Characterization of the Dynamic Gastrointestinal Digests of the Preserved Eggs and Their Effect and Mechanism on HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Gastrointestinal Digestive Properties of Preserved Eggs
2.2.1. Preparation of Simulated Digestion Fluids
2.2.2. In Vitro Dynamic Digestion of Preserved Eggs
Oral Digestion
The Dynamic Human Gastrointestinal-IV (DHGI-IV)
Gastrointestinal Digestion
2.2.3. Determination of Protein and Fat Digestibility
Protein Digestibility
Fat Digestibility
2.2.4. Determination Particle Size
2.2.5. In Vitro Gastric Emptying Assay of PED
2.2.6. Determination of Antioxidant Activity
2.3. Effect of PED on HepG2 Cells
2.3.1. Cell Culture and Activity Assay
2.3.2. Cell Morphological Observation
2.3.3. Colony Formation Assay
2.3.4. Cellular Apoptosis Assay
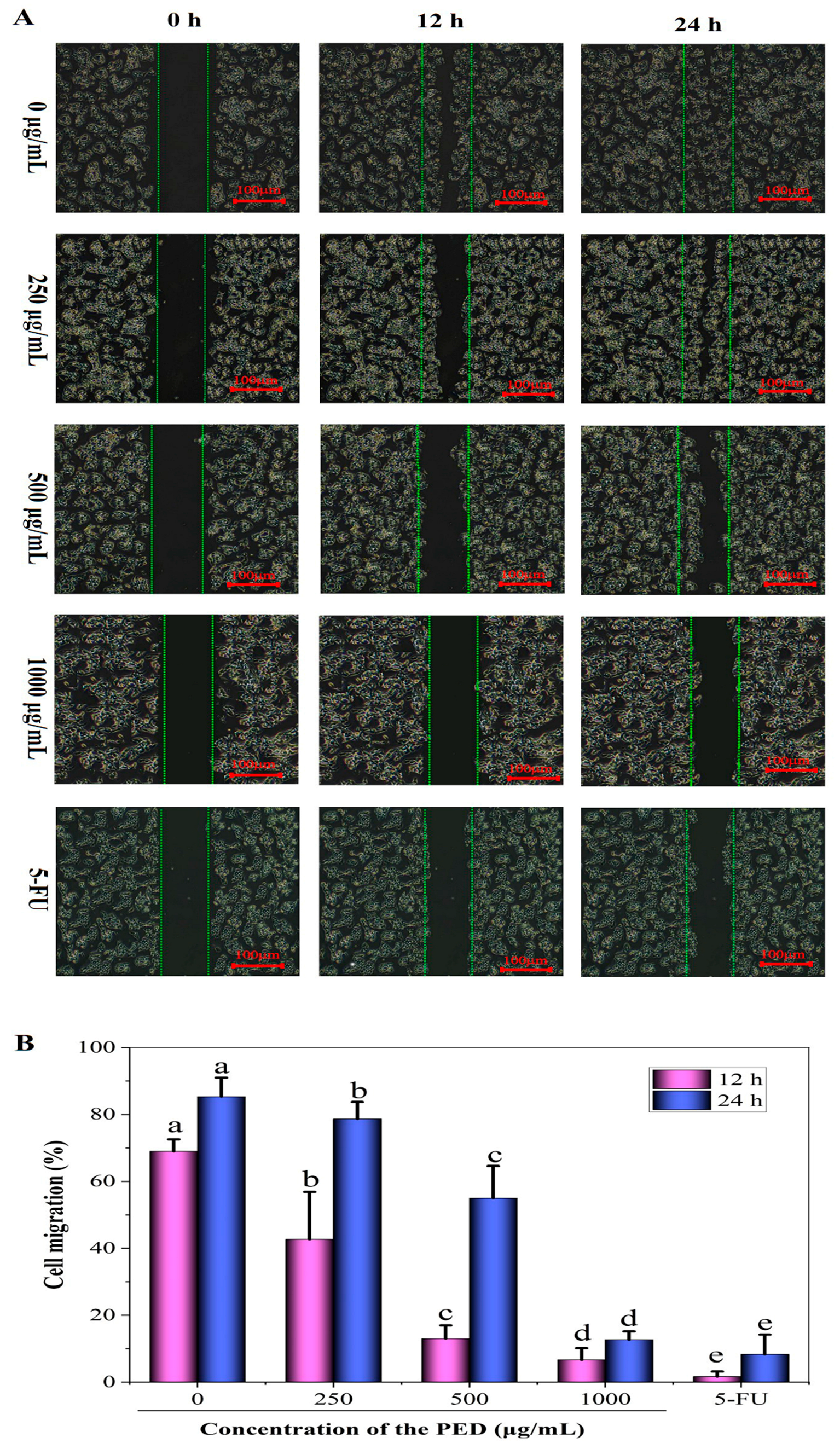
2.3.5. Cell Migration Examination
2.3.6. Measurement of Intracellular ROS
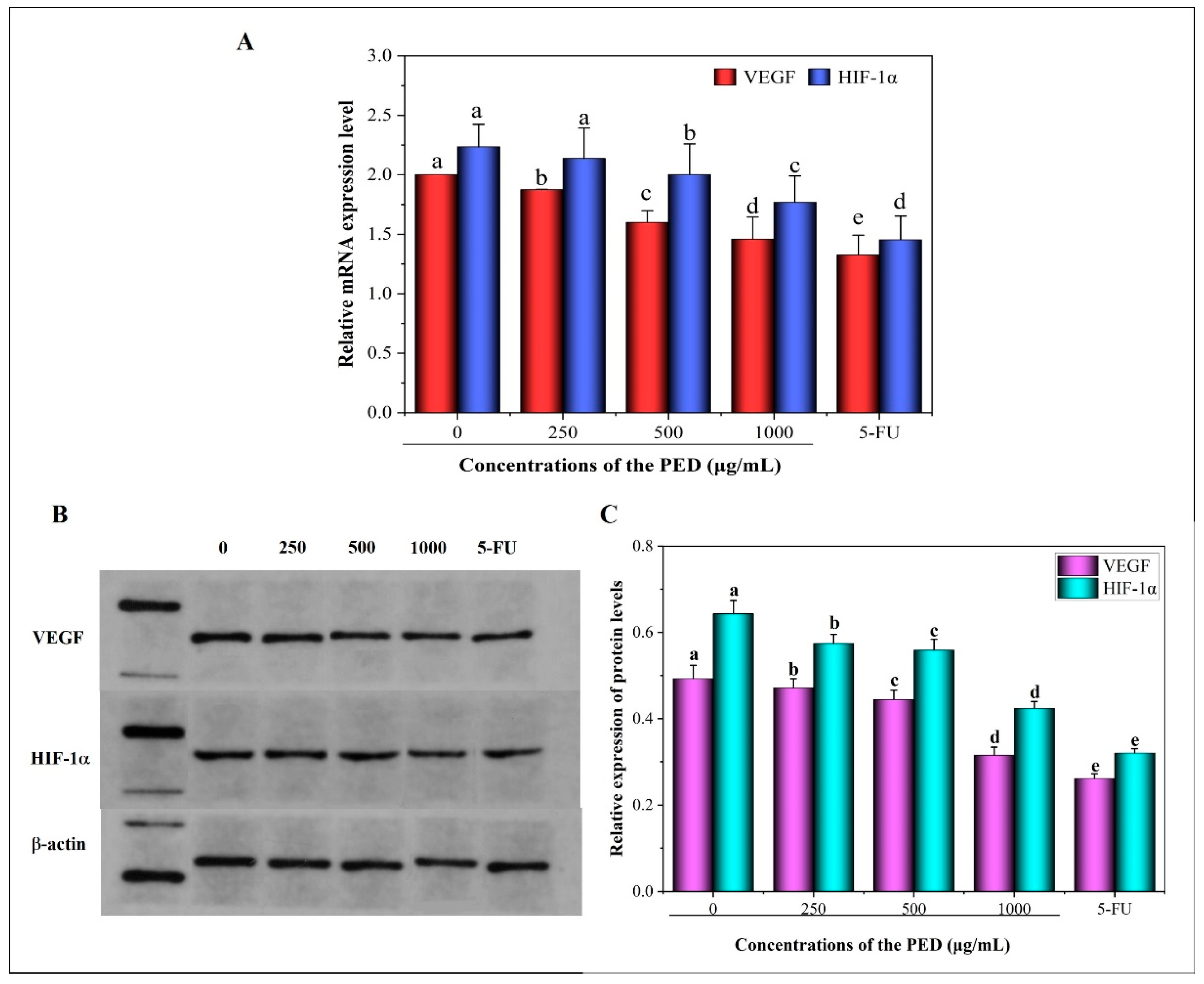
2.3.7. Real-Time PCR
2.3.8. Western Blot Analysis
2.4. Statistics and Analysis
3. Results and Discussion
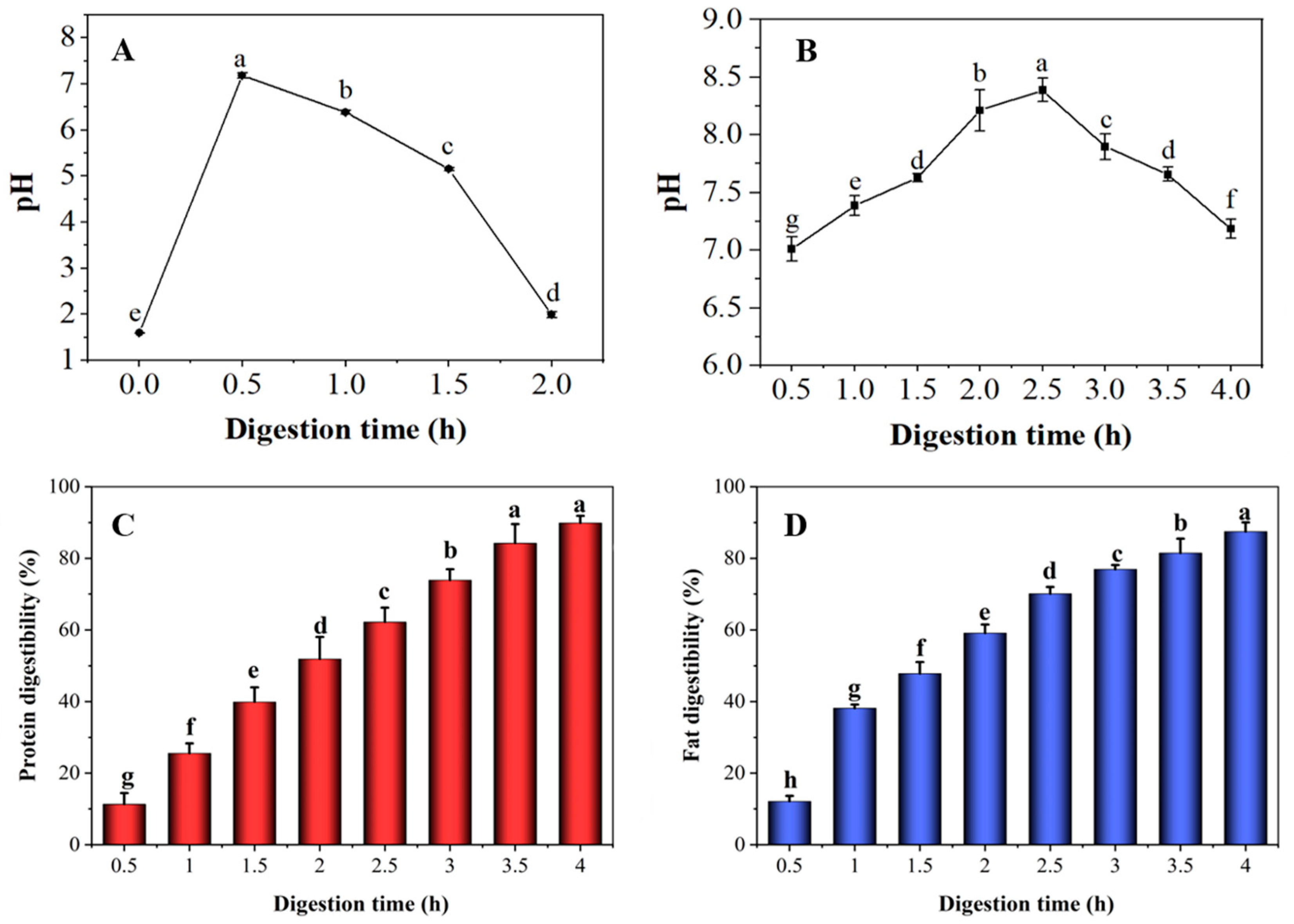
3.1. Changes in pH, Protein and Fat Digestibility of Preserved Eggs during Digestion
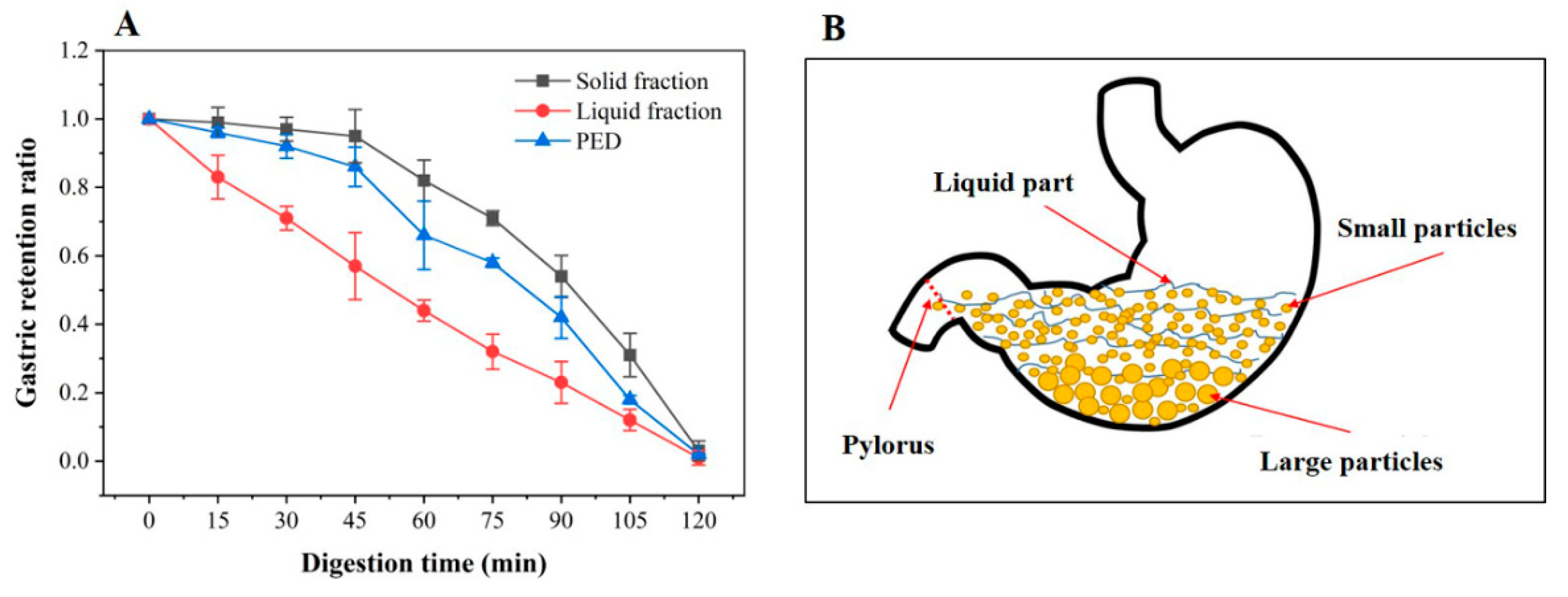
3.2. In Vitro Gastric Emptying Properties of PED
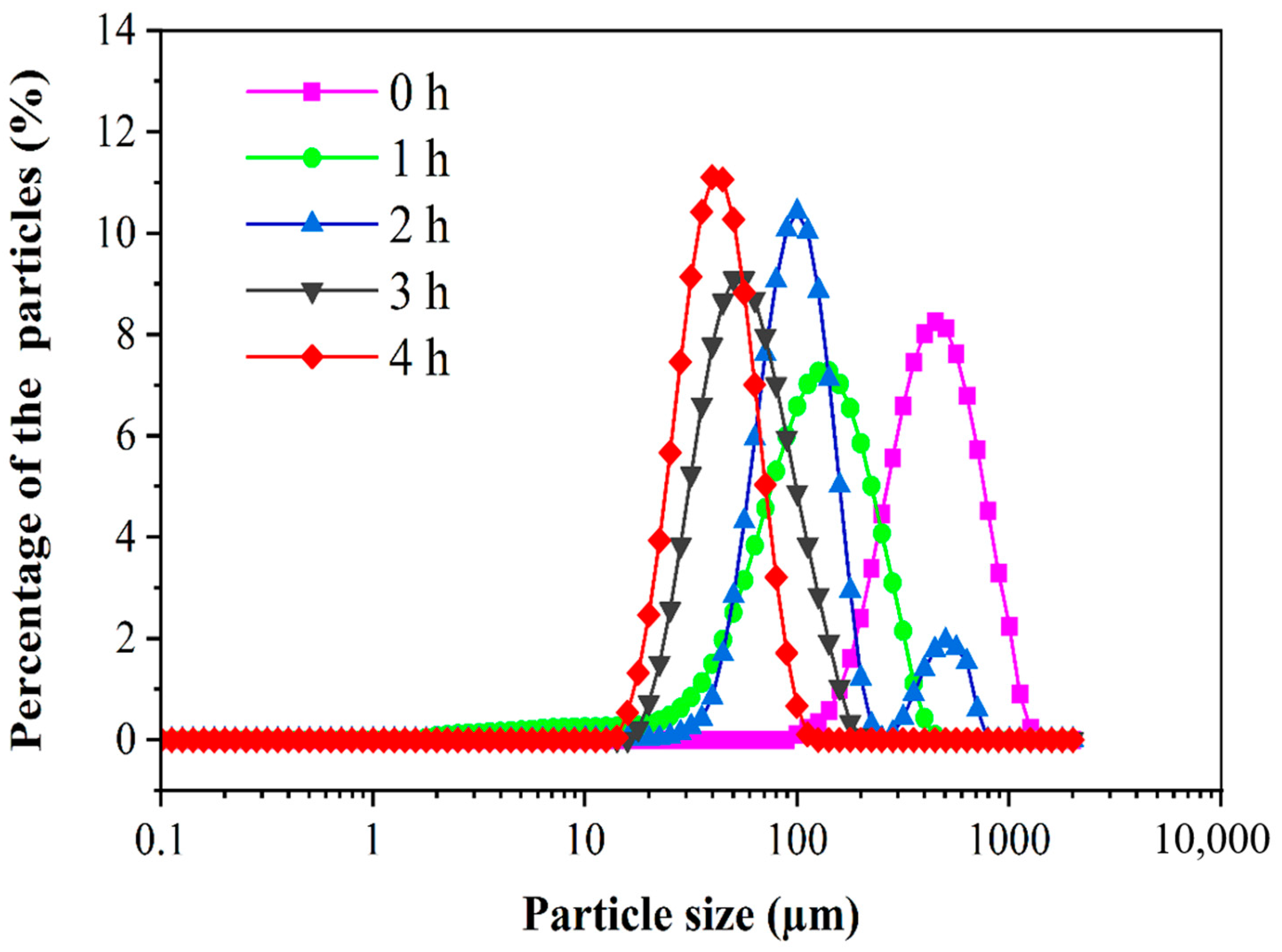
3.3. Particle Size Distribution of PED
3.4. In Vitro Antioxidant Activity of PED
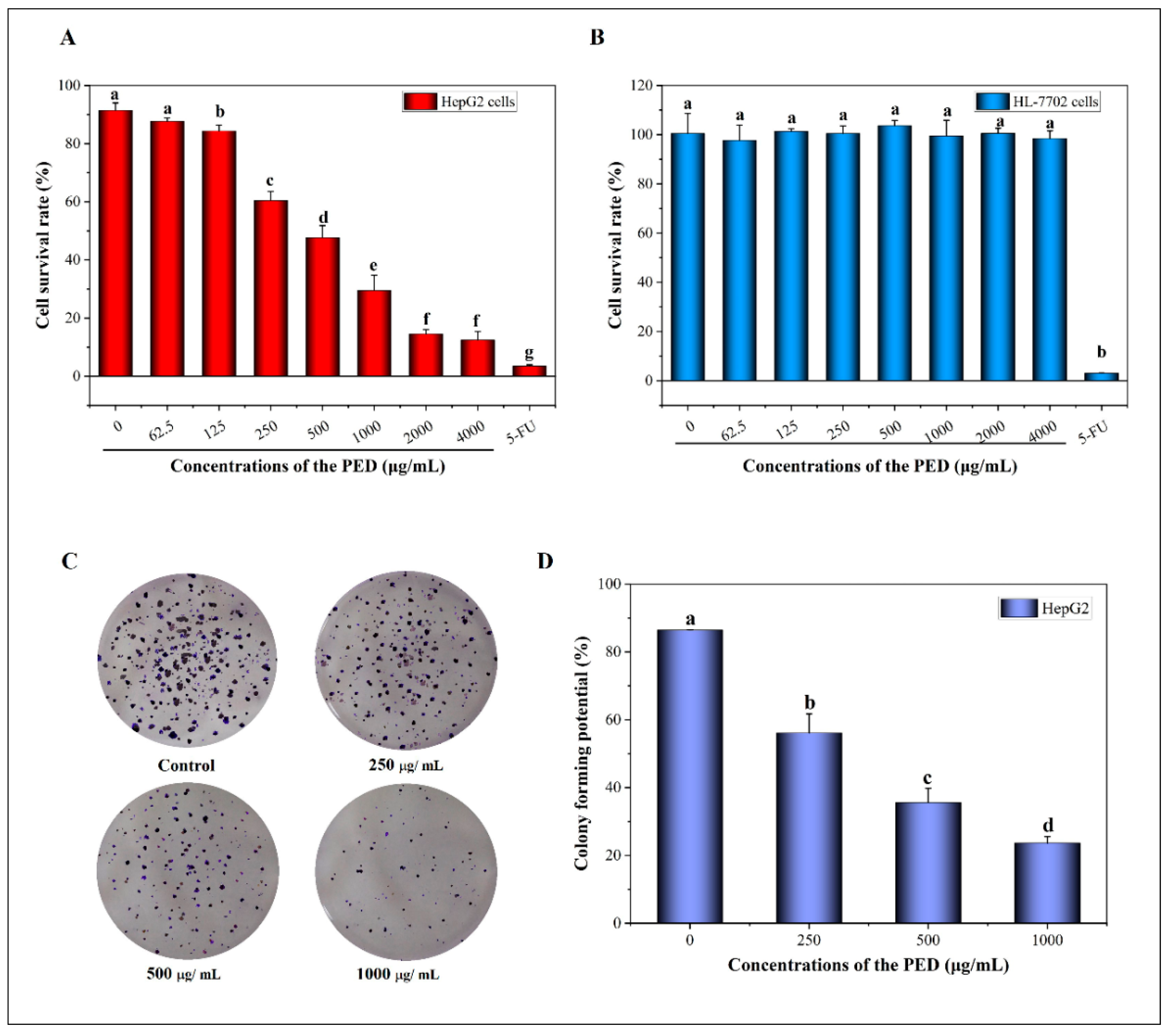
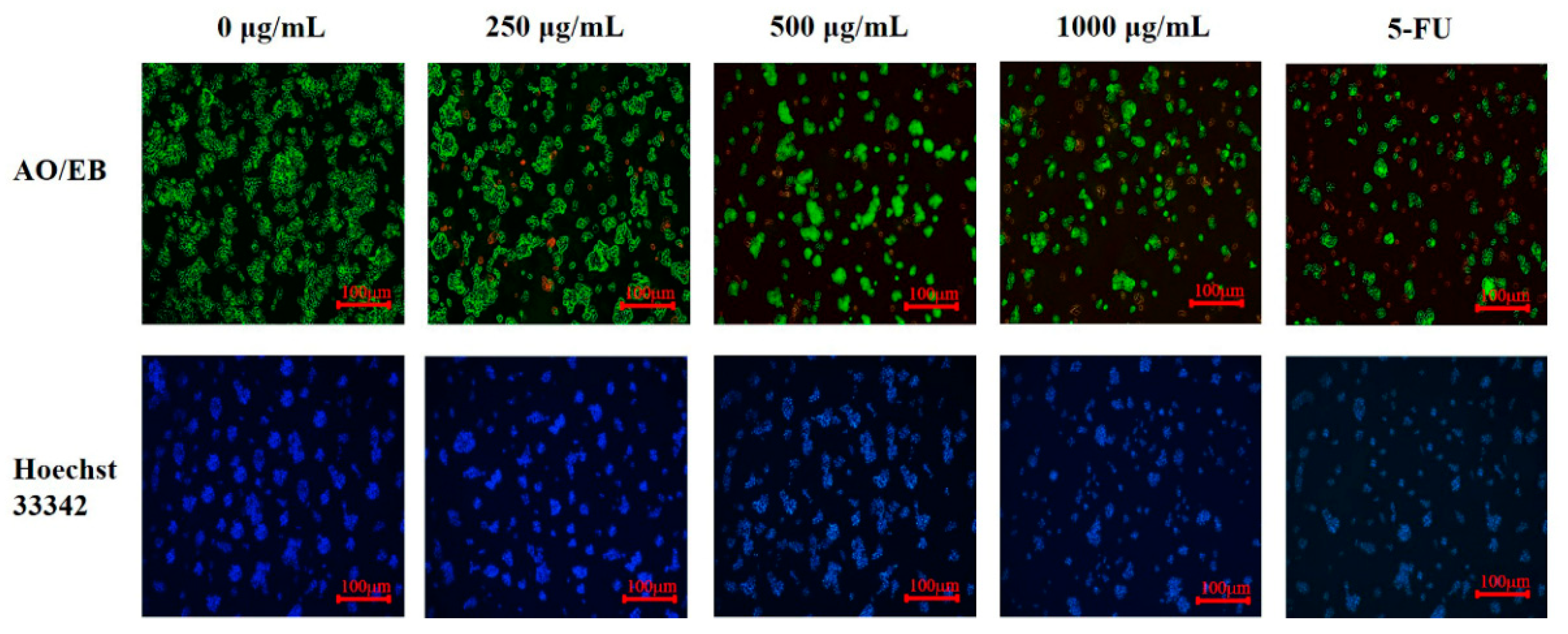
3.5. The Inhibition Effects of PED on Cell Growth
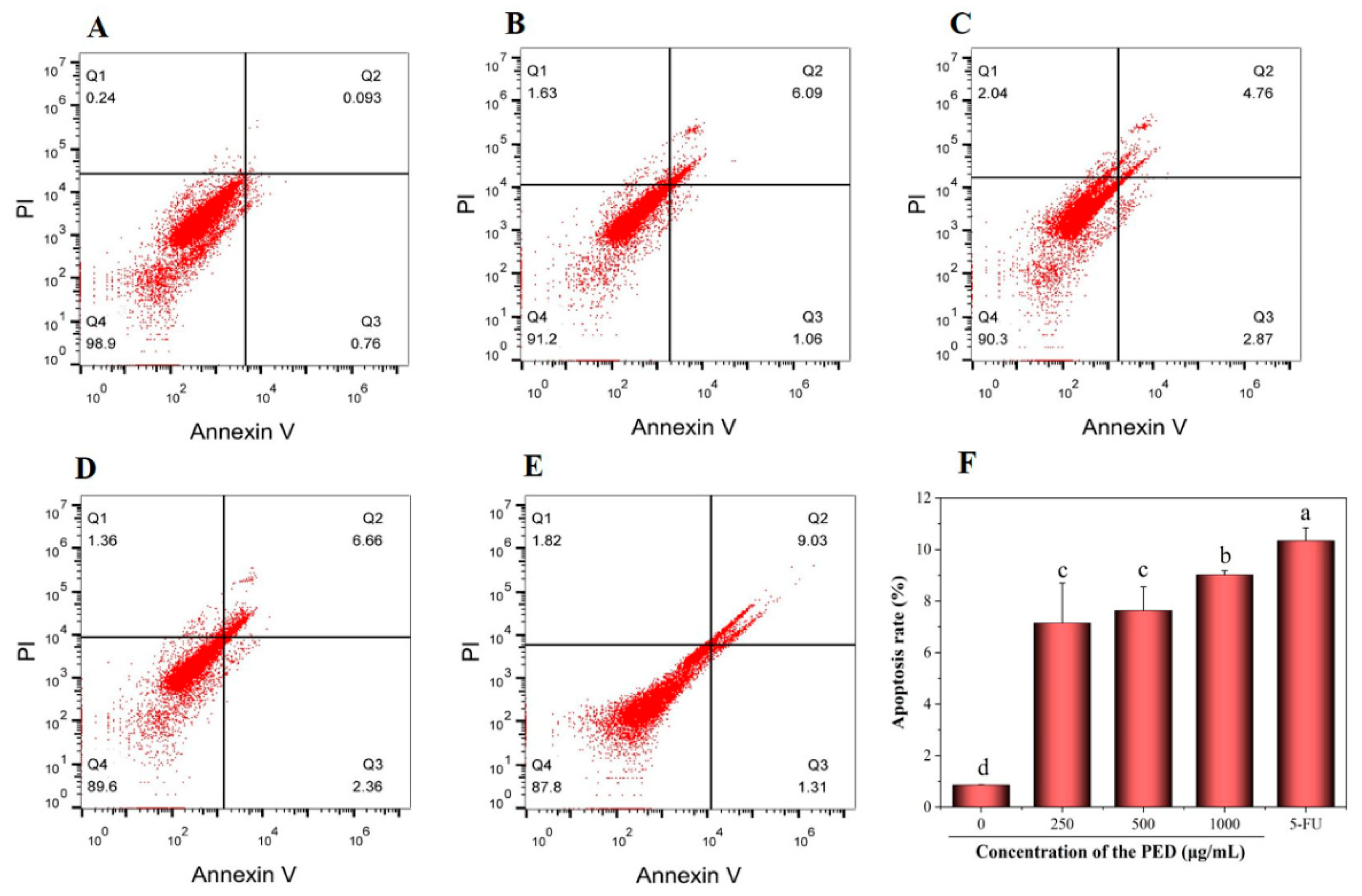
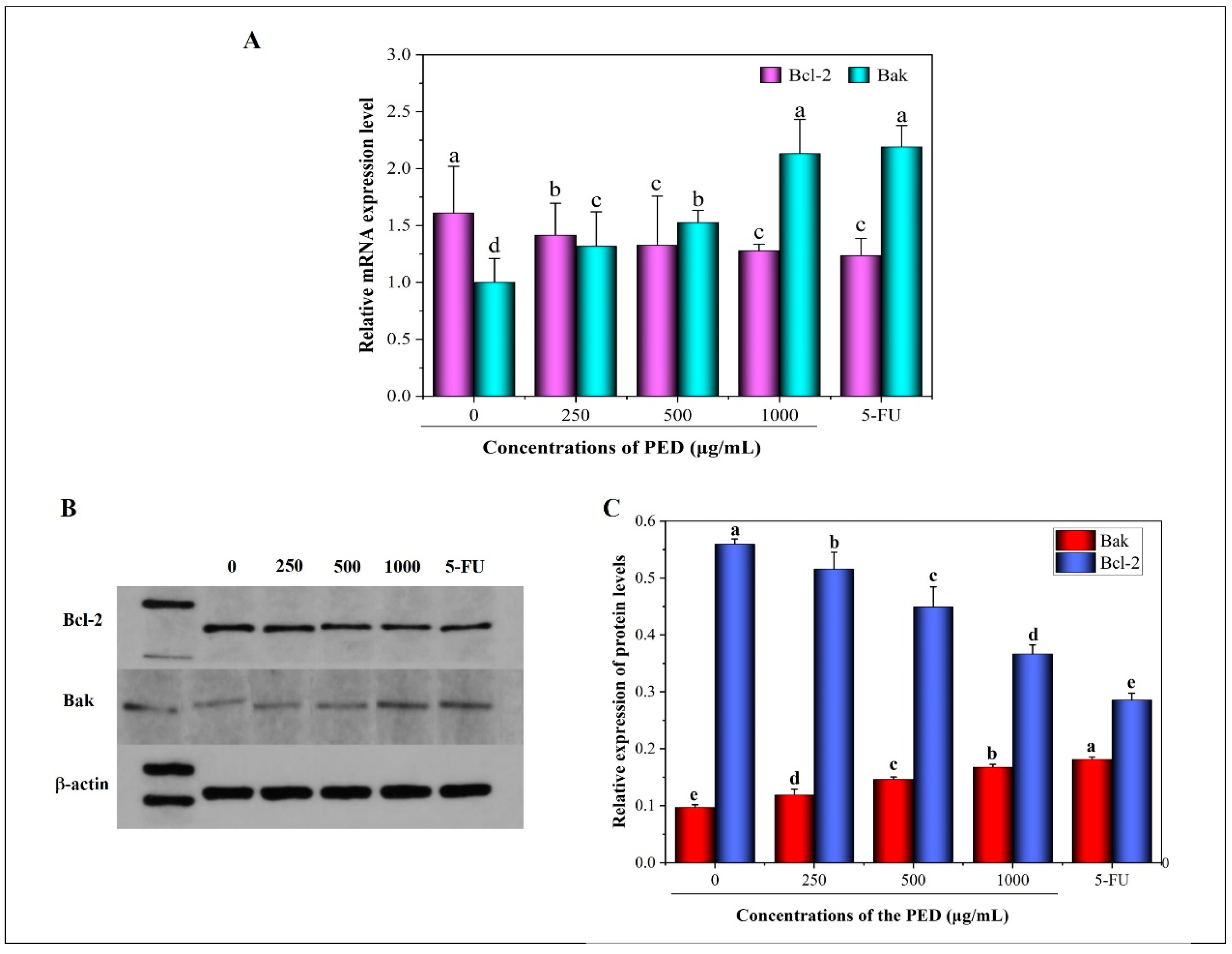
3.6. The Induction of PED on Cell Apoptosis through the Mitochondrial Pathway
3.7. The Induction of PED on Cell Apoptosis through Exacerbates Oxidative Stress
