Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis
2.3. Quantification of Fermentable Oligo-, Di-, Mono-Saccharides and Polyols (FODMAPs)
2.4. pH and Total Titratable Acidity (TTA)
2.5. Foaming Capacity and Stability
2.6. Fat Absorption Capacity
2.7. Protein Profile Analysis
2.8. Particle Size
2.9. Minimum Gelation Concentration
2.10. Rheological Characteristics
2.11. Surface Hydrophobicity
2.12. Protein Solubility
2.13. Zeta Potential
2.14. Emulsifying Characteristics
2.15. Colour
2.16. Ultrastructure
2.17. Statistical Analysis
3. Results
3.1. Nutritional Characteristics
3.2. Protein Characteristics
3.2.1. Amino Acid Composition
3.2.2. Protein Profiles
3.3. Physical Properties
3.3.1. pH and Total Titratable Acidity
3.3.2. Protein Solubility, Surface hydrophobicity and Zeta Potential
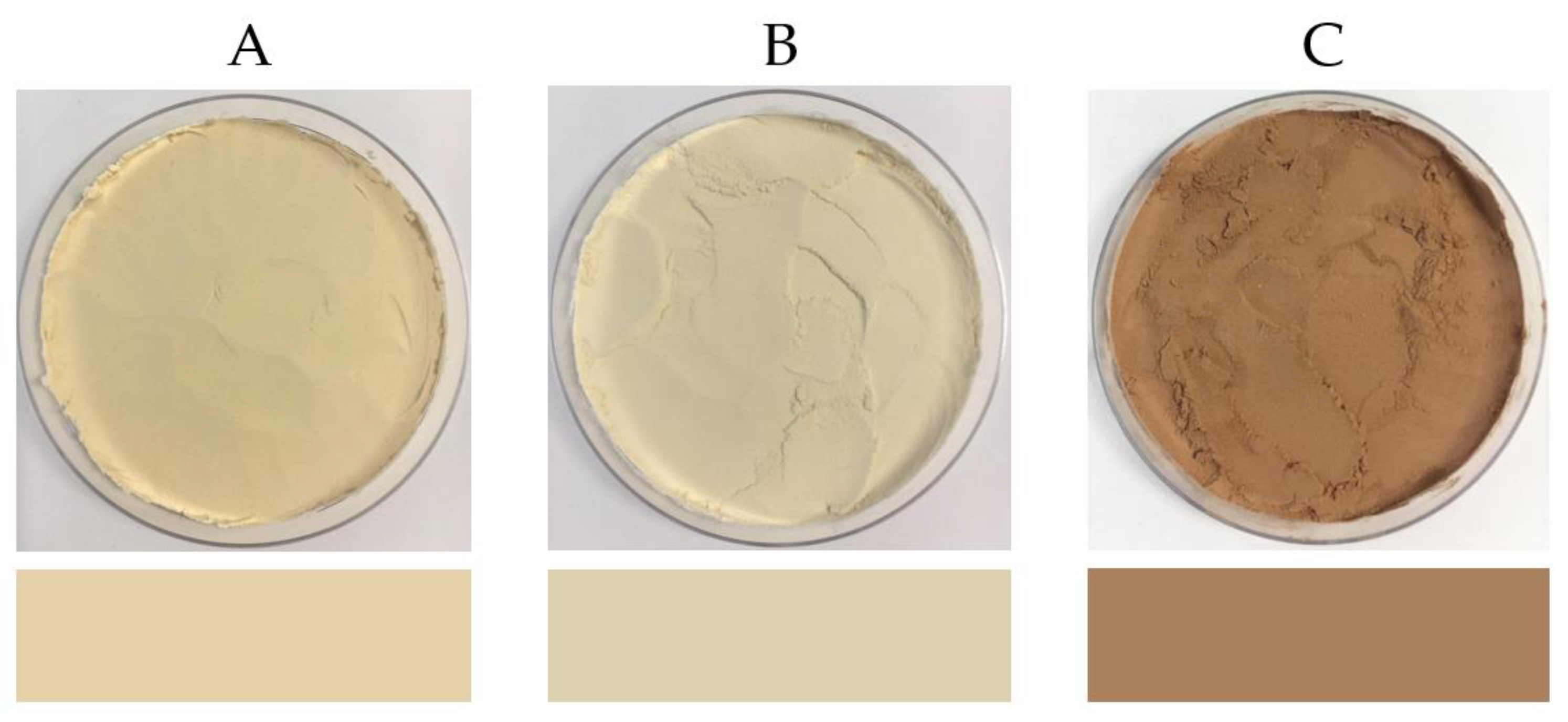
3.3.3. Colour
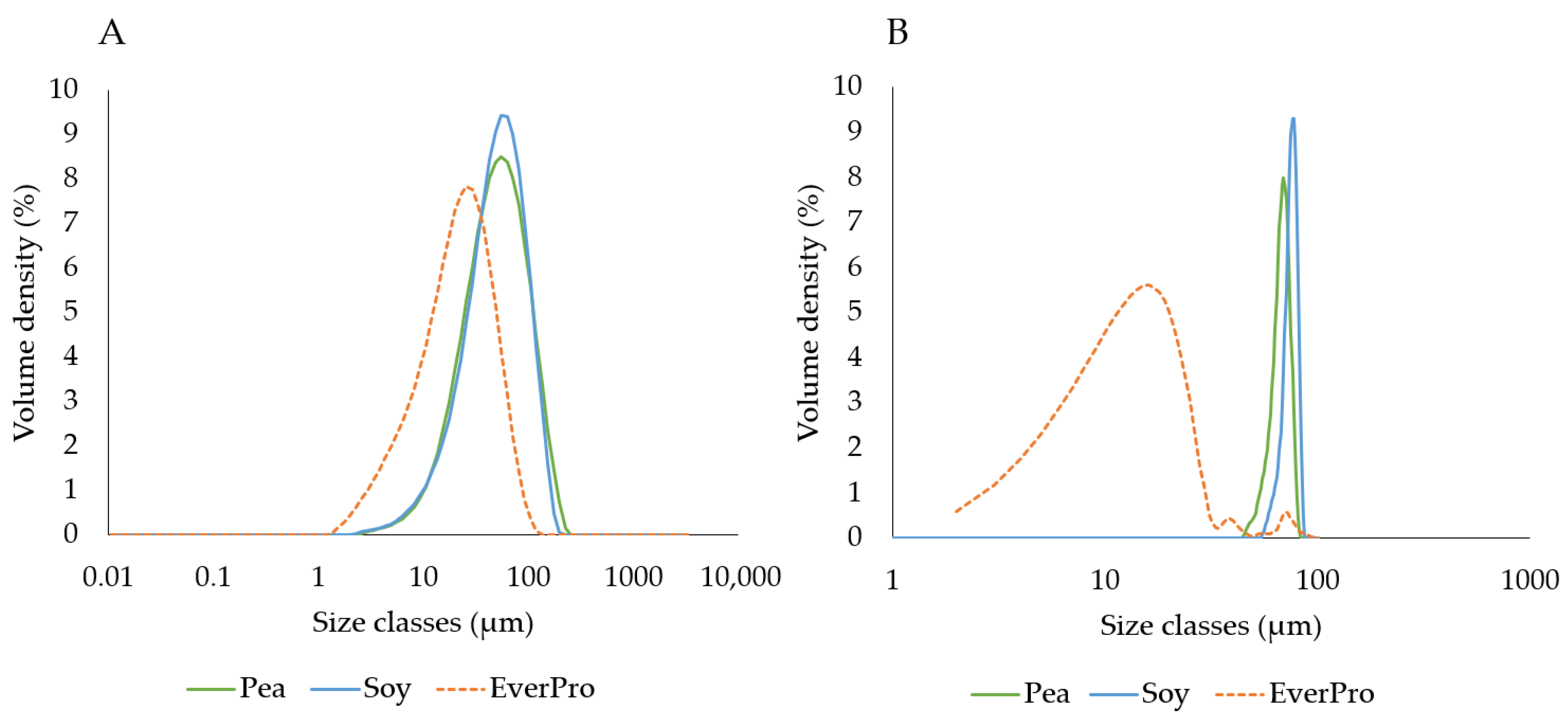
3.3.4. Particle Size
3.3.5. Fat holding Capacity
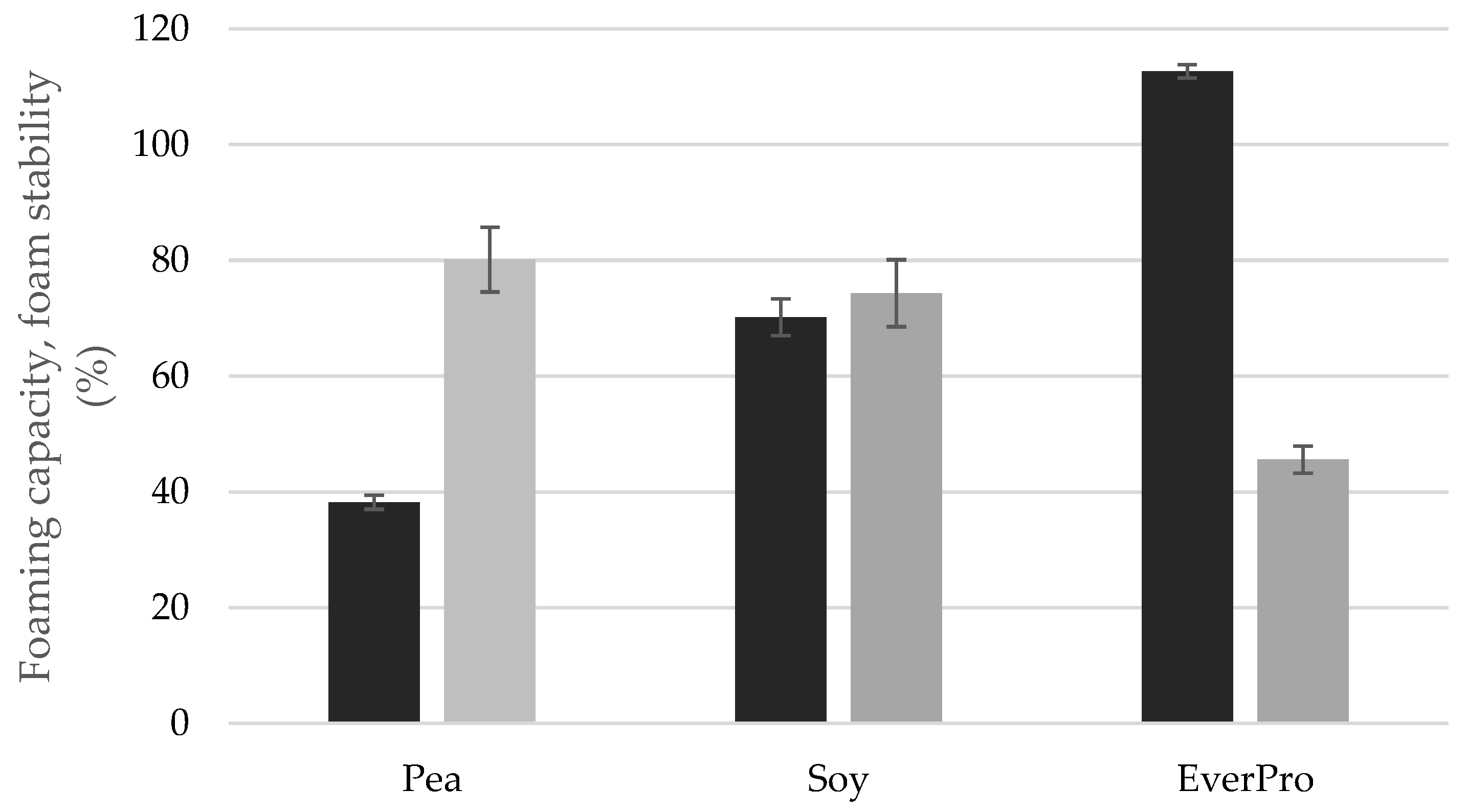
3.3.6. Foaming Capacity and Stability
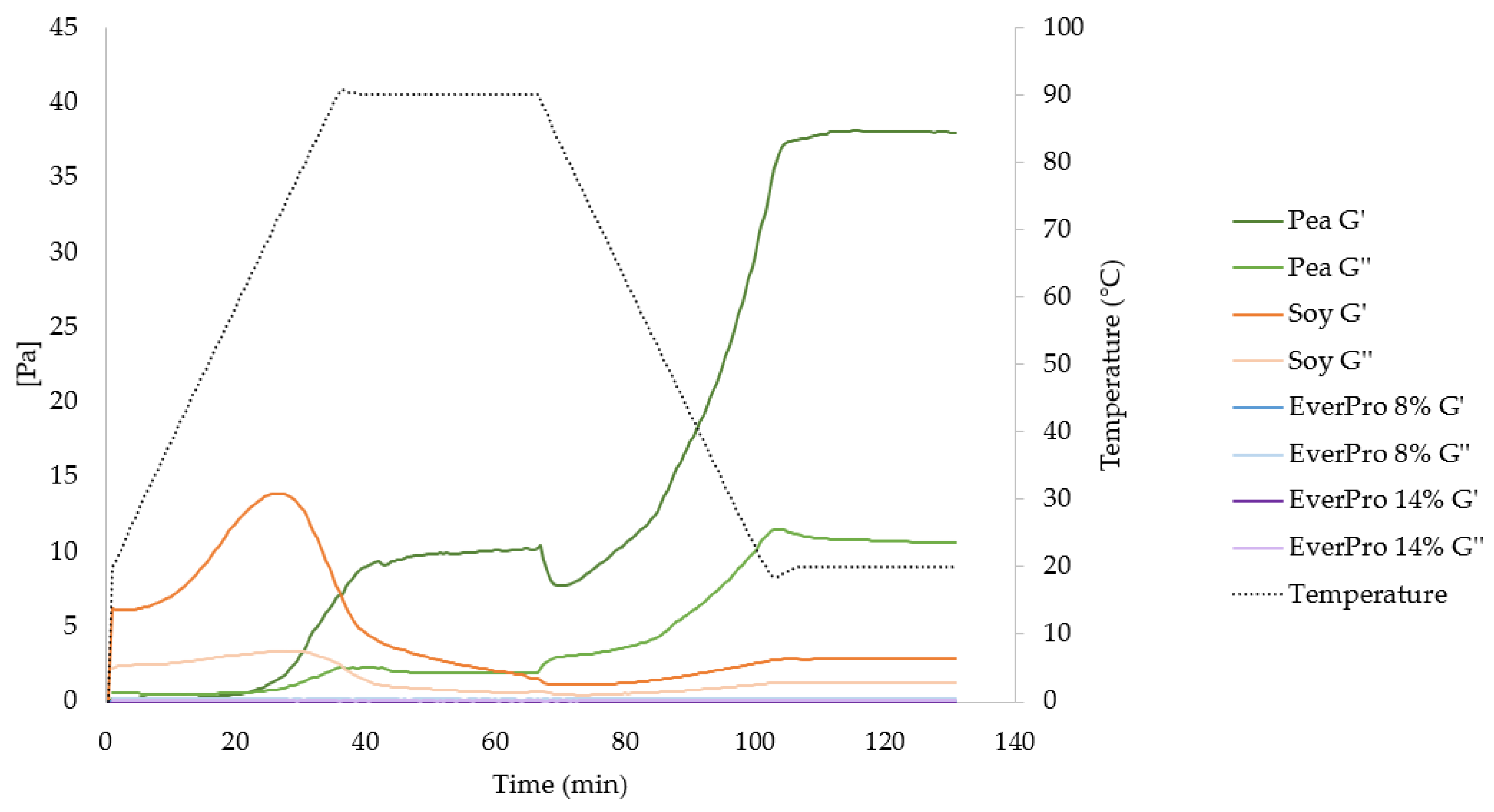
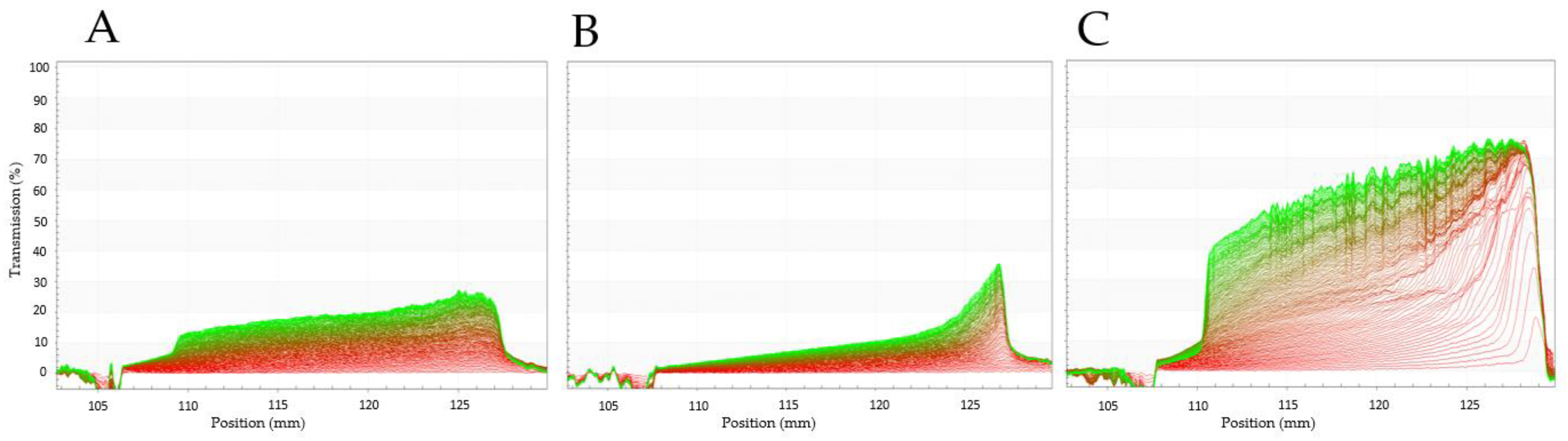
3.3.7. Gelation Characteristics and Rheological Properties
3.3.8. Emulsifying Characteristics
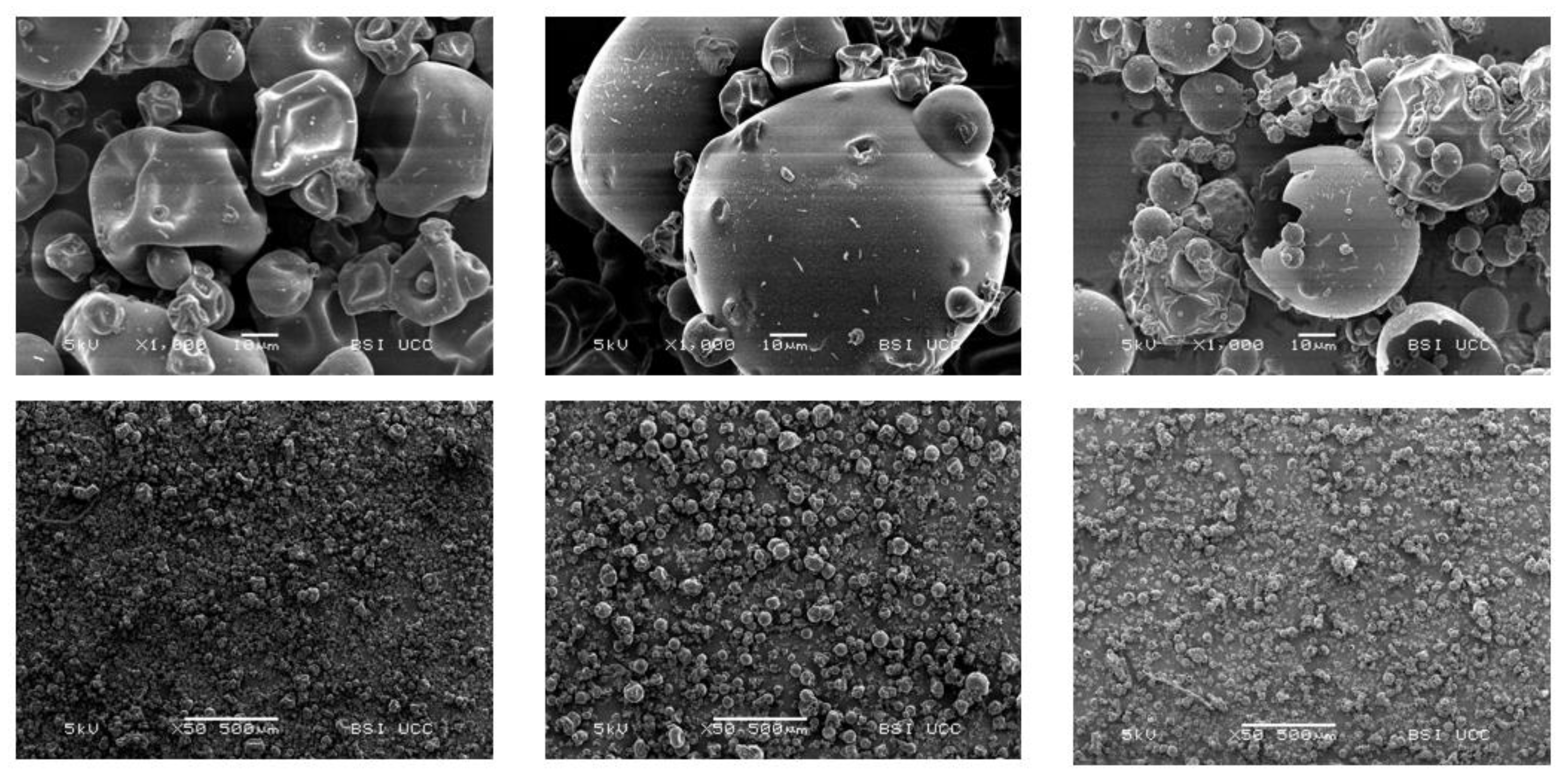
3.3.9. Ultrastructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boukid, F. Plant-Based Meat Analogues: From Niche to Mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Aschemann-witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-cueto, F.J.A.; Gantriis, R.F.; Fraga, P. Plant-Based Food and Protein Trend from a Business Perspective: Markets, Consumers, and the Challenges and Opportunities in the Future. Crit. Rev. Food Sci. Nutr. 2020, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef]
- Stewart, G.G.; Russell, I.; Anstruther, A. Handbook of Brewing, 3rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 9781498751919. [Google Scholar]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fundamental Study of the Application of Brewers Spent Grain and Fermented Brewers Spent Grain on the Quality of Pasta. Food Struct. 2021, 30, 100225. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 1, jib363. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’ s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 12, 767–778. [Google Scholar] [CrossRef]
