Abstract
Although bioprotection is now recognised as an alternative to SO2 for limiting microbial spoilage, it does not guarantee protection against oxidation. This limits its application, more specifically for rosé winemaking. Oenological tannins present antioxidant properties, which could represent an interesting alternative to SO2 to protect must and wines against oxidation. A combination of the inoculation of a bioprotectant yeast strain and the addition of oenological tannins was tested to eliminate sulfites during the pre-fermentative step of rosé winemaking. In this experiment carried out in a winery, two oenological tannins were compared: quebracho and gall nut tannins. The antioxidant efficiency of tannins was compared to that of SO2. Colorimetric assays associated with chemical analyses of anthocyanins and phenolic compounds confirmed that the use of bioprotection alone did not protect the wine from oxidation. An addition of oenological tannins on musts stabilized the colour of bioprotected rosé wine in a similar way that SO2 addition did. Quebracho tannins appeared more efficient than gall nut tannins. The colour differences observed cannot be explained either by the concentration or forms of anthocyanins. However, the addition of tannins led to better protection of oxidation-sensitive phenolic compounds comparable to that obtained with the addition of sulfites.
1. Introduction
Reducing chemical inputs during winemaking has become a priority from a legal and societal point of view. The bioprotection of musts or grapes is a strategy to limit sulfiting during winemaking, and more specifically, during the pre-fermentative step. The bioprotectants currently proposed to winemakers are non-Saccharomyces yeast strains (most often belonging to the genus Metschnikowia) mainly used as pure cultures [1]. In both red and white winemaking, previous studies have demonstrated that bioprotectant strains colonise the matrix [2,3,4,5]. Their dominance suppresses or limits the development of indigenous yeasts, potential agents of wine spoilage [2,3,4], but remains unsatisfactory to ensure the protection of musts against oxidation.
The oxidation of a must is mainly an enzymatic phenomenon [6,7] due to tyrosinase, which belongs to the polyphenol oxidase family [7,8]. The presence of O2 in must also induces redox reactions through the interactions between phenolic compounds and oxygen, which can alter wine colour and taste through the production of off-flavours [9,10,11,12]. Oxidation of must compounds such as caftaric, coutaric, or hydroxy cinnamoyl tartaric acids generates the corresponding quinones. Those molecules can polymerise with each other and form brown pigments, producing enzymatic browning in wines [8,13]. Phenolic compounds can also be subjected to enzymatic oxido-reduction reactions mediated by Fe(III) and Cu(II) ions leading to production of quinones and hydrogen peroxide [14]. H2O2 is then used in the Fenton reaction, which oxidises wine’s compounds such as ethanol and phenolic compounds.
The addition of SO2 protects must and wine against enzymatic and chemical oxidation. It can interact with hydrogen peroxide and reduce it to water, thereby inhibiting the oxidation of other wine compounds [14,15,16,17]. SO2 can also minimise the production of quinones by converting them back into their corresponding phenols and then inhibiting quinone polymerization and subsequent wine browning [14].
To substitute the antioxidant and antioxidasic properties of SO2, the use of oenological tannin was investigated. Tannins are traditionally used to prevent protein haze and can promote colour stability [18]. Recently, the 2022 International code of good oenological practice of the International Organization of Vine and Wine recommended their use on must as an antioxidant [19]. Oenological tannins can be classified into two families: hydrolysable tannins and condensed tannins, also named proanthocyanidins. Hydrolysable tannins can be divided into two subfamilies: ellagitannins and gallotannins [20,21]. Ellagitannins are generally polymers of ellagic acid, gallic acid, and/or hexahydroxydiphenic acid such as castalgins or vescalgins and are mainly extracted from oak and chestnut wood [18,22]. Gallotannins are polymers of D-glucose and gallic acid. In most cases, they are obtained from gall nuts [18]. There are many different condensed tannins, which vary according to the monomeric units from which they are derived, their polymerisation degree, their galloylation level as a consequence of their botanical origin. They are derived from monomeric units of flavan-3-ols like catechin, epicatechin, epigallocatechin, or fistinidiol. Polymerisation of these compounds leads to the production of different condensed tannins such as profisetinidins (quebracho tannins), procyanidins (seed grape tannins), or a mixture of procyanidins and prodelphinidins (skin-grape tannins) [23].