3.8. The Inhibition Effects of PED on Cell Migration and Angiogenesis-Related Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perumal, A.; AlSalhi, M.S.; Kanakarajan, S.; Devanesan, S.; Selvaraj, R.; Tamizhazhagan, V. Phytochemical evaluation and anticancer activity of rambutan (Nephelium lappaceum) fruit endocarp extracts against human hepatocellular carcinoma (HepG-2) cells. Saudi J. Biol. Sci. 2021, 28, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, M.; Zhu, J.; Qu, J.; Qin, K.; Zhao, D.; Wang, L.; Dong, L.; Zhang, X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 2020, 132, 110797. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yan, S.-M.; Cai, C.-B.; Yu, X.-P.; Jiang, J.-H.; Wu, H.-L.; Yu, R.-Q. Nonlinear multivariate calibration of shelf life of preserved eggs (Pidan) by near infrared spectroscopy: Stacked least squares support vector machine with ensemble preprocessing. J. Spectro. 2013, 2013, 797302. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, Y.; Xu, M.; Li, J.; Du, H. Physicochemical and nutritional characteristics of preserved duck egg white. Poult. Sci. 2014, 93, 3130–3137. [Google Scholar] [CrossRef]
- Batool, Z.; Hu, G.; Huang, X.; Wu, Y.; Fu, X.; Cai, Z.; Huang, X.; Ma, M. Dietary therapeutic treatment of renal carcinoma cell lines by down-regulating cFlip, Mcl-1, Bcl-XL and STAT3 gene expression under the influence of up-regulated Bax and intrinsic apoptotic pathway. Food Biosci. 2021, 43, 101319. [Google Scholar] [CrossRef]
- Mao, C.; Yu, Z.; Li, C.; Jin, Y.; Ma, M. The functional properties of preserved eggs: From anti-cancer and anti-inflammatory aspects. Korean J. Food Sci. Anim. Resour. 2018, 38, 615. [Google Scholar] [CrossRef]
- Ding, N.; Mao, C.; Cai, Z.; Ma, M. Anti-inflammatory effect of preserved egg with simulated gastrointestinal digestion on LPS-stimulated RAW264. 7 cells. Poult. Sci. 2019, 98, 4401–4407. [Google Scholar] [CrossRef]