- Klose, C.; Thiele, F.; Arendt, E.K. Changes in the Protein Profile of Oats and Barley during Brewing and Fermentation. J. Am. Soc. Brew. Chem. 2010, 68, 119–124. [Google Scholar] [CrossRef]
- Osman, A.M.; Coverdale, S.M.; Cole, N.; Hamilton, S.E.; de Jersey, J. Characterisation and Assessment of the Role of Barley Malt Endoproteases During Malting and Mashing1. J. Inst. Brew. 2002, 108, 62–67. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 1–23. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea Protein Isolates: Structure, Extraction, and Functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsi Fi Cation. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Arcand, Y. Food Chemistry and Toxicology Composition and Functional Properties of Soy Protein Isolates Prepared Using Alternative Defatting and Extraction Procedures. Food Chem. Toxicol. 2006, 71, C137–C145. [Google Scholar]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with Starchy Adjuncts: Its Influence on the Sensory and Nutritional Properties of Beer. Foods 2021, 10, 2911. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Hoseney, R.C. Principles of Cereal Science and Technology, 3rd ed.; AACC International: St Paul, MN, USA, 2010. [Google Scholar]
- AACC. Method 46-12—Crude Protein–Kjeldahl Method, Boric Acid Modification. In International Approved Methods of Analysis, 11th ed.; AACC: St. Paul, MN, USA, 2011. [Google Scholar]
- AACC. Method 30-25.01—Crude Fat in Wheat, Corn, Soy Flour and Feed Mixes. In International Approved Methods of Analysis, 11th ed.; AACC: St Paul, MN, USA, 2011. [Google Scholar]
- AACC. Method 44-15.02—Moisture-Air-Oven Method. In International Approved Methods of Analysis, 11th ed.; AACC: St Paul, MN, USA, 2011. [Google Scholar]