The redox activity of gallotannins seems to be due to their ability to scavenge free radicals from the medium. Condensed tannins are able to scavenge peroxyl radicals, while ellagitannins chelate iron ions [24,25]. A previous study demonstrated that oenological tannins are able to inhibit polyphenol oxidases, such as laccase synthesised by the phytopathogen Botrytis cinerea [26]. This work demonstrated an antioxidasic efficiency of tannins on red and white wines, but with a larger effect on synthetic red wines than white ones. In addition, tannins can act as co-pigments by interacting with must anthocyanins [23,27] and can produce new pigments by direct or indirect condensation reactions with anthocyanins [28]. Because of the diversity of their properties, oenological tannins could represent an interesting alternative to replace or decrease the amount of SO2 used during winemaking and wine ageing.
To our knowledge, the antioxidant properties of tannins have not yet been studied in natural rosé wine, but only in white and red wines or in white wines supplemented with anthocyanins to simulate red or rosé wines [26]. Furthermore, tannins are not traditionally added during pre-fermentative steps in rosé winemaking. The present study, conducted in real conditions of production in a winery, aimed at assessing the efficiency of combining the antimicrobial properties of bioprotection with the antioxidasic/antioxidant properties of tannins (gall nuts or quebracho tannins) in comparison with bioprotection combined with SO2 addition at the pre-fermentative step during rosé elaboration. The implantation of bioprotection in musts and its effect on the development of alteration microorganisms during alcoholic fermentation were verified. The phenolic composition of wines and their colorimetric characteristics were determined to investigate the ability of oenological tannin addition to protect musts against oxidation and to stabilise the colour of bioprotected rosé wines.
2. Materials and Methods
2.1. Yeast Strains and Experimental Conditions
The bioprotectant strain Metschnikowia pulcherrima MCR 24 (Primaflora VB®) (AEB group—Kaysersberg, France). and the Saccharomyces cerevisiae strain Fermol Tropical® (AEB group—Kaysersberg, France) were used. These strains were provided in dried form and were rehydrated before inoculation according to the manufacturer’s instructions. Fermentations were performed on must obtained from Vitis vinifera L. cv. Pinot Noir grape vines, grown in the vineyards of the University of Burgundy in Marsannay-La-Côte (Côte d’Or, France) and harvested in 2019. The vines were treated according to conventional viticulture conditions.
The grapevines were harvested by a mechanical grape harvester. The bioprotectant yeast was added in harvest containers at 10 g/hL (5 × 105 CFU/mL) concentration, corresponding to the manufacturer’s recommendations. The juice obtained after pressing was distributed in eight 100 L flat-bottomed stainless steel tanks for alcoholic fermentation. Four modalities were designed (in duplicate): bioprotection (control modality named BP); bioprotection + addition of 5 g/hL of gall nuts tannin (GALLOVIN®) (named BPG); bioprotection + addition of 15 g/hL of quebracho tannin (PROTAN BIO Q®) (named BPQ); and bioprotection + addition of 5 g/hL of SO2 (named BPS) (Figure 1). Tannins were provided by AEB Group® (Kaysersberg, France) and the concentrations used were those recommended by the manufacturer, based on the higher antioxidant capacity (three-fold) of gall nut tannins than quebracho tannins, in accordance with the literature [29].
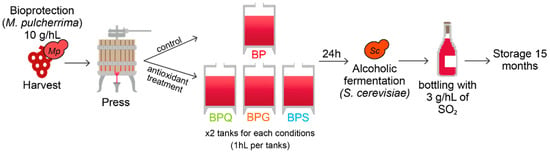
Figure 1.