- Farjami, T.; Babaei, J.; Nau, F.; Dupont, D.; Madadlou, A. Effects of thermal, non-thermal and emulsification processes on the gastrointestinal digestibility of egg white proteins. Trends Food Sci. Technol. 2021, 107, 45–56. [Google Scholar] [CrossRef]
- De Boever, P.; Wouters, R.; Vermeirssen, V.; Boon, N.; Verstraete, W. Development of a six-stage culture system for simulating the gastrointestinal microbiota of weaned infants. Microb. Ecol. Health Dis. 2001, 13, 111–123. [Google Scholar] [CrossRef]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trends Food Sci. Technol. 2021, 114, 510–520. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liao, Z.; Luo, T.; Chen, L.; Chen, X.D. Enhancement of digestibility of casein powder and raw rice particles in an improved dynamic rat stomach model through an additional rolling mechanism. J. Food Sci. 2017, 82, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro-and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Goncalves, R.F.; Madalena, D.A.; Vicente, A.A. Towards the understanding of the behavior of bio-based nanostructures during in vitro digestion. Curr. Opin. Food Sci. 2017, 15, 79–86. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Ménard, O. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Liu, M.; Liao, Z.; Wang, Y.; Dong, Z.; Chen, X.D. An advanced near real dynamic in vitro human stomach system to study gastric digestion and emptying of beef stew and cooked rice. Food Funct. 2019, 10, 2914–2925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Z.; Li, J.; Xu, M.; Shao, Y.; Tu, Y. Changes of microstructure characteristics and intermolecular interactions of preserved egg white gel during pickling. Food Chem. 2016, 203, 323–330. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, Y.; Xu, M.; Wang, S.; Wang, X.; Tu, Y. Simulated gastrointestinal digest from preserved egg white exerts anti-inflammatory effects on Caco-2 cells and a mouse model of DSS-induced colitis. J. Funct. Foods 2017, 35, 655–665. [Google Scholar] [CrossRef]