- Hoehnel, A.; Bez, J.; Petersen, I.L.; Amarowicz, R.; Juśkiewicz, J.; Arendt, E.K.; Zannini, E. Enhancing the Nutritional Profile of Regular Wheat Bread While Maintaining Technological Quality and Adequate Sensory Attributes. Food Funct. 2020, 11, 4732–4751. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef]
- Waters, D.M.; Kingston, W.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Wheat Bread Biofortification with Rootlets, a Malting by-Product. J. Sci. Food Agric. 2013, 93, 2372–2383. [Google Scholar] [CrossRef]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Food Ingredients for the Future: In-Depth Analysis of the Effects of Lactic Acid Bacteria Fermentation on Spent Barley Rootlets. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Bez, J.; Petersen, I.L.; Joehnke, M.S.; Detzel, A.; Busch, M.; Krueger, M.; Ispiryan, L.; O’Mahony, J.A.; Arendt, E.K.; et al. Techno-Functional, Nutritional and Environmental Performance of Protein Isolates from Blue Lupin and White Lupin. Foods 2020, 9, 230. [Google Scholar] [CrossRef]
- Vogelsango, D.; Petersen, M.; Joehnke, I.L.; Sørensen, M.S.; Jill, C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Mahony, J.A.O.; et al. Comparison of Faba Bean Protein Ingredients Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Silva, J.V.C.; O’Mahony, J.A. Flowability and Wetting Behaviour of Milk Protein Ingredients as Influenced by Powder Composition, Particle Size and Microstructure. Int. J. Dairy Technol. 2017, 70, 277–286. [Google Scholar] [CrossRef]
- Vogelsang, M.; Aylin, O.D.; Francesca, W.S.; James, B.; Juergen, A.O.M.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Lentil Protein Concentrate for Modification of Physicochemical and Techno-Functional Properties. Eur. Food Res. Technol. 2022, 1, e04152. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Physicochemical and Nutritional Properties of High Protein Emulsion-Type Lupin-Based Model Milk Alternatives: Effect of Protein Source and Homogenization Pressure. J. Sci. Food Agric. 