Shema of experimental conditions. BP: bioprotection (control modality), BPG: bioprotection and gall nut tannin addition (5 g/hL), BPQ: bioprotection and quebracho tannin addition (15 g/hL), BPS: bioprotection and SO2 addition (5 g/hL).
Initial juice composition was 232 g/L of sugar, 493 mg/L of Yeast Assimilable Nitrogen (YAN) sources, with a total acidity of 6.83 g/L of tartaric acid and a pH value of 3.5. These values were obtained by Fourier transform infrared spectroscopy with OenoFoss© (FOSS—Nanterre, France) analyser.
At the end of alcoholic fermentation, wines were filtered through 0.22 µm filter cartridges with nitrogen gas. 30 mg/L of SO2 was added at bottling for each condition. Wines were stored in bottles with DIAM® corks away from light for 15 months (samples named aged wines).
2.2. Experimental Sampling
Grape juice was collected directly from harvest containers before addition of the bioprotectant yeast (named T0 sample) and after pressing in the presence of the bioprotectant yeast (named T0 + BP). During alcoholic fermentation, samples were collected from both tanks for each condition: 24 h after vatting and before S. cerevisiae strain inoculation (named D1), 48 h after vatting (corresponding to 24 h after S. cerevisiae strain inoculation) (named D2), 72 h after vatting (named D3), 96 h after vatting (named D4), and at the end of alcoholic fermentation (D6).
2.3. Microbiological Analysis
Total yeasts counting was carried out on an agar plate medium WL Oxoid CM0309 with an addition of 0.2 g/L of chloramphenicol (Sigma-Aldrich, St. Louis, MO, USA). Yeast morphological diversity on WL medium allows discrimination between Saccharomyces genus, M. pulcherrima and Hanseniaspora genus [30] after an incubation of 48 h at 28 °C. On this medium, M. pulcherrima colonies appear red because of the production of a red pigment named pulcherrimin [31,32].
Enumerations of Brettanomyces bruxellensis were specifically made on agar plate ITV medium according to Gerbaux et al., [33] with some modifications (20 g/L glucose, 10 g/L yeast extract, 20 g/L tryptone, 0.1 g/L para-coumaric acid, 0.1 g/L ferulic acid, 0.03 g/L green bromocresol, 0.2 g/L chloramphenicol, 20 g/L agar, pH 5, with addition of cycloheximide 0.006% (v/v)) after an incubation of 7 days at 28 °C. Acetic acid bacteria (AAB) populations were determined by enumeration on Mannitol medium (25 g/L mannitol, 10 g/L yeast extract, 0.004% (w/v) Delvocid©, 0.002% (w/v) penicillin), and lactic acid bacteria (LAB) populations on Lac medium (78 mL grape juice, 33 g/L yeast extract, 0.6 mL/L Tween 80, 0.08 g/L MnSO4, 0.004% (w/v) Delvocid©, pH 5.1) after an incubation of 48 h at 28 °C.
2.4. Fermentation Kinetics and Wine Composition
Fermentation kinetics were monitored daily by densitometry. At the end of alcoholic fermentation (D6), each sample was analysed with Fourier transform infrared spectroscopy by OenoFoss® for residual sugar (g/L), ethanol (% (v/v)), total acidity (g/L tartaric acid), and volatile acidity (g/L tartaric acid) concentrations of wines. SO2 analyses were realised on aged wines with a Sulfilyser+® analyzer (Dujardin-Salleron, Noizay, France), according to the manufacturer’s instructions.