- Liang, Y.; He, L.; Zhang, M.; Liu, X.; Jin, G.; Jin, Y.; Ma, M. Preserved egg digests promote the apoptosis of HT29 and HepG2 cells. Food Biosci. 2020, 36, 100661. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, T.; Qin, W.; Huang, B.; Chen, W.; Li, S.; Li, J. Upregulated PTPN2 induced by inflammatory response or oxidative stress stimulates the progression of thyroid cancer. Biochem. Biophys. Res. Commun. 2020, 522, 21–25. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Ren, Y.; Ai, T.; Zhou, P.; Hu, L.; Wang, L.; Li, J.; Li, B. In vitro gastric emptying characteristics of konjac glucomannan with different viscosity and its effects on appetite regulation. Food Funct. 2020, 11, 7596–7610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Wang, J.; Wang, J.; Gu, B.; Ge, F.; Chen, X.D. In vitro gastric digestion and emptying of cooked white and brown rice using a dynamic human stomach system. Food Struct. 2022, 31, 100245. [Google Scholar] [CrossRef]
- Bellmann, S.; Lelieveld, J.; Gorissen, T.; Minekus, M.; Havenaar, R. Development of an advanced in vitro model of the stomach and its evaluation versus human gastric physiology. Food Res. Int. 2016, 88, 191–198. [Google Scholar] [CrossRef]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef]
- Fang, M.; You, J.; Yin, T.; Hu, Y.; Liu, R.; Du, H.; Liu, Y.; Xiong, S. Peptidomic analysis of digested products of surimi gels with different degrees of cross-linking: In vitro gastrointestinal digestion and absorption. Food Chem. 2022, 375, 131913. [Google Scholar] [CrossRef]
- Kong, F.; Oztop, M.H.; Singh, R.P.; McCarthy, M.J. Physical changes in white and brown rice during simulated gastric digestion. J. Food Sci. 2011, 76, E450–E457. [Google Scholar] [CrossRef]
