according to the number of marinade recycling cycles. To produce LD-tofu, dried tofu (10 pieces/time) was marinated by RHM (90 °C, 120 min) in a cooking pot and VPM (80 °C, 80 min; vacuum: 0.03 MPa; no. of pulses: 3) using vacuum pulse equipment (Kangdeli Machinery Equipment Manufacturing Co., Ltd., China). The ratio of dried tofu and marinade was 1:40 (g/mL). A marinade sample (100 mL) was taken from each group after the marinating process to determine the nutritional components, textural properties and TVC. The fresh marinade was added for the next marinating process according to the volume. LD-tofu was dried in a dryer at 85 °C for 30 min and after cooling the samples were collected for analysis. Additionally, LD-tofu and marinade samples were analysed for bacterial community composition during the early (50), middle (100) and late (150) stages.
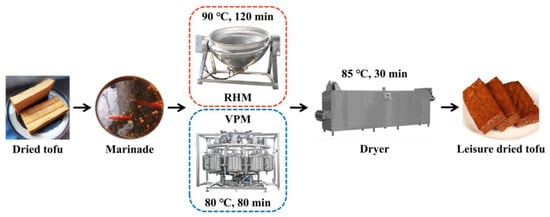
Figure 1.
Marinating process flow chart of LD-tofu by RHM and VPM.
2.2. Chemical Analysis
The moisture, protein and fat contents of LD-tofu were determined by using direct drying method, Kjeldahl method and ether extraction method, respectively. Additionally, the protein conversion factor was 6.25 [16].
2.3. Textural Properties
The hardness, chewiness and springiness of LD-tofu were measured at four corners and the centre of the LD-tofu using a Texture Analyzer (LS-5, AMETEK, Berwyn, PA, USA) [17]. The following are the main parameters: P/0.5 cylindrical flat bottom probe; triggering force of 0.05 N; sample flat surface height of 20 mm; test front, middle and final speeds of 2.0 mm/s, 1.0 mm/s and 1.0 mm/s; acquisition rate of 50.0 PPS (pulses per second).
2.4. Total Viable Counts
Five grams of LD-tofu were homogenized with sterilized saline solution (0.85%, 45 mL) for 3 min (10,000 r/min). All homogenates were diluted ten-fold serially using saline (0.85%). Then, the diluted sample (0.1 mL) was spread onto plate count agar (Beijing Aoboxing Bio-tech Co., Ltd., Beijing, China) and incubated at 37 °C for 48 h to determine the TVC [18].
2.5. DNA Extraction and PCR Amplification
Microbial DNA was extracted using the HiPure Soil DNA Kit (Magen, Guangzhou, China) according to the manufacturer’s protocols [19]. The 16S rDNA V3–V4 region of the ribosomal RNA was amplified by PCR (95 °C for 2 min, followed by 27 cycles at 98 °C for 10 s, 62 °C for 30 s and 68 °C for 30 s, then a final extension at 68 °C for 10 min) using primers 341F: CCTACGGGNGGCWGCAG and 806R: GGACTACHVGGGTATCTAAT, where the barcode was an eight-base sequence unique to each sample. The PCR reactions were performed in triplicate using a 50 μL mixture containing KOD Buffer (10×, 5 μL), dNTPs (2.5 mM, 5 μL), primers (5 μM, 1.5 μL of each), KOD polymerase (1 μL) and template DNA (100 ng).
2.6. Illumina HiSeq Sequencing
Amplicons were extracted from 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using the ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA, USA). Purified amplicons were pooled equimolarly and paired-end sequenced (2 × 250 bp) on an Illumina platform according to the standard protocols [19].
2.7. Analysis of Bacterial Community Structure
To obtain high-quality clean reads, raw reads were filtered according to the following rules using FASTP (https://github.com/OpenGene/fastp; accessed on 24 March 2021). Then, paired-end clean reads were merged as raw tags using FLSAH (version 1.2.11) with a minimum overlap of 10 bp and a mismatch error rate of 2%. Noisy sequences of raw tags were filtered by the QIIME (version 1.9.1) pipeline to obtain high-quality clean tags. All chimeric tags were removed to obtain effective tags for further analysis. The effective tags were clustered into operational taxonomic units (OTUs) with ≥97% similarity using the UPARSE pipeline. The tag sequence with the highest abundance was selected as the representative sequence within each cluster. The α-diversity indexes (Shannon, Simpson, Chao1 and ACE) and β-diversity index (principal component analysis (PCA)) were calculated by QIIME.
2.8. Statistical Analysis
Each measurement was repeated three times, and the results were expressed as the mean ± standard deviation (SD). SPSS 22.0 software (IBM, Chicago, IL, USA) and Origin 9.1 software (OriginLab, Northampton, MA, USA) were used for data analysis. ANOVA and Duncan’s multiple range test (p < 0.05) were used to determine the statistical difference between the groups.