Combined SO2 (mg/L) can be calculated as the difference between total and free SO2 as follows:
2.5. Colorimetric Analyses by Tristimulus Coordinates (L*a*b*)
Colorimetric analysis of Tristimulus coordinates (L*a*b*) was performed on a spectrophotometer CM-5 Konica Minolta. The visible absorption spectrum was measured between 380 and 700 nm. The CIELab parameters (L*, a*, and b*) were obtained following the recommendations of the Commission Internationale de L’Eclairage (CIE, 2004) and the OIV-MA-AS2-11 method, using the standard illuminant D65 and the 10° standard observer on aged wines. Three millilitres of samples were transferred to a glass spectrophotometer cuvette (10 mm). The calculation of colorimetric parameters is done according to the OIV-MA-AS2-11 method.
Colorimetric variation between two samples ΔE* is calculated as follows:
Chroma of sample are calculated as follows:
Hue of samples is calculated as follows:
2.6. Anthocyanins Analyses
For anthocyanins analyses, 500 mL of each wine was collected before bottling from each tank, and 0.2 g/L of sodium benzoate was added to the samples. Analyses of total anthocyanins by SO2 discoloration were carried out according to Ribéreau-Gayon & Stonestreet [34] methods. The concentration of total anthocyanins in mg/L was calculated as follows:
The analysis of free and combined anthocyanins was carried out by PVPP column methods [35]. PVPP index (%) was calculated as follows:
Combined anthocyanins concentration (mg/L) were calculated:
The ionisation index allows to determine the percentage of anthocyanins contributing to sample coloration [36]. Samples were centrifuged for 5 min at 9000× g. For each sample, Abs520 was measured in four different sample tubes by spectrophotometric analysis. The four sample tubes contained, respectively:
Tube 1 (Abs1): 5 mL of supernatant + 1 mL of distilled water
Tube 2 (Abs2): 5 mL of supernatant + 1 mL of NaHSO3 15% (v/v)
Tube 3 (Abs3): 1 mL of supernatant + 7 mL of HCl 0.1 M + 2 mL of distilled water
Tube 4 (Abs4): 1 mL of supernatant + 7 mL of HCl 0.1 M + 2 mL NaHSO3 15% (v/v)
Ionisation index (%) was calculated according to:
2.7. UHPLC Analysis of Phenolic Compounds
Wine samples (bottles labeled as aged wines) were filtered on 0.45 µm Polytetrafluoroethylene (TPFE) filters and placed directly in UHPLC vials. The analyses were carried out in an Ultra High Performance Liquid Chromatography (UHPLC) system from Waters Acquity (Waters, Milford, MA, USA) with a Raptor ARC-18 (Restek) column (150 mm × 2.1 mm) with 2.7 µm of granulometry. The temperature of the column oven was regulated at 35 °C and the sample system was at 12 °C with an injection volume of 2 µL. Two eluants are used: a mix of water, 0.28% trifluoroacetic acid (TFA), and 5% methanol as eluant A, and methanol as eluant B, according to Popîrdă et al. [37].
The PDA (photodiode array) detector screens the UV-visible spectrum between 210 and 610 nm wavelength, with a 1.2 nm resolution and an acquisition frequency of 20 point/s. The fluorimeter records three pairs of excitation/emission wavelengths: 270 nm/322 nm, 270 nm/420 nm, and 330 nm/374 nm.
2.8. Statistical Analysis
The cellar condition of this experiment allowed two biological replicates for each condition. One-Way ANOVA test was performed for each analysis, followed by a Tukey test for group determination (α = 0.05). Statistical analyses and graphs were drawn with RStudio software (4.0.3 version).
Colorimetric similarity was analysed using multidimensional scaling (MDS). This multivariate statistical technique analyses the similarity relationships among samples, representing them as points on a map. The distances between pairs of points reflect the distances between the pairs of products. MDS was carried out on the symmetrical square distance matrix, where the rows and columns are the samples of the study, and ΔE* values are presented between each pair of samples.