- Malagelada, J.-R.; Go, V.L.; Summerskill, W. Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals. Digest. Dis. Sci. 1979, 24, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Elashoff, J.D.; Reedy, T.J.; Meyer, J.H. Analysis of gastric emptying data. Gastroenterology 1982, 83, 1306–1312. [Google Scholar] [CrossRef]
- Xiao, H.; Fu, X.; Cao, C.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Amini, N.; Supriyanto, E.; Abdul Majid, F.A.; Kumar Jaganathan, S.; Jamil, S.; Hamzehalipour Almaki, J.; Nasiri, R. Antioxidant activity and ROS-dependent apoptotic effect of Scurrula ferruginea (Jack) danser methanol extract in human breast cancer cell MDA-MB-231. PLoS ONE 2016, 11, e0158942. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, L.; Wu, X.; Chen, X.D. Digestive behaviours of large raw rice particles in vivo and in vitro rat stomach systems. J. Food Eng. 2014, 142, 170–178. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A.J.F. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef]
- Rajasekhar, M.D.; Badri, K.R.; Kumar, K.V.; Babu, K.R.; Fatima, S.S.; Kumar, M.T.S.; Rao, C.A. Corrigendum to “Isolation and characterization of a novel antihyperglycemic protein from the fruits of Momordica cymbalaria”[Journal of Ethnopharmacology 128 (1)(2010) 58–62]. Saudi J. Biol. Sci. 2012, 1, 315. [Google Scholar] [CrossRef]
- Renaud, S.d.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Li, L.; Somerset, S. Digestive system dysfunction in cystic fibrosis: Challenges for nutrition therapy. Digest. Liver Dis. 2014, 46, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroent. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Camilleri, M.; Malagelada, J.; Brown, M.; Becker, G.; Zinsmeister, A. Relation between antral motility and gastric emptying of solids and liquids in humans. Am. J. Physiol-Gastro. Liver Physiol. 1985, 249, G580–G585. [Google Scholar] [CrossRef]