3. Results and Discussion
3.1. Effects of Different Marinating Processes and Different Marinade Recycling Cycles on the Basic Nutrients in LD-tofu
The changes in the basic nutrients of the LD-tofu and marinade under different marinating processes and different marinade recycling cycles are shown in Figure 2. With the increase in marinade recycling cycles, the protein content of the RHM LD-tofu and VPM LD-tofu decreased at 0–30 recycling cycles and increased at 30–150 recycling cycles. Moreover, the protein content of the RHM LD-tofu changed more significantly than that of VPM LD-tofu. The fat content of the RHM LD-tofu and VPM LD-tofu decreased significantly with the decline in marinade recycling cycles (p < 0.05), from 15.03 g/100 g to 13.07 g/100 g and 14.30 g/100 g, respectively. With the increase in marinade recycling cycles (p < 0.05), the moisture content of the VPM LD-tofu changed marginally, while the moisture content of the RHM LD-tofu significantly decreased, reaching 54.07 g/100 g after 150 recycling cycles. The decrease in LD-tofu fat and protein content could be attributed to the fact that the soluble protein and fatty acid in the LD-tofu gradually dissolved or hydrolysed during the marinating process. Another reason could be that the protein oxidative denaturation was induced by peroxide and free radicals produced by the lipid oxidation of the LD-tofu under the high-temperature marinating process [20,21]. The LD-tofu is a highly gelatinous product that forms by protein–protein and protein–water interactions [22,23]. Therefore, the oxidative denaturation of LD-tofu protein is bound to destroy the protein gel structure, resulting in a reduction in its water-holding capacity (WHC) and water content [24]. Due to the higher temperature of the RHM process and the open environment, the moisture content of the RHM LD-tofu decreased significantly compared with the VPM groups. The above results also confirmed that there was a direct relationship between lipid oxidation, protein oxidation and the WHC [25,26].
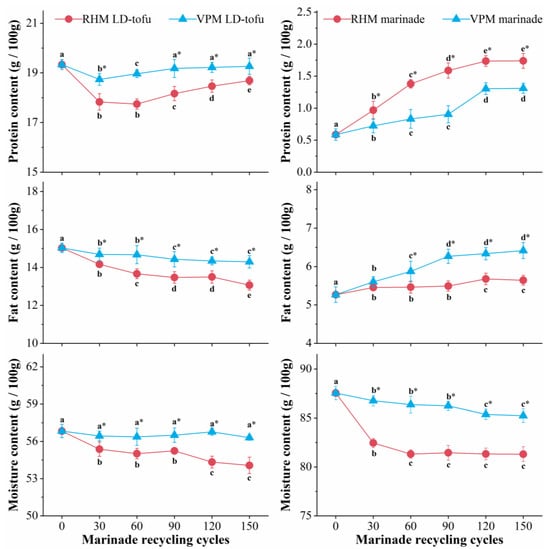
Figure 2.
Effects of marinating method and marinade recycling cycles on the basic nutrients of LD-tofu and marinade. Superscripts with different letters in a row are significantly different between groups (p < 0.05). * indicates a significant difference between RHM groups and VPM groups (p < 0.05).
With the increase in marinade recycling cycles, the moisture content of the marinade in the VPM groups decreased marginally and significantly decreased (p < 0.05) in the RHM groups from the initial value of 87.54 g/100 g to 81.31 g/100 g. This was mainly due to the evaporation of water caused by the open, high-temperature marinating process. With the increase in marinade recycling cycles, the marinade protein and fat content in the RHM and VPM groups significantly increased (p < 0.05). At 150 recycling cycles, the protein content of VPM marinade and RHM marinade was 1.74 g/100 g and 1.31 g/100 g, while the fat content was 6.42 g/100 g and 5.64 g/100 g, respectively. This could be attributed to the dissolution of nutrients from the LD-tofu and the reduction in the moisture content of the marinade. It is interesting to note that while the protein and moisture content of the marinade in the RHM groups increased more than in the VPM groups, the fat content increased less than in the VPM groups. This phenomenon may have been caused primarily by the difference in marinating temperatures between the RHM and VPM, which resulted in the difference in the degree of water evaporation and fat oxidation in the marinade. Additionally, the low-fat content of the marinade in the RHM groups was attributed to the floating oil droplets, which were produced in the upper layer of the marinade under VPM processing and were required to be removed before the initiation of a new marinating process.
3.2. Effects of Different Marinating Processes and Different Marinade Recycling Cycles on the Textural Characteristics of LD-tofu
Hardness, chewiness and springiness are the most commonly used indicators to evaluate the textural characteristics of LD-tofu. Hardness refers to the force required to deform the food to a certain extent, chewiness refers to the energy required to chew the solid sample for swallowing, and springiness refers to the ability of the food to quickly recover its original shape following the application of a force [22,27]. The springiness, chewiness and hardness of the LD-tofu in the RHM and VPM groups were measured by a texture analyser in this study (Table 1). With the increase in the marinade recycling cycles, the hardness and chewiness of the LD-tofu in the RHM and VPM groups first marginally decreased and then significantly increased. Additionally, the hardness and chewiness of the LD-tofu in the VPM groups were significantly higher than in the RHM groups (p < 0.05). The changes in the hardness and chewiness of the LD-tofu were consistent with the changes in protein content but opposite to those in water content. These results indicated that the protein and water contents of the LD-tofu were closely related to hardness and chewiness, which was consistent with the results of Bu et al. [4]. This was primarily attributed to the denaturation of LD-tofu protein and the decline in water content, which ultimately increased the density of the protein gel and enhanced the hardness as well as chewiness of the LD-tofu.

Table 1.
Effects of different marinating methods and marinade recycling cycles on the textural characteristics of LD-tofu.
Additionally, with the increase in marinade recycling cycles, the springiness of the RHM LD-tofu and VPM LD-tofu increased marginally. High-quality LD-tofu has good springiness, a good taste and soft texture [4,28]. These results showed that the VPM LD-tofu had better gel properties and textural characteristics than the RHM LD-tofu.