3. Results
3.1. Microbial Behavour
Microbial populations were analysed in the grape juice collected directly from the harvest containers before bioprotection addition (T0) and at the end of pressing (T0 + BP). Before bioprotection addition, the indigenous yeast population in the must was approximately 4.8 × 106 CFU/mL, and no M. pulcherrima was detected. Lactic and acetic acid bacteria populations were 1.0 × 103 CFU/mL and 1.2 × 103 CFU/mL, respectively. After bioprotection addition and grape pressing, the total yeast population reached 1.1 × 107 CFU/mL with 70.5% (7.9 × 106 CFU/mL) of M. pulcherrima yeasts.
The results of microbial analysis after juice transfer into tanks and the addition of tannins or sulfites are presented in Table 1. These analyses were performed at one (D1-before S. cerevisiae strain inoculation), two (D2-24 h after S. cerevisiae strain), and four days (mid-fermentation D4).

Table 1.
Microbial populations during winemaking.
At D1, the M. pulcherrima population was approximately 3.1 × 106 CFU/mL. This yeast was undetectable at D2 in BP and BPS conditions, whereas its concentration was maintained at 5 × 105 CFU/mL in BPG and BPQ conditions. No M. pulcherrima colony was detectable at D4, whatever the condition (Table 1). Indigenous S. cerevisiae yeasts were detected at D1 in high concentration (around 6.4 × 107 CFU/mL) without significant differences between conditions. At D2 (24 h after S. cerevisiae addition), S. cerevisiae concentration varied between 2.2 × 108 and 2.8 × 108 CFU/mL, and between 2.7 × 108 and 3.3 × 108 CFU/mL at D4. B. bruxellensis populations remained low (maximum of 3.50 × 102 CFU/mL) at D1 and D2, whatever the modalities. From D4, no B. bruxellensis were detected. Hanseniaspora populations represented a small proportion of total yeasts (≈2% corresponding to 1 × 106 CFU/mL) during D1 but they were undetectable from D2. Lactic acid bacteria populations decreased in all modalities to reach concentrations under 103 CFU/mL at D4, except for the BPS condition (concentration of 1.48 × 103 CFU/mL). Concerning acetic acid bacteria, the populations were below 1.0 × 103 CFU/mL for BP and BPS conditions and 5.0 × 103 CFU/mL for BPG and BPQ conditions, but those differences were not statistically significant (Table 1).
3.2. Fermentation Kinetics Analysis and Must and Wine Composition
Alcoholic fermentation ended in all tanks after 6 days (0.992 of density) (Table S1). At the end of fermentation, the residual sugar concentration was 1.71 (±0.26) g/L with a total acidity of 5.49 (±0.08) g/L of tartaric acid. The ethanol concentration was 13.71 (±0.07) % (v/v), and pH values were 3.25 (±0.03) (Table S1) (means values for the eight tanks). No significant differences were found in the evolution of density during alcoholic fermentation or in the final wine’s composition between the different modalities.
Concerning sulfite concentrations in wines after ageing (Table 2), the total SO2 concentrations were significantly different between conditions. BPS wines had the highest SO2 concentration (27 mg/L). BPG and BPQ wines presented lower levels at around 19 mg/L. The average concentration of free SO2 was 6 mg/L in all conditions, without statistical differences. Differences in total SO2 concentration could not be explained by the free SO2 form but by the combined SO2 form, where significant differences were found for BPS wines with the higher combined SO2 concentration (20.55 ± 2.83 mg/L).

Table 2.
SO2 composition of wines after ageing.