- Mackie, A.; Mulet-Cabero, A.-I.; Torcello-Gómez, A. Simulating human digestion: Developing our knowledge to create healthier and more sustainable foods. Food Funct. 2020, 11, 9397–9431. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. Disintegration of solid foods in human stomach. J. Food Sci. 2008, 73, R67–R80. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Fan, T.; Liao, Z.; Wu, P.; Wu, X.; Chen, X.D. Gastric emptying and morphology of a ‘near real’in vitro human stomach model (RD-IV-HSM). J. Food Eng. 2016, 183, 1–8. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Kostlan, K.; Singh, R.P. Particle size distribution of brown and white rice during gastric digestion measured by image analysis. J. Food Sci. 2013, 78, E1383–E1391. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Sun, Q.; Um, B.H.; Park, Y.; McClements, D.J. In vitro and in vivo study of fucoxanthin bioavailability from nanoemulsion-based delivery systems: Impact of lipid carrier type. J. Funct. Foods 2015, 17, 293–304. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohyd. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Abedin, M.; Wang, D.; McDonnell, M.; Lehmann, U.; Kelekar, A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007, 14, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-R.; Shen, S.-C.; Lin, H.-Y.; Hou, W.-C.; Yang, L.-L.; Chen, Y.-C.J.B.P. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem. Pharmacol. 2002, 63, 225–236. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, L.-L.; Liu, H.-P.; Wu, Y.-R.; Li, M.-Y.; Jia, Q. Structural characterization of a novel polysaccharide from Pleurotus citrinopileatus and its antitumor activity on H22 tumor-bearing mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef]