3.3. Effects of Different Marinating Processes and Different Marinade Recycling Cycles on the TVC of LD-tofu
The TVC is a core index for monitoring food quality and safety, and is related to the raw materials, processing technology and storage conditions [18,29]. The TVC of the LD-tofu and marinade under different marinating processes and marinade recycling cycles was determined (Figure 3). The initial TVC values of the LD-tofu and marinade were 4.41 and 3.97 lg cfu/g, respectively. With the increase in marinade recycling cycles, the TVC of the LD-tofu and marinade decreased significantly (p < 0.05). This indicated that marinating (continuous heating) could effectively reduce the TVC in the product. The higher initial TVC values were caused by the presence of a higher number of microorganisms in the raw materials of LD-tofu and marinade than after marination. This was mutually verified with the findings of Jeong et al. [9]. Numerous studies have shown that spices and herbs (e.g., cinnamon, ginger, bay leaf, cumin, anise) have antibacterial effects [30,31,32]. The marinade used in this study contained more than 20 spices and herbs, and the repeated heating process promoted the release of active substances from them, thereby inhibiting the growth of microorganisms.
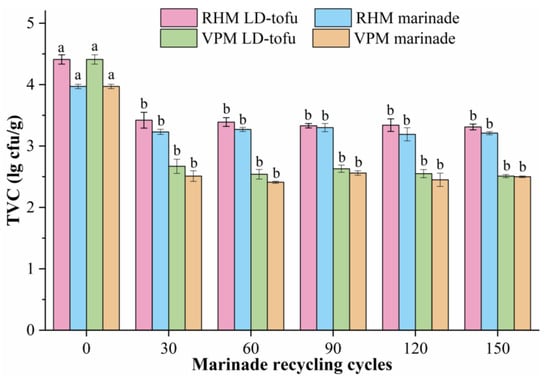
Figure 3.
The effects of marinating methods and marinade recycling cycles on the TVC of LD-tofu and marinade. Superscripts with different letters in a row are significantly different between the same sample groups (p < 0.05).
As the LD-tofu was subjected to drying and cooling processes after marination, it readily introduced additional microorganisms and hence, the TVC was slightly higher than that of the marinade. With the increase in marinade recycling times, VPM LD-tofu’s TVC decreased from 4.41 lg cfu/g to 2.51–2.67 lg cfu/g as a result of the marinating process, which had a significant inhibitory effect. The TVC of the VPM LD-tofu and VPM marinade was significantly lower than that of RHM LD-tofu and RHM marinade. This indicated that the quality and safety of the VPM LD-tofu and VPM marinade were higher, meaning that conducting the marinating process in a confined space could play a key role in the TVC of the LD-tofu and marinade.
3.4. Changes in Bacterial α-Diversity and β-Diversity of LD-tofu and Marinade under Different Marinating Processes and Different Marinade Recycling Cycles
Bacterial α-diversity analysis can reflect the diversity, evenness and richness of bacterial communities in a sample [33]. The Shannon, Simpson, Chao1 and ACE indexes of bacterial α-diversity, effective tags and operational taxonomic units (OTUs) in the LD-tofu and marinade were analysed in this study (Table 2). With the increase in marinade recycling cycles, the effective tags in the LD-tofu and marinade decreased significantly, which was consistent with the trend in TVC. With the increase in marinade recycling time, the number of OTUs in the LD-tofu decreased slightly and then significantly increased, while the number of OTUs in the marinade significantly increased (p < 0.05). This showed that the marinating process reduced the number of bacterial communities in the LD-tofu and marinade but increased the diversity of bacterial community species.

Table 2.
The effects of marinating methods and marinade recycling cycles on the microbial α-diversity of LD-tofu and marinade.
The Simpson and Shannon indexes can comprehensively reflect the evenness and richness of sample species. The larger these values, the greater the richness and evenness [33]. The Chao1 and ACE indexes mainly reflect the diversity information of sample species. The higher these values, the greater the number of species [34]. With the increase in marinade recycling cycles, the Shannon, Simpson, Chao1 and ACE indexes of bacterial communities in the LD-tofu and marinade showed different increasing trends, which was consistent with the trend in the number of OTUs. These results indicated that the marinating process could improve the diversity and richness of bacterial communities in the LD-tofu and marinade.
Principal component analysis (PCA) is a common method for β-diversity analysis. It can describe the similarity–difference relationships of bacterial communities between different samples [35]. In the LD-tofu and marinade, PC1 and PC2 contributed 41.82% and 19.79%, respectively. This means that PC1 and PC2 explained 61.61% of the difference between groups (Figure 4). The greater the distance between sample points in the PCA, the greater the bacterial community difference between them. In contrast, the closer the sample points, the more similar the bacterial community composition between the two samples [35]. With the increase in the marinade recycling cycles, the marinade points separated. This indicated that there were significant differences between marinade samples and that the bacterial community was in a state of dynamic change during the marinating process. However, all the LD-tofu points overlapped, indicating that the difference between LD-tofu samples was not significant and the similarity of bacterial communities was high.
