1. Introduction
Listeria monocytogenes is a bacterial pathogen that is mainly transmitted through food. The pathogen is notorious worldwide, and can grow in high salinity (10%), low water activity (<0.9), low temperature (4 °C), and in a pH range of 4.1–9.6 [
1]. Therefore, it can survive in processing equipment, packaging materials, food contact surfaces, etc. Food processing, in particular, is considered to be the most important pathway for
L. monocytogenes contamination [
2].
L. monocytogenes can cause disease in certain groups, such as the elderly, infants, pregnant women, and people with weakened immunity, with a fatality rate of up to 30% in some countries [
3,
4], including the European Union (EU), the United States, and China [
5,
6,
7].
At present,
L. monocytogenes is frequently found in a variety of food products, especially those of animal origin, with a plethora of isolated strains being resistant to antibiotics [
8,
9]. Therefore, various foods or food products, especially meat products, have been investigated in recent years [
10]. In 2018, the highest contamination rate of
L. monocytogenes in the EU was reported for RTE (ready to eat) food products, accounting for 37.5% of all contamination [
4]. From 2016 to 2018,
L. monocytogenes was detected in 5996 raw meat products in Poland, with a prevalence rate of 2.1% and the rate among different types of meats varying [
11]. The prevalence of
L. monocytogenes in livestock and poultry meat in 28 provinces in China has been investigated, with the highest prevalence of 8.91% in meat and poultry [
12]. The prevalence of
L. monocytogenes in ruminant farms and slaughter environments in China was also tested, and
L. monocytogenes was detected in multiple processes in the slaughter environment [
13]. Hence, authorities around the world were devoted to the study of the prevalence of
L. monocytogenes in meat products, covering different types of meat and over different periods, and even incorporating research studies in the production chain [
14,
15]. However, based on case studies, there are still differences in prevalence between countries and even between cities, and it is hard to see the whole picture in terms of the prevalence of this pathogen in a certain area.
Meanwhile, the Food and Agriculture Organization (FAO), the World Organization for Animal Health (WOAH)), and the World Health Organization (WHO) have collected and provided data about the emergence of antibiotic resistant
L. monocytogenes isolates, and the hazard of
L monocytogenes in food was further elaborated upon [
16].
L. monocytogenes isolated in different countries developed resistance to first-line antibiotics such as rifampin, kanamycin, streptomycin and erythromycin [
17,
18,
19,
20]. The heavy use of prescription drugs in livestock and clinics is one of the reasons for the increased frequency of antibiotic resistance [
10]. Additionally, multi-drug resistant strains were beginning to emerge in food and environmental isolates [
21]. Some case studies found a high frequency of antibiotic resistance to cefotaxime, ciprofloxacin and tetracycline in certain
L. monocytogenes [
22,
23,
24,
25,
26]. Another study found that all 25 strains, isolated from pig slaughtering sites in Romania, were resistant to penicillin, imipenem and fusidiac acid and seven other antibiotics [
27]. However, the overall understanding of the prevalence of the antibiotic resistance of
L. monocytogenes in various geographical regions has also been less studied.
In recent years, more and more meta-analyses have been conducted in the field of food safety [
28,
29,
30,
31,
32,
33]. A meta-analysis can deal with the overall and sub-sets of the prevalence of certain pathogens in food and show the pre- and post-intervention effects on food microbes, and it is therefore a powerful tool for evaluating, identifying and summarizing the results of a large number of studies [
34]. A meta-analysis of the prevalence of
Listeria spp. in foods was performed in Iran, and a pooled prevalence of
L. monocytogenes in meat products of 2.6% (95% CI: 0.2–35.0%) was reported [
35]. In another study, a meta-analysis of the prevalence of
L. monocytogenes in meat products in China was conducted from 2007 to 2017, and it found that the prevalence of
L. monocytogenes in raw and RTE meat products was 8.5% (95% CI: 7.1–10.3%) and 3.2% (95% CI: 2.7–3.9%), respectively [
34]. This is despite the fact that some meta-analyses of
L. monocytogenes were already conducted in meat products and clear information on the prevalence of the pathogen in different types of meat was provided. China and the EU can partially reflect the meat safety levels for the developing and developed countries since both of them are big meat consumers and producers [
36,
37]. Considering that no meta-analysis has been conducted on the prevalence and antibiotic resistance of
L. monocytogenes in livestock and poultry meat products between China and the EU over a 20-year period to date, an updated systematic comparative review on the prevalence and antibiotic resistance of
L. monocytogenes in these regions is urgently needed.
Therefore, the objective of this meta-analysis was to assess the prevalence and antibiotic resistance of L. monocytogenes in livestock and poultry (beef, pork and chicken) meat products in China and the EU by extracting data from numerous literature sources collected from various databases. In the quantitative analysis, the differences between China and the EU were compared through the data and the discrepancies were discussed. The results obtained in this study can further help to formulate reasonable preventive measures against L. monocytogenes and to select effective drugs for the treatment of listeriosis through the understanding of antibiotic resistance.