- Jeon, M.-Y.; Min, K.-j.; Woo, S.M.; Seo, S.U.; Kim, S.; Park, J.-W.; Kwon, T.K. Volasertib enhances sensitivity to TRAIL in renal carcinoma Caki cells through downregulation of c-FLIP expression. Int. J. Mol. Sci. 2017, 18, 2568. [Google Scholar] [CrossRef]
- Hussain, S.S.; Zhang, F.; Zhang, Y.; Thakur, K.; Naudhani, M.; Cespedes-Acuña, C.L.; Wei, Z. Stevenleaf from Gynostemma Pentaphyllum inhibits human hepatoma cell (HepG2) through cell cycle arrest and apoptotic induction. Food Sci. Hum. Wellness 2020, 9, 295–303. [Google Scholar] [CrossRef]
- Prenek, L.; Boldizsár, F.; Kugyelka, R.; Ugor, E.; Berta, G.; Németh, P.; Berki, T. The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaboration with Bcl-2 family proteins in developing T cells. Apoptosis 2017, 22, 239–253. [Google Scholar] [CrossRef]
- Warren, C.F.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Glab, J.A.; Cao, Z.; Puthalakath, H. Bcl-2 family proteins, beyond the veil. Int. Rev. Cell Mol. Biol. 2020, 351, 1–22. [Google Scholar] [CrossRef]
- Sprick, M.R.; Walczak, H. The interplay between the Bcl-2 family and death receptor-mediated apoptosis. BBA-Mol. Cell Res. 2004, 1644, 125–132. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R.J.O.M. Understanding of ROS-inducing strategy in anticancer therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, D.; Liu, J.; Ye, S.; Xiao, S.; Wang, W.; Sun, Z.; Xie, Y.; Wang, J. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother. Radiopharm. 2013, 28, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Bianchini, K.; Bourckhardt, G.F.; Ammar, D.; Nazari, E.M.; Müller, Y.M.R. Homocysteine-induced changes in cell proliferation and differentiation in the chick embryo spinal cord: Implications for mechanisms of neural tube defects (NTD). Reprod. Toxicol. 2017, 69, 167–173. [Google Scholar] [CrossRef]