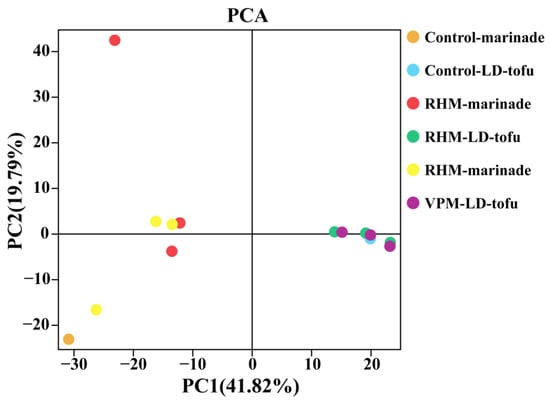
Figure 4.
Principal component analysis of the effects of marinating methods and marinade recycling cycles on the microbial β-diversity of LD-tofu and marinade.
According to the α-diversity and β-diversity analysis results, the initial bacterial diversity in the LD-tofu was higher than that in the marinade. The reason could be attributed to the fact that LD-tofu is a reprocessed tofu product, and tofu becomes rich in beneficial microorganisms after being curdled by fermented soybean whey (mixed fermentation with multiple strains) [17]. Additionally, the diversity and richness of microbial communities in the VPM LD-tofu and VPM marinade were significantly higher than that in the RHM LD-tofu and RHM marinade. However, the TVC in the VPM LD-tofu and VPM marinade was significantly lower than that in the RHM LD-tofu and RHM marinade. This indicated that the VPM could not only reduce the TVC more effectively but also protect the structural balance of microbial communities.
3.5. Changes in Bacterial Community Composition of LD-tofu and Marinade under Different Marinating Processes and Different Marinade Recycling Cycles
In this study, 16S rDNA was extracted from the LD-tofu and marinade for PCR amplification and sequencing. The changes in the bacterial community composition of the LD-tofu and marinade under different marinating processes and different marinade recycling cycles were determined by database comparison (Figure 5). At the phylum level, Proteobacteria, Firmicutes and Actinobacteria were the predominant bacteria in all samples, accounting for 81.20–94.77% (Figure 5A). The top 10 bacteria at the phylum level also included Deinococcus-Thermus, Bacteroidetes, Verrucomicrobia, Chloroflexi and Planctomycetes. The Proteobacteria and Firmicutes in the marinade accounted for 80.50–93.66%. The higher abundance of Proteobacteria and Firmicutes in the marinade could be due to the spices and herbs as they are often present in plant soil [36,37]. The Firmicutes and Deinococus-Thermus in the LD-tofu accounted for 80.35–83.47%. The presence of Firmicutes in the control LD-tofu could be due to soybean picking or tofu residue. The increase in the Firmicutes in the LD-tofu after the marinating process might be due to the marinade’s high Firmicutes content [19,38]. The predominant phylum-level bacteria of the LD-tofu or marinade exhibited a high degree of similarity under different marinating processes and marinade recycling cycles. However, there were significant differences between the dominant bacteria of the LD-tofu and marinade. Additionally, the relative abundance of each dominant bacteria differed significantly.
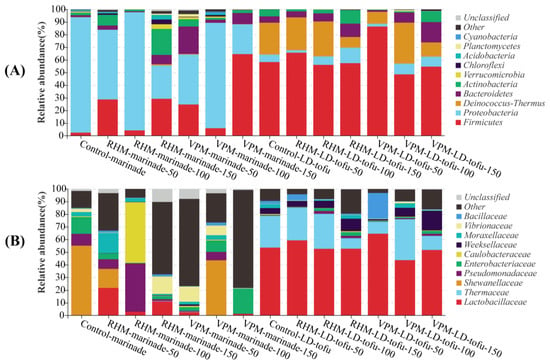
Figure 5.
Effects of marinating methods and marinade recycling cycles at the (A) phylum and (B) family bacterial community level in LD-tofu and marinade.
At the family level, Lactobacillaceae were the predominant bacteria in the LD-tofu (Figure 5B). It was inevitable that the tofu and its reprocessed products would contain considerable Lactobacillus because the tofu was curdled by Lactobacillus-fermented soybean whey [17]. Additionally, Lactobacillaceae are widely found in plants, equipment surfaces and water. They can also be introduced into products during processing [39,40]. The abundance of Lactobacillaceae decreased with the increase in marinade recycling cycles. It was 65.23%, 48.13% and 44.32% in the control LD-tofu, VPM LD-tofu and RHM LD-tofu, respectively. These results indicated that the marinating process had a greater intervention effect on Lactobacillaceae. The Shewanellaceae in the control marinade accounted for 54.92%. Shewanellaceae are extremely adaptable to the environment and can survive and reproduce under different salt concentrations, temperatures and other environments [41]. The identification of Shewanellaceae in this work provides a theoretical basis for the sterilisation and safety control of LD-tofu. With the increase in marinade recycling cycles, Lactobacillaceae, Pseudomonaceae, Caulobacteraceae and Moraxellaceae in the RHM marinade replaced Shewanellaceae as the predominant bacteria, whereas Enterobacteriaceae, Hewanellaceae and Vibrionaceae became the predominant bacteria in the VPM marinade.