3.3. Colorimetric Analysis by Tri-Dimensional Coordinates L*a*b*
Parameters L*, a*, and b* correspond respectively to brightness parameters, red/green and yellow/blue hue of wine (Figure 2, Table S2a). BP wines had a higher L* value (L* = 67.56 ± 2.92) compared to those resulting from BPG (L* = 62.96 ± 0.81), BPQ (L* = 58.99 ± 1.92), or BPS conditions (L* = 60.61 ± 1.52), as well as lower a* and b* values than the wines that combined bioprotection and other treatments (Figure 2, Table S2a). The colorimetric variation (ΔE*) values, calculated with L*, a*, and b* parameters, were used to detect differences between samples. The ΔE* value is considered significantly different and distinguishable for the human eye (non-expert) at a threshold value of 3 [38]. The ΔE* values calculated for aged wines are presented in Table S2b. Comparisons between BPG/BPQ, BPG/BPS, and BPQ/BPS conditions showed ΔE* values up to the threshold value of 3 but close to it. Comparisons between BP and the three other conditions (BPQ, BPG, and BPS) show ΔE* values that are considerably higher than the threshold. The chroma (C*) and hue (h*) values (Table S2a) were also calculated from the a* and b* values. The hue (p-value = 0.10) and chroma (p-value = 0.0595) were not statistically different, but trends can be drawn for the chroma. BP wine had the lowest value (41.06 ± 3.46), BPG and BPQ wines had intermediate values, and BPS wine had the highest (47.70 ± 0.1).
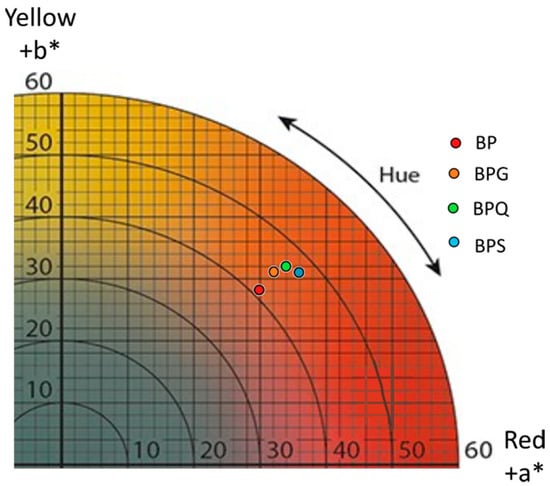
Figure 2.
Graphic representing the CIELab coordinated a* (red hue) and b* (yellow hue) of the wines issued from four conditions. BP: bioprotection, BPG: bioprotection and gall nut tannin addition, BPQ: bioprotection and quebracho tannin addition, BPS: bioprotection and SO2 addition.
Figure 2 shows the two-dimensional MDS space translating colorimetric differences between samples based on ΔE* computations. The stress value is very low, indicating a very good fit of the data.
Figure 3 shows a clear visual proximity between the two BPS and the two BPQ replicates. BP is located on the opposite side of BPQ and BPS. BPG replicates are located between the two previous groups. Moreover, BP samples show low reproducibility, as visualised by the large distance between them.
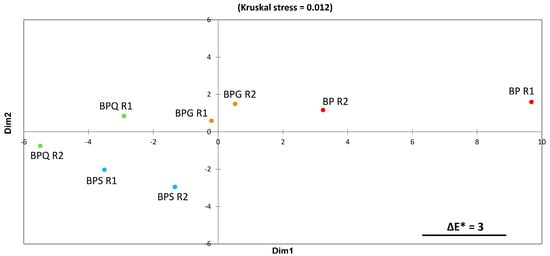
Figure 3.
Colorimetric differences between samples obtained by MDS of the ΔE* distance matrix. The segment represented in the bottom right corner represents the distance corresponding to ΔE* of 3, which is the average human difference threshold for colour differences [38]. R1 and R2 correspond, respectively, to the two replicates of each condition. BP: bioprotection, BPG: bioprotection and gall nut tannin addition, BPQ: bioprotection and quebracho tannin addition, BPS: bioprotection and SO2 addition.
3.4. Anthocyanin Analysis and Phenolic Compound Determination
3.4.1. Anthocyanin Analysis by Spectrophotometric Analyses
After ageing, the mean total anthocyanin concentration in the wines was 17.2 (±2.24) mg/L (Table 3). Concentrations of free and combined anthocyanins were 2.92 (±1.90) mg/L and 14.20 (±1.95) mg/L, respectively. The ionization index allows determining the percentage of anthocyanins contributing to sample colouration. In wines, the ionisation index was about 37.9 (±20) %. No statistical differences were found between the four conditions.