4. Discussion
Food safety is a multi-faceted international issue [
44]. Both developing and developed countries are actively looking for ways to prevent food safety problems. Microbial foodborne risk is usually defined as the possibility and severity of adverse effects on human health [
45]. Based on the data obtained in this review, the prevalence of
L. monocytogenes from livestock and poultry (beef, pork and chicken) meat in China remained at low levels throughout almost the last 20 years, and it is slightly lower than that in EU countries.
Chronologically, the prevalence of
L. monocytogenes showed an overall downward trend. The prevalence peaked in 2002–2005, and gradually declined and stabilized in 2006, following the intensive regulation and legislation on food safety in China’s 10th Five-Year Plan [
46] and the establishment of the General Food Law by the European Food Safety Authority in 2002 [
47]. This indicates that governance at the national level plays an effective role. Among different meat products, the prevalence of
L. monocytogenes in chicken and pork was higher than that in beef. Meanwhile, the meat consumption percentages for pork and poultry meat are above 60% and 20%, respectively, in China. Therefore, measures taken to control
L. monocytogenes in pork and poultry meat are essential to improve the meat safety level [
48].
The prevalence of
L. monocytogenes in raw meat is, as expected, much higher than in RTE and cooked meat products. This suggests that we need to strengthen the monitoring of
L. monocytogenes contamination in raw meat. However, the opposite is true: in recent years, more attention has been paid to foodborne pathogens in RTE and cooked meat products, while the high prevalence of
L. monocytogenes in raw meat products has been overlooked [
49,
50,
51]. In the vast majority of cases, raw meat is eaten after being heated or cooked; however, attention should be paid to the heating temperature, heating time, and secondary contamination. Therefore, the addition of cooking labels to different raw meat packages is a useful measure that can also effectively avoid the survival of pathogenic bacteria due to the use of incorrect processing methods or the failure to meet the required heating temperature and time. Meanwhile, RTE meat products and cooked meat products may be exposed to pathogenic bacteria and cause their spread through cross-contamination during storage, transportation, packaging, and consumption [
52,
53]. The food processing environment is considered to be the main source of RTE food contamination by
L. monocytogenes. Specifically, it can survive for a long time under adverse conditions such as low temperature and is an important cause of persistent outbreaks of human listeriosis [
54,
55]. At the same time, the boundary between RTE meat and cooked meat is not clear in many studies, and the classification of RTE meat is not detailed. This has resulted in the inability to set appropriate subgroups for analysis. Therefore, developing a more detailed classification of RTE meat is important to determine the effect of different processed types of products with regard to prevalence and to further understand the effect of physicochemical properties on them.
The detection method of
L. monocytogenes also has an impact on the identification results, with a slightly lower prevalence reported when biochemical identification methods were used compared to the molecular methods. The discrepancy may be due to false positives in the PCR primer design process where the amplification sequence has the same sequence as the non-target gene amplification sequence, the difficulty in distinguishing dead from live bacteria, or the false positive results due to contamination [