To comprehensively analyse the bacterial community composition in the LD-tofu and marinade, genus-level bacteria with a relative abundance of greater than 0.1% were selected for cluster analysis (Figure 6). The relative abundance of genus-level bacteria in the marinade of the control, VPM and RHM groups changed most dynamically, which was similar to the results of Correa-Galeote et al. [40]. Shewanella and Psychrobacter were the predominant bacteria in the control marinade, while Brevundimonas, Acinetobacter, Kocuria, Pseudomonas, Bacillus and Akkermania were the predominant bacteria in the RHM marinade. Escherichia-Shigella, Weissella, Eubacterium_ruminantium_group, Eubacterium_coprostanoligenes_group, Dubosiella and Photobacterium were the predominant bacteria in the VPM marinade. There might be several reasons for the significant difference in the composition of dominant bacteria in the marinade samples. First, the marinade is rich in nutrients and high in water activity, which can promote bacterial growth and reproduction [42]. Second, a certain proportion of control marinade should be added before a new marinating process. Therefore, the control marinade can readily breed bacteria when it is not stored properly. Third, the predominant microorganisms in the LD-tofu could move into the marinade during the marinating process. Fourth, the differences in the marinating process parameters, such as temperature and open vs. closed environment, could have a significant impact on the microorganisms [42,43].
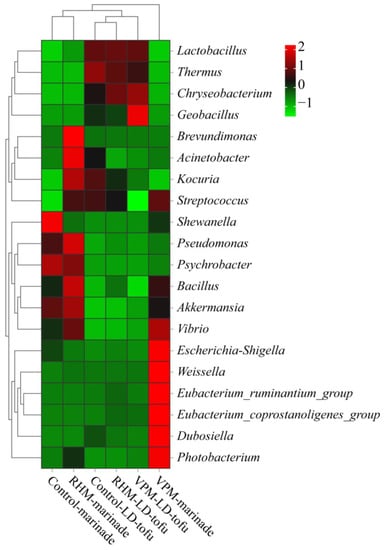
Figure 6.
Effects of marinating methods and marinade recycling cycles at the bacterial community genus level in LD-tofu and marinade.
The relative abundance of genus-level bacteria in the control, VPM group and RHM group LD-tofu were highly similar. Since Lactobacillus was the crucial bacterium for fermented soybean whey (tofu coagulant) preparation, the relative abundance of Lactobacillus in the LD-tofu was relatively high and did not change significantly [17,44]. Compared with the control LD-tofu group, the relative abundance of Thermus in the RHM LD-tofu and VPM LD-tofu groups decreased slightly, while the relative abundance of Streptococcus decreased significantly. These phenomena occurred because the RHM and VPM were performed at 80 °C and 90 °C, respectively, both of which were much higher than the optimal growth temperature of Streptococcus (37 °C) and Thermus (70–72 °C) [45,46]. The relative abundance of Chryseobacterium and Geobacillus in the RHM LD-tofu and VPM LD-tofu was higher than that in the control LD-tofu. These results showed that the bacterial community of the LD-tofu samples was similar under different marinating processes. However, there were significant differences in the content of specific bacteria at the genus level.
3.6. Correlation Analysis of Bacterial Community and Quality Characteristics of LD-tofu and Marinade
Pearson’s correlation test was used to determine the correlation between the family-level bacterial community and the quality characteristics of the LD-tofu and marinade (Figure 7). Pseudomonadaceae and Thermaceae in the LD-tofu were negatively correlated with the hardness, chewiness, springiness, protein content and fat content, but positively correlated with the water content and TVC (Figure 7A). This could be because Pseudomonadaceae can produce lipase and protease [47,48], which could reduce the fat content and protein while changing the textural characteristics. Previous studies have confirmed that equalisin (the thermo-active serine peptidase of Thermous aquaticus) can deteriorate the texture of food [49], which was verified by the results of this study. The presence of Lactobacillacea in LD-tofu was positively correlated with the water content and TVC. This might be because the Lactobacillacea in the LD-tofu gradually infiltrated the marinade during the marinating process, which was consistent with the change in water content and TVC in LD-tofu.
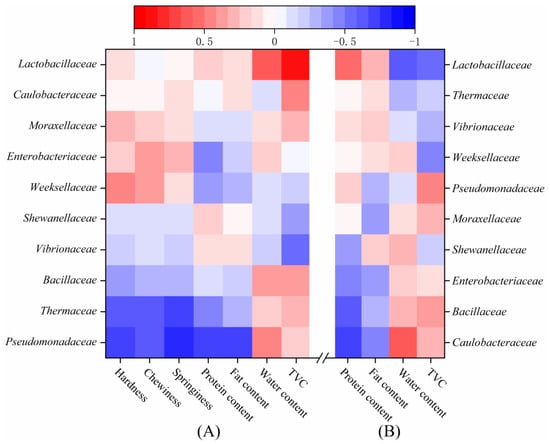
Figure 7.
Pearson correlation between the bacterial community family and the quality characteristics of (A) LD-tofu and (B) marinade.
Caulobacteriaceae, Bacillaceae and Enterobacteriaceae in the marinade were negatively correlated with the protein and fat content but positively correlated with the water content and TVC (Figure 7B). Caulobacteriaceae, Bacillaceae and Enterobacteriaceae are often found in raw materials (including spices, herbs and drinking water) [42,50,51]. Although heating can effectively control these bacteria, a certain proportion of control marinade was added before commencing a new marinating process. This resulted in a high content of these bacteria in the marinade. Lactobacillaceae in the marinade was negatively correlated with the water content and TVC. This might be because Lactobacillaceae in the LD-tofu infiltrated the marinade during the marinating process. Its change trend was opposite to the change in the water content and TVC in the marinade.