2021, 5, 11230. [Google Scholar] [CrossRef] [PubMed]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative Analysis of Plant-Based High-Protein Ingredients and Their Impact on Quality of High-Protein Bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Atzler, J.J.; Sahin, A.W.; Gallagher, E.; Zannini, E.; Arendt, E.K. Investigation of Different Dietary-Fibre-Ingredients for the Design of a Fibre Enriched Bread Formulation Low in FODMAPs Based on Wheat Starch and Vital Gluten. Eur. Food Res. Technol. 2021, 247, 1939–1957. [Google Scholar] [CrossRef]
- WHO. Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Gläser, P.; Mittermeier-Kleßinger, V.K.; Spaccasassi, A.; Hofmann, T.; Dawid, C. Quantification and Bitter Taste Contribution of Lipids and Their Oxidation Products in Pea-Protein Isolates (Pisum sativum L.). J. Agric. Food Chem. 2021, 69, 8768–8776. [Google Scholar] [CrossRef]
- Nemeth, C.; Andersson, A.A.M.; Andersson, R.; Mangelsen, E.; Sun, C.; Åman, P. Relationship of Grain Fructan Content to Degree of Polymerisation in Different Barleys. Food Nutr. Sci. 2014, 05, 581–589. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-Profile in Cereal-Product Ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Ispiryan, L.; Kuktaite, R.; Zannini, E.; Arendt, E.K. Fundamental Study on Changes in the FODMAP Profile of Cereals, Pseudo-Cereals, and Pulses during the Malting Process. Food Chem. 2021, 343, 128549. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. FODMAP Modulation as a Dietary Therapy for IBS: Scientific and Market Perspective. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1491–1516. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; Fitzgerald, R.J. Characterisation of Protein-Rich Isolates and Antioxidative Phenolic Extracts from Pale and Black Brewers’ Spent Grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. The Effects of Malting and Mashing on Barley Protein Extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zhong, Z.; Wang, D.; Sun, X. Soybean Glycinin Subunits: Characterization of Physicochemical and Adhesion Properties. J. Agric. Food Chem. 2006, 54, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E.; Barbeau, W.E.; Kella, N.K. Effect of Oxidative Sulfitolysis of Disulfide Bonds of Glycinin on Solubility, Surface Hydrophobicity, and in Vitro Digestibility. J. Agric. Food Chem. 1986, 34, 251–256. [Google Scholar] [CrossRef]