56]. However, due to the high cost of biochemical identification and long testing period, this method is not commonly used [
57]. On the contrary, molecular methods are increasingly used for the identification of
L. monocytogenes [
58]. Moreover, new techniques such as chromatography and immuno- and aptamer techniques are now being used more frequently in the detection of
L. monocytogenes, but there is still a long way to go before they are widely used [
59].
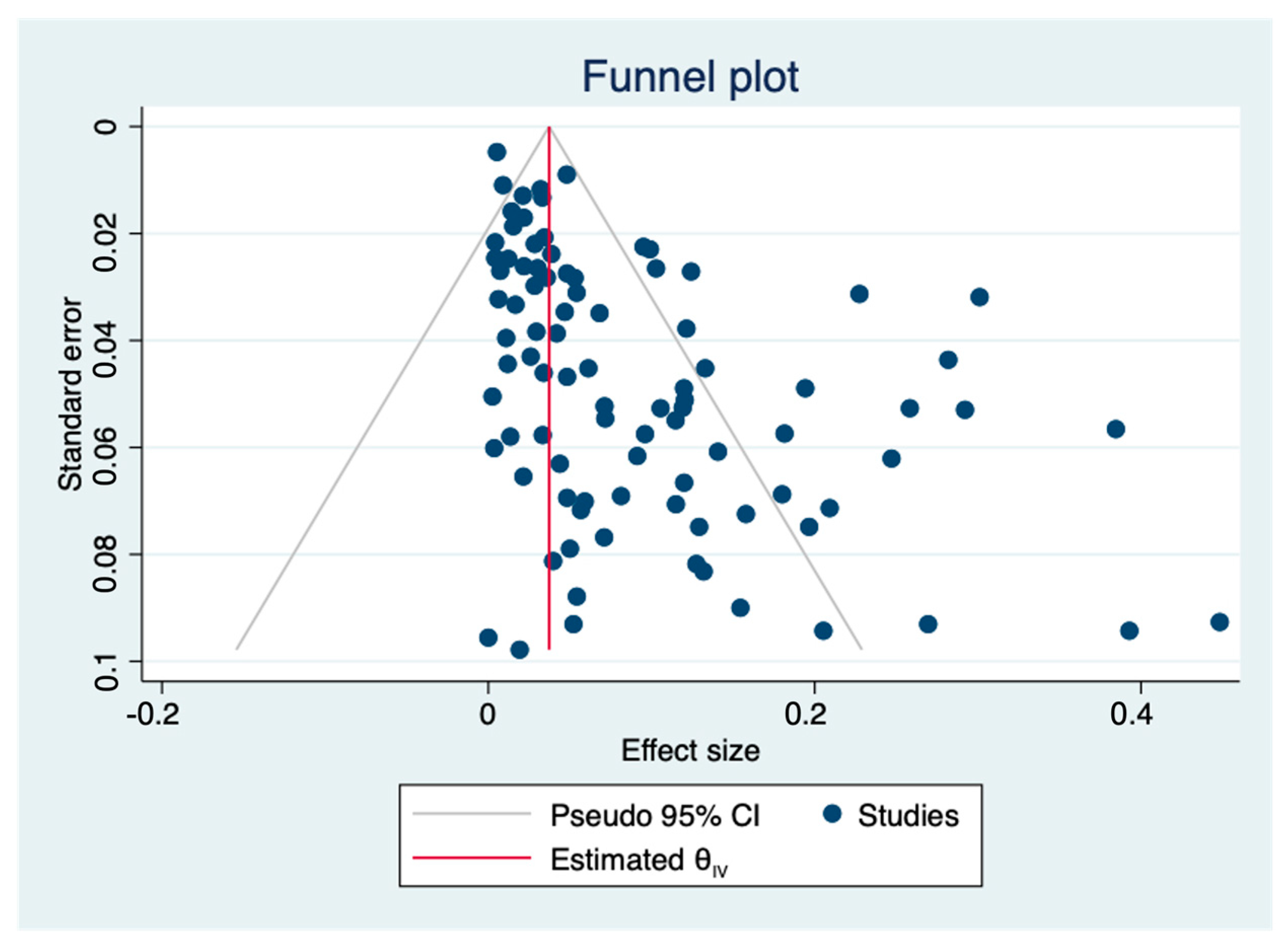
In this study, the pooled prevalence of antibiotic resistance of
L. monocytogenes in livestock and poultry between China and EU was 5.8% (95% CI: 3.1–9.1%), which was much lower than that of RTE foods globally (38.1%; 95% CI: 36.1–39.7%) [
60]. The reason for the difference may be due to the nature and diversity of the food types, their nutrient content, status, water activity, etc., as well as differences in processing methods. Internationally, RTE foods cover a wider range of food types, including fish products, dairy products, and salad products, which may undergo multiple food processing steps which increase the risk of cross-contamination. It is also relevant that under the sublethal environmental stresses encountered during the food processing, bacteria can develop a stress response and increase the resistance to the subsequent exposure to antibiotics [
61,
62]. In order to come to a conclusion, the number of studies regarding antibiotic resistance in the present meta-analysis, which is quite small (nine studies with 356 samples), should also be taken into account. Ampicillin and oxacillin (β-lactam antibiotics) can inhibit the synthesis of polysaccharide peptides in bacterial cell walls [
63].
L. monocytogenes is naturally susceptible to β-lactam antibiotics, and the standard antibiotic regimen prescribed for listeriosis includes penicillin/ampicillin alone or in combination with aminoglycosides (gentamicin) [
64]. The results of this study showed that
L. monocytogenes was still sensitive to ampicillin and gentamicin, confirming the feasibility of treatment criteria. However, there was a high resistance rate (61.2%; 95% CI: 19.4–95.4%) to oxacillin. This suggests that
L. monocytogenes has begun to develop resistance, which may be attributed to the overuse of this drug in the veterinary field [
65].
In order to reduce the phenomenon of pathogenic bacteria resistance to antibiotics in animal-derived food, the EU implemented an EU-wide ban on the use of antibiotics as growth promoters in animal feed on 1 January 2006 [
64]. China has also developed a corresponding catalog of banned veterinary drugs [
66]. The antibiotics involved in this study, with the exception of vancomycin, are not included in the list of prohibited veterinary drugs. However, this does not imply that antibiotic resistance of pathogenic bacteria in meat products can be greatly reduced, since treatment with antibiotics may also be an option when animals are sick. During food production and processing, there is also a risk of exposure to antibiotic-resistant bacteria [
67]. Taking globalization into consideration, the import and export of food between countries also contributes to the spread of antibiotic resistance [
68]. The increase in antibiotic resistance rates over time was also demonstrated in the present study. To deal with this situation, governmental authorities should first strengthen the supervision of the use of antibiotics in animals bred for food production. When animals are sick, drugs other than antibiotics should be prescribed for treatment. Additionally, antibiotic susceptibility testing is necessary prior to the use of antibiotics.