4. Conclusions
In this study, the effects of different marinating processes on the nutritional components, textural characteristics, TVC and bacterial community of LD-tofu were analysed. The marinating process promoted the loss of nutrients in LD-tofu, resulting in a significant increase in the marinade protein and fat content. The textural characteristics of LD-tofu were improved under different marinating processes. The TVC values of the LD-tofu and marinade were significantly reduced following marination. The nutrient content, textural characteristics and microbial contamination of the VPM LD-tofu were superior to those of the RHM LD-tofu. There were also significant differences in the bacterial communities of the LD-tofu and marinade under different marinating processes. Pseudomonadaceae, Thermaceae and Lactobacillaceae were closely related to the quality characteristics of LD-tofu, while Caulobacteriaceae, Bacillaceae and Enterobacteriae were closely related to the quality characteristics of the marinade. The present work provides producers with a solid theoretical basis to maintain the quality and safety of LD-tofu.
Author Contributions
Z.H. and L.Z.; methodology: T.W.; validation, X.Z. (Xiaohu Zhou) and H.C.; investigation: X.Z. (Xiaojie Zhou) and M.L.; resources, J.Z. and Y.L.; writing—original draft: T.W.; writing—review and editing: Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hunan Province (No. 2021JJ40514) and the Science and Technology Innovative Program of Hunan Province (No. 2022NK2039, 2019TP1028).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the College of Food and Chemical Engineering, Shaoyang University, and the Hunan Provincial Key Laboratory of Soybean Products Processing and Safety Control for providing facilities and equipment.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Wu, T.; Peng, X.L.; Zhao, L.Z.; Chen, H.; Zhou, X.J.; Huang, Z.R.; Zhou, X.H. Variation law of Xiangpai dried tofu and brine during marinating process. Food Ferment. Ind. 2021, 47, 146–154. (In China) [Google Scholar]
- Zhao, L.Z.; Yin, L.B.; Lei, Z.M.; Bu, Y.F.; Zhang, C.Y.; Yang, Y.; Kong, Y.Z. Evaluation of quality changes of leisure dried tofu during storage based on electronic nose. Nanosci. Nanotech. Let. 2017, 9, 705–711. [Google Scholar] [CrossRef]
- Xia, A.L.; Zhang, Y.; Zhao, L.Z.; Qin, P. Simultaneous, rapid and nondestructive determination of moisture, fat content and storage time in leisure dried tofu using LF-NMR. Anal. Sci. 2021, 37, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.F.; Li, W.Q.; Xie, L.L.; Zhao, L.Z. Research on quality change of leisure dried Tofu during storage. Food Mach. 2016, 32, 115–118. (In Chinese) [Google Scholar]
- Li, Q.R.; Hua, Y.F.; Li, X.F.; Kong, X.Z.; Zhang, C.M.; Chen, Y.M. Effects of heat treatments on the properties of soymilks and glucono-δ-Lactone induced tofu gels. Food Res. Int. 2022, 161, 111912. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.X.; Li, C.G.; Dong, H.; Yuan, H.R.; Ye, S.L.; Huang, X.L.; Zhang, X.L.; Wang, Q. Analysis of key fungi and their effect on the edible quality of HongJun tofu. a Chinese fermented okara food. LWT-Food Sci. Technol. 2022, 172, 114151. [Google Scholar] [CrossRef]
- Wang, F.; Meng, J.; Sun, L.; Weng, Z.B.; Fang, Y.; Tang, X.Z.; Zhao, T.J.; Shen, X.C. Study on the tofu quality evaluation method and the establishment of a model for suitable soybean varieties for Chinese traditional tofu processing. LWT-Food Sci. Technol. 2020, 117, 108441. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Zhao, L.Z.; Yue, Z.J.; Yin, L.B.; Chen, H.F. The safety monitoring and early-warning model construction for Xiangpai brine recycling. Food Ferment. Ind. 2020, 46, 181–187. (In China) [Google Scholar]
- Jeong, K.; Hyeonbin, O.; Shin, S.Y.; Kim, Y.S. Effects of different marination conditions on quality, microbiological properties, and sensory characteristics of pork ham cooked by the sous-vide method. Korean J. Food Sci. Anim. Resour. 2018, 38, 506–514. [Google Scholar]
- Wen, X.Y.; Liang, C.; Zhang, D.Q.; Li, X.; Chen, L.; Zheng, X.C.; Fang, F.; Cheng, Z.; Wang, D.Y.; Hou, C.L. Effects of hot or cold boning on the freshness and bacterial community changes of lamb cuts during chilled storage. LWT-Food Sci. Technol. 2022, 170, 114063. [Google Scholar] [CrossRef]
- Chen, X.; Dong, P.C.; Li, K.; Zhu, L.X.; Yang, X.Y.; Mao, Y.W.; Niu, L.B.; Hopkins, D.L.; Luo, X.; Liang, R.R.; et al. Effect of the combination of superchilling and super-chilled storage on shelf-life and bacterial community dynamics of beef during long-term storage. Meat Sci. 2022, 192, 108910. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT-Food Sci. Technol. 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Huang, Z.R.; He, W.Y.; Zhao, L.Z.; Liu, H.Y.; Zhou, X.J. Processing technology optimization for tofu curded by fermented yellow whey using response surface methodology. Food Sci. Nut. 2021, 9, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kwon, K.H.; Chai, C.; Oh, S.W. Microbial contamination of tofu in Korea and growth characteristics of Bacillus cereus isolates in tofu. LWT-Food Sci. Technol. 2017, 78, 63–69. [Google Scholar] [CrossRef]