- Maruyama, N.; Salleh, M.R.M.; Takahashi, K.; Yagasaki, K.; Goto, H.; Hontani, N.; Nakagawa, S.; Utsumi, S. Structure-Physicochemical Function Relationships of Soybean β-Conglycinin Heterotrimers. J. Agric. Food Chem. 2002, 50, 4323–4326. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 3, 198–224. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food Proteins: A Review on Their Emulsifying Properties Using a Structure—Function Approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic Hydrolysis of Brewers’ Spent Grain Proteins and Technofunctional Properties of the Resulting Hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Trends in Food Science & Technology Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspensionstability: Why Particle Size Zeta Potentialand Rheology Are Important. Ann. Trans. Nord. Rheol. Soc. 2 2012, 20, 209–214. [Google Scholar]
- Stone, A.K.; Avarmenko, N.A.; Warkentin, T.D.; Nickerson, M.T. Functional Properties of Protein Isolates from Different Pea Cultivars. Food Sci. Biotechnol. 2015, 24, 827–833. [Google Scholar] [CrossRef]
- Lam, M.; Paulsen, P.; Corredig, M. Interactions of Soy Protein Fractions with High-Methoxyl Pectin. J. Agric. Food Chem. 2008, 56, 4726–4735. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure Formation and Rheological Properties of Pea Protein-Based Gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef]
- Klost, M.; Brzeski, C.; Drusch, S. Effect of Protein Aggregation on Rheological Properties of Pea Protein Gels. Food Hydrocoll. 2020, 108, 106036. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Induced by Heat Treatment. Food Res. Int. 2010, 43, 509–515. [Google Scholar] [CrossRef]