- Granci, V.; Dupertuis, Y.M.; Pichard, C. Angiogenesis as a potential target of pharmaconutrients in cancer therapy. Curr. Opin. Clin. Nutr. 2010, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Ordoñez, R.; Benet, M.; Jover, R.; García-Palomo, A.; Mauriz, J.; González-Gallego, J. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br. J. Cancer 2013, 109, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Tarnawski, A.S. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.L.; Ryan, C.K.; Francis, C.W.; Kohli, M.; Taubman, M.B.; Khorana, A.A. Tissue factor and VEGF expression in prostate carcinoma: A tissue microarray study. Cancer Investig. 2009, 27, 430–434. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, T.; Li, J.; Deng, H.; Song, Y.; Zhai, D.; Peng, Y.; Lu, X.; Liu, M.; Zhao, Y. Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the Notch signaling. PLoS ONE 2014, 9, e113830. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus peel flavonoids as potential cancer prevention agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, X.; Ma, M.; Hu, G.; Fu, X.; Liu, J. Characterization of the Dynamic Gastrointestinal Digests of the Preserved Eggs and Their Effect and Mechanism on HepG2 Cells. Foods 2023, 12, 800. https://doi.org/10.3390/foods12040800
Wu Y, Li X, Ma M, Hu G, Fu X, Liu J. Characterization of the Dynamic Gastrointestinal Digests of the Preserved Eggs and Their Effect and Mechanism on HepG2 Cells. Foods. 2023; 12(4):800. https://doi.org/10.3390/foods12040800
Chicago/Turabian StyleWu, Yan, Xiujuan Li, Meihu Ma, Gan Hu, Xing Fu, and Jihong Liu. 2023. "Characterization of the Dynamic Gastrointestinal Digests of the Preserved Eggs and Their Effect and Mechanism on HepG2 Cells" Foods 12, no. 4: 800. https://doi.org/10.3390/foods12040800
APA StyleWu, Y., Li, X., Ma, M., Hu, G., Fu, X., & Liu, J. (2023). Characterization of the Dynamic Gastrointestinal Digests of the Preserved Eggs and Their Effect and Mechanism on HepG2 Cells. Foods, 12(4), 800. https://doi.org/10.3390/foods12040800