- Rossi, F.; Felis, G.E.; Martinelli, A.; Calcavecchia, B.; Torriani, S. Microbiological characteristics of fresh tofu produced in small industrial scale and identification of specific spoiling microorganisms (SSO). LWT-Food Sci. Technol. 2016, 70, 280–285. [Google Scholar] [CrossRef]
- Shin, W.K.; Yokoyama, W.H.; Kim, W.; Wicker, L.; Kim, Y. Change in texture improvement of low-fat tofu by means of low-fat soymilk protein denaturation. J. Sci. Food Agric. 2015, 95, 1000–1007. [Google Scholar] [CrossRef]
- Huang, Z.R.; Liu, H.Y.; Zhao, L.Z.; He, W.Y.; Zhou, X.J.; Chen, H.; Zhou, X.H.; Zhou, J.S.; Liu, Z.X. Evaluating the effect of different processing methods on fermented soybean whey-based tofu quality, nutrition, and flavour. LWT-Food Sci. Technol. 2022, 158, 113139. [Google Scholar] [CrossRef]
- Feng, C.H. Quality evaluation and mathematical modelling approach to estimate the growth parameters of total viable count in sausages with different casings. Foods 2022, 11, 634. [Google Scholar] [CrossRef]
- He, Z.; Chen, H.Y.; Wang, X.Y.; Lin, X.P.; Ji, C.F.; Li, S.J.; Liang, H.P. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT-Food Sci. Technol. 2020, 118, 108773. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rahman, M.S.; Kim, A.N.; Gul, K.; Kang, S.W.; Chun, J.; Kerr, W.L.; Choi, S.G. Quality characteristics and storage stability of low-fat tofu prepared with defatted soy flours treated by supercritical-CO2 and hexane. LWT-Food Sci. Technol. 2018, 100, 237–243. [Google Scholar] [CrossRef]
- Huang, Z.R.; Zhou, H.P.; Jiang, Q.H.; He, W.Y.; Zhou, X.H.; Chen, H.; Zhou, X.J.; Li, M.; Liu, B.B.; Zhou, J.S.; et al. Study on the quality change and deterioration mechanism of leisure dried tofu under different storage temperature conditions. LWT-Food Sci. Technol. 2022, 172, 114257. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Bara, M.B.; Pei, M.B.; Vucelic-Radovic, B.V. Protein composition and textural properties of inulin-enriched tofu produced by hydrothermal process. LWT-Food Sci. Technol. 2020, 126, 109309. [Google Scholar] [CrossRef]
- Huang, Z.R.; Sun, J.; Zhao, L.Z.; He, W.Y.; Liu, T.Y.; Liu, B.B. Analysis of the gel properties, microstructural characteristics, and intermolecular forces of soybean protein isolate gel induced by transglutaminase. Food Sci. Nutr. 2022, 10, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Kyriakopoulou, K.; Rahmani, A.; Venema, P.; Goot, A.J. Isochoric moisture heating as a tool to control the functionality of soy protein. LWT-Food Sci. Technol. 2021, 150, 111979. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in proteolysis, protein oxidation, flavor, color and texture of dry-cured mutton ham during storage. LWT-Food Sci. Technol. 2021, 149, 111860. [Google Scholar] [CrossRef]
- Tazi, S.; Plantevin, F.; Falco, C.D.; Puigserver, A.; Ajandouz, E.H. Effects of light, temperature and water activity on the kinetics of lipoxidation in almond-based products. Food Chem. 2009, 115, 958–964. [Google Scholar] [CrossRef]
- Zuo, F.; Chen, Z.; Shi, X.; Wang, R.; Guo, S. Yield and textural properties of tofu as affected by soymilk coagulation prepared by a high-temperature pressure cooking process. Food Chem. 2016, 213, 561–566. [Google Scholar] [CrossRef]
- Sun, D.; Li, T.; Ma, L.; Zhang, F.; Li, A.; Jiang, Z. Effect of selective thermal denaturation and glycosylation on the textural properties and microstructure of vegetable tofu. J. Food Process. Eng. 2019, 42, e13001. [Google Scholar] [CrossRef]
- Cho, E.R.; Kim, S.S.; Kang, D.H. Inactivation kinetics and membrane potential of pathogens in soybean curd subjected to pulsed ohmic heating depending on applied voltage and duty ratio. Appl. Environ. Microb. 2020, 86, e00656-20. [Google Scholar] [CrossRef]
- Haute, S.V.; Raes, K.; Meeren, P.V.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control. 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Korley, K.N.; Teye, D.B.; Okraku, T.C.; Ofori, A.A.; Enoch, A.; Ken, E.E.; Agyemang, B.A.; Theophilus, A. Toxicogenic fungi, aflatoxins, and antimicrobial activities associated with some spices and herbs from three selected markets in Ho Municipality, Ghana. Int. J. Food Sci. 2022, 2022, 7195890. [Google Scholar]
- Zheng, R.H.; Xu, X.R.; Xing, J.L.; Cheng, H.; Zhang, S.F.; Shen, J.; Li, H.S. Quality evaluation and characterization of specific spoilage organisms of spanish mackerel by high-throughput sequencing during 0 °C cold chain logistics. Foods 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Q.; Xu, J.H.; Sun, F.D.; Liu, H.T.; Kong, B.H. Effects of modified atmosphere packaging with various CO2 concentrations on the bacterial community and shelf-Life of smoked chicken legs. Foods 2022, 11, 559. [Google Scholar] [CrossRef]