- Shand, P.J.; Ya, H.; Pietrasik, Z.; Wanasundara, P.K.J.P.D. Physicochemical and Textural Properties of Heat-Induced Pea Protein Isolate Gels. Food Chem. 2007, 102, 1119–1130. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Gonzalez, R.; Subirade, M. Formation of Soy Protein Isolate Cold-Set Gels: Protein and Salt Effects. Food Chem. Toxicol. 2005, 70, 67–73. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Chand, J. Structural and Functional Characterization of Kidney Bean and Fi Eld Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Coghe, S.; Gheeraert, B.; Michiels, A.; Delvaux, F.R. Development of Maillard Reaction Related Characteristics during Malt Roasting. J. Inst. Brew. 2006, 112, 148–156. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Liu, H.; Sun, X.; Ye, Y.; Cao, X. Effect of PH Regulation on the Components and Functional Properties of Proteins Isolated from Cold-Pressed Rapeseed Meal through Alkaline Extraction and Acid Precipitation. Food Chem. 2020, 327, 126998. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.; Sutar, A.K.; Maharana, T. Graft Copolymerization of Soy Protein Isolate with Polylactide via Ring Opening Polymerization. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Harbin, China, 4–6 May 2018; Volume 410. [Google Scholar]
- Yan, G.; Cui, Y.; Lia, D.; Ding, Y.; Han, J.; Wang, S.; Yang, Q.; Zheng, H. The Characteristics of Soybean Protein Isolate Obtained by Synergistic Modification of High Hydrostatic Pressure and Phospholipids as a Promising Replacement of Milk in Ice Cream. LWT 2022, 160, 113223. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional Properties and Structural Characteristics of Phosphorylated Pea Protein Isolate. Int. J. Food Sci. Technol. 2020, 55, 2002–2010. [Google Scholar] [CrossRef]

| PPI | SPI | EverPro | |
|---|---|---|---|
| g/100 g DM | |||
| Moisture | 7.29 ± 0.11 a | 6.62 ± 0.08 b | 6.10 ± 0.05 c |
| Protein | 81.22 ± 0.43 a | 89.22 ± 0.33 b | 83.22 ± 1.11 c |
| Fat | 8.51 ± 0.07 a | 1.72 ± 0.17 b | 0.37 ± 0.06 c |
| Total Sugars (Sucrose, Glucose, Fructose, Maltose) | 0.19 ± 0.00 a | 0.06 ± 0.00 b | 0.30 ± 0.00 c |
| Total Starch (digestible) | 2.83 ± 0.06 a | 1.67 ± 0.03 b | 1.42 ± 0.04 c |
| FODMAPs (Total) | 0.53 ± 0 a | 0.04 ± 0 b | n.d.*c |
| Essential Amino Acids | PPI | SPI | EverPro | |||
|---|---|---|---|---|---|---|
| % Requirement * | % Requirement * | % Requirement * | ||||
| Histidine | 2.227 ± 0.311 | 182.8 | 2.255 ± 0.314 | 168.5 | 1.768 ± 0.181 | 141.7 |
| Isoleucine | 3.955 ± 0.550 | 162.3 | 3.116 ± 0.434 | 116.4 | 2.673 ± 0.266 | 107.1 |
| Leucine | 7.422 ± 1.032 | 154.9 | 6.689 ± 0.931 | 127.1 | 6.145 ± 0.618 | 125.2 |
| Lysine | 6.399 ± 0.891 | 175.1 | 5.343 ± 0.743 | 133.1 | 3.035 ± 0.277 | 81.1 |
| Methionine + cysteine | 0.605 ± 0.075 | 33.9 | 0.942 ± 0.118 | 48.0 | 2.769 ± 0.296 | 151.3 |
| Phenylalanine + tyrosine ** | 8.456 ± 0.843 | 273.9 | 8.16 ± 0.814 | 240.8 | 8.317 ± 0.597 | 263.1 |
| Threonine | 3.137 ± 0.437 | 167.9 | 3.281 ± 0.456 | 159.9 | 3.45 ± 0.351 | 180.3 |
| Tryptophan | 0.398 ± 0.043 | 81.7 | 0.690 ± 0.075 | 128.8 | 1.171 ± 0.128 | 234.7 |
| Valine | 4.433 ± 0.617 | 139.9 | 3.358 ± 0.468 | 96.5 | 4.004 ± 0.405 | 123.4 |
| Non-essential amino acids | ||||||
| Alanine | 3.66 ± 0.509 | 3.571 ± 0.497 | 4.228 ± 0.426 | |||
| Arginine | 8.422 ± 1.172 | 7.327 ± 1.019 | 4.707 ± 0.469 | |||
| Aspartic acid | 9.286 ± 1.292 | 8.963 ± 1.248 | 7.699 ± 0.767 | |||
| Glutamic acid | 14.549 ± 2.025 | 15.555 ± 2.164 | 20.234 ± 2.023 | |||
| Proline | 4.035 ± 0.562 | 4.511 ± 0.628 | 8.019 ± 0.799 | |||
| Serine | 4.694 ± 0.654 | 4.907 ± 0.683 | 4.164 ± 0.415 | |||
| Glycine | 3.842 ± 0.535 | 3.688 ± 0.514 | 3.663 ± 0.373 | |||
| Free Amino Acids | ||||||
| Glutamic acid | 0 | 0 | 0.168 ± 0.028 | |||
| Alanine | 0 | 0 | 0.085 ± 0.016 | |||
| Arginine | 0.053 ± 0.009 | 0 | 0.018 ± 0.004 | |||
| Asparagine | n.d. | n.d. | 0.012 ± 0.003 | |||
| Citrulline | 0 | 0 | 0.044 ± 0.010 | |||
| Phenylalanine | 0 | 0 | 0.132 ± 0.022 | |||
| Glycine | n.d. | n.d. | 0.007 ± 0.002 | |||
| Glutamine | 0.013 ± 0.008 | 0 | 0.038 ± 0.008 | |||
| Isoleucine | 0 | 0 | 0.086 ± 0.016 | |||
| Histidine | 0 | 0 | 0.014 ± 0.003 | |||
| Leucine | 0.013 ± 0.008 | 0 | 0.217 ± 0.036 | |||