- Mao, J.J.; Liu, X.L.; Gao, T.; Gu, S.B.; Wu, Y.; Zhao, L.N.; Ma, J.L.; Li, X.; Zhang, J. Unraveling the correlations between bacterial diversity, physicochemical properties and bacterial community succession during the fermentation of traditional Chinese strong-flavor Daqu. LWT-Food Sci. Technol. 2022, 154, 112764. [Google Scholar] [CrossRef]
- Solanki, M.K.; Wang, Z.; Wang, F.Y.; Li, C.N.; Gupta, C.L.; Singh, R.K.; Malviya, M.K.; Singh, P.; Yang, L.T.; Li, Y.R. Assessment of diazotrophic proteobacteria in sugarcane rhizosphere when intercropped with legumes (Peanut and Soybean) in the field. Front. Microbiol. 2020, 11, 1814. [Google Scholar] [CrossRef]
- Zhang, G.L.; Chu, X.B.; Zhu, H.Y.; Zou, D.S.; Li, L.C.; Du, L.S. The response of soil nutrients and microbial community structures in long-term tea plantations and diverse agroforestry intercropping systems. Sustainability 2021, 13, 7799. [Google Scholar] [CrossRef]
- Tamang, J.P.; Das, S.; Kharnaior, P.; Pariyar, P.; Thapa, N.; Jo, S.W.; Yim, E.J.; Shin, D.H. Shotgun metagenomics of Cheonggukjang, a fermented soybean food of Korea: Community structure, predictive functionalities and amino acids profile. Food Res. Int. 2022, 151, 110904. [Google Scholar] [CrossRef]
- Daniela, B.; Giovann, M.; Viviana, B.D.M.; Federica, B.; Chiara, M.; Silvia, L.; Vida, S.; Gardini, F.G.; Giulia, T. Taxonomical identification and safety characterization of Lactobacillaceae from mediterranean natural fermented sausages. Foods 2022, 11, 2776. [Google Scholar]
- Correa-Galeote, D.; Ghomari, I.; Asehraou, A.; Gonzalez-Lopez, J. Revealing the bacterial abundance and diversity in brines from started Spanish-style green table olives. LWT-Food Sci. Technol. 2022, 160, 113212. [Google Scholar] [CrossRef]
- Symanzik, C.; Esser, J.; Pfennigwerth, N.; Reuter, C.; Bronnert, J.; Grade, M. Shewanella algae bacteraemia in a patient with a chronic ulcer after contact with seawater on vacation in turkey: A case report from a German maximum-care hospital. New Microb. New Infec. 2022, 48, 101016. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Rupert, C.L.; Chapman, B.; Porto, F.A.C.S.; Luchansky, J.B. Assessment of microbiological safety and quality of marinades used to treat beef and that were collected over a 12-month period from specialty retailers near Raleigh, North Carolina. J. Food Protect. 2018, 81, 490–496. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.J.E. Effect of different marinating conditions on the evolution of spoilage microbiota and metabolomic profile of chicken breast fillets. Food Microbiol. 2017, 66, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, J.J.; Dai, Y.Q.; Liu, X.L.; Zhou, J.Z.; Xia, X.D. Effects of lactic acid bacteria fermented yellow whey on the protein coagulation and isoflavones distribution in soymilk. Food Chem. 2021, 334, 127484. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Peng, J.Y.; Kwok, L.Y.; Zhang, W.Y.; Sun, T.S. Metabolomic analysis of Streptococcus thermophilus S10-fermented milk. LWT-Food Sci. Technol. 2021, 161, 113368. [Google Scholar] [CrossRef]
- Li, M.M.; Lv, A.P.; Zhao, Z.Y.; Xian, W.D.; Lian, Z.H.; OuYang, Y.T.; Ming, H.; Tan, S.; Jiao, J.Y.; Zhou, E.M.; et al. Description of five novel thermophilic species of the genus Thermus: Thermus hydrothermalis sp. nov., Thermus neutrinimicus sp. nov., Thermus thalpophilus sp. nov.; Thermus albus sp. nov., and Thermus altitudinis sp. nov., isolated from hot spring sediments. Syst. Appl. Microbiol. 2022, 45, 126361. [Google Scholar]
- Behera, S.; Pattnaik, S. Persister cell development among enterobacteriaceae, pseudomonadaceae, mycobacteriaceae and staphylococcaceae biotypes: A review-sciencedirect. Biocatal. Agric. Biotech. 2019, 22, 101401. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef]
- Verbauwhede, A.E.; Lambrecht, M.A.; Fierens, E.; Hermans, S.; Shegay, O.; Brijs, K.; Delcour, J.A. Thermo-reversible inhibition makes aqualysin 1 from Thermus aquaticus a potent tool for studying the contribution of the wheat gluten network to the crumb texture of fresh bread. Food Chem. 2018, 264, 118–125. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Koskinen, K.; Laine, P.; Hultman, J.; Sade, E.; Paulin, L.; Paloranta, A.; Johansson, P.; Bjorkroth, J.; Auvinen, P. Comparison of microbial communities in marinated and unmarinated broiler meat by metagenomics. Int. J. Food Microbiol. 2012, 157, 142–149. [Google Scholar] [CrossRef]
- Durek, J.; Frohling, A.; Bußler, S.; Hase, A.; Ehlbeck, J.; Schluter, O.K. Pilot-scale generation of plasma processed air and its influence on microbial count, microbial diversity, and selected quality parameters of dried herbs. Innov. Food Sci. Emerg. 2022, 75, 102890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).