| Lysine | 0 | 0 | 0.024 ± 0.005 | |||
| Methionine | 0 | 0 | 0.054 ± 0.012 | |||
| Ornithine | n.d. | n.d. | 0.01 ± 0.002 | |||
| Serine | n.d. | n.d. | 0.039 ± 0.009 | |||
| Tyrosine | n.d. | n.d. | 0.108 ± 0.019 | |||
| Threonine | n.d. | n.d. | 0.026 ± 0.006 | |||
| Aspartic acid | n.d. | n.d. | 0.081 ± 0.015 | |||
| Valine | 0 | 0 | 0.085 ± 0.016 | |||
| Total free amino acid | 0.079 ± 0.014 | 0 ± 0 | 1.248 ± 0.066 | |||
| Pea | Soy | EverPro | |
|---|---|---|---|
| pH | 6.360 ± 0.030 a | 6.770 ± 0.010 b | 7.900 ± 0.000 c |
| TTA (mL 0.1 M NaOH) | 11.470 ± 0.020 a | 12.060 ± 0.260 a | 17.850 ± 0.100 b |
| Protein solubility (%) | 22.267 ± 1.457 a | 51.960 ± 3.354 b | 101.714 ± 2.898 c |
| Surface Hydrophobicity (a.u.) | 4292.467 ± 500 a | 7471.367 ± 324 b | n.a. |
| Zeta potential at pH7 (mV) | −22.600 ± 1.633 a | −33.778 ± 1.524 b | −30.033 ± 2.035 b |
| Separation rate (%/min) | 1.327 ± 0.110 a | 0.990 ± 0.008 a | 4.135 ± 0.373 b |
| Fat Absorption (%) | 157.723 ± 3.202 a | 120.050 ± 17.847 b | 182.350 ± 1.909 a |
| Colour L* | 84.688 ± 1.164 a | 83.821 ± 0.603 a | 57.415 ± 2.379 b |
| Colour a* | 2.180 ± 0.036 a | 0.734 ± 0.049 b | 13.400 ± 0.455 c |
| Colour b* | 22.338 ± 0.215 a | 18.498 ± 0.129 b | 24.976 ± 0.804 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeger, A.; Sahin, A.W.; Nyhan, L.; Zannini, E.; Arendt, E.K. Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio. Foods 2023, 12, 798. https://doi.org/10.3390/foods12040798
Jaeger A, Sahin AW, Nyhan L, Zannini E, Arendt EK. Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio. Foods. 2023; 12(4):798. https://doi.org/10.3390/foods12040798
Chicago/Turabian StyleJaeger, Alice, Aylin W. Sahin, Laura Nyhan, Emanuele Zannini, and Elke K. Arendt. 2023. "Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio" Foods 12, no. 4: 798. https://doi.org/10.3390/foods12040798
APA StyleJaeger, A., Sahin, A. W., Nyhan, L., Zannini, E., & Arendt, E. K. (2023). Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio. Foods, 12(4), 798. https://doi.org/10.3390/foods12040798