Table 3.
Anthocyanins analyses by spectrophotometric and UHPLC methods.
3.4.2. Phenolic Compound Analyses (UHPLC Analyses)
The concentration of mono-glycoside anthocyanins was 1.02 (±0.11) mg/L (Table 3). Other peaks were detected at 520 nm between the malvidin-3-glucoside peak and the end of the chromatograms, which corresponded to non-identified anthocyanin derivatives formed during winemaking and ageing. The same peaks at the same concentrations were detected for all the conditions.
The analysis of the aged wines revealed 14 phenolic compounds in addition to anthocyanins (Table 4). Significative differences between conditions were found only for gentisic acid (p-values ≈ 0.009), coumaric acid (p-values ≈ 0.037), and gallic acid (p-values ≈ 0.0002). Concerning gallic acid, a higher concentration (11.99 mg/L) was found in wines resulting from the BPG condition. Gentisic acid was detected at higher concentrations in wines resulting from the BPS and BPQ conditions (0.48 and 0.40 mg/L, respectively). For coumaric acid, the BP condition presented the lowest concentration (0.30 mg/L) and the BPQ condition the highest (0.38 mg/L), while the BPG and BPS conditions showed an intermediate concentration.

Table 4.
Aged wines’ phenolic composition.
4. Discussion
4.1. Microbial Analyses
The bioprotective strain added to grapes in harvest containers was predominant and well established in the must after pressing (representing more than 70% of the total yeast population), despite the high concentration of indigenous yeasts in the must (4.8 × 106 CFU/mL). This result confirms the implantation of the strain, as previously reported in the literature [2,3,4]. A decrease in the bioprotectant yeast was observed after 24 h. This drop in concentration could be explained by the quick start of fermentation.
Brettanomyces was detected in low concentrations and undetectable from D4, like the Hanseniaspora population, which was undetectable 24 h after vatting, regardless of conditions (Table 1). Bacterial populations (AAB and LAB) remained at low concentrations. Concentrations of undesirable microorganisms measured in this experiment were consistent with data previously observed in unaltered wines [39]. This lack of spoilage microorganisms in must and wine could be attributed to the implantation of the bioprotective yeast linked to the healthy state of grapevines.
4.2. Oenological Analysis of Wines
Our results indicated that the combined addition of M. pulcherrima and oenological tannins leads to a wine composition similar to that with SO2 (Table 2 and Table S1). Concerning sulfite concentrations in wines, the higher total SO2 concentration in the BPS condition can be explained by the addition of 50 mg/L in this condition in pre-fermentative steps. The free SO2 form was similar in all conditions, which suggests that the difference detected in total SO2 was due to a higher combined SO2 proportion in the BPS condition. These results could be attributed to higher acetaldehyde production by yeast. Studies have demonstrated that a higher initial SO2 concentration leads to increased acetaldehyde release by yeasts [40,41]. Acetaldehyde is the main compound that combines with SO2. However, the final concentrations appeared lower than expected. These results could be explained by the oxidation of SO2 and its transformation into other chemical forms (such as SO4−) during fermentation, and during ageing from the oxygen added during bottling [42].
4.3. Wine Colour
Wines resulting from the condition with tannins had higher a*, b*, and C* values, indicating higher colour intensity (Figure 2, Table S2a) and lower brightness (L* value) than the control BP wine (Table S2a), in accordance with the effect of tannins on wine colour previously observed in red wines and model wine solutions [43,44,45,46]. Both co-pigmentation [45] and the formation of anthocyanin-tannin adducts by direct or indirect condensation reactions could be responsible for the higher colour expression of the wines [22].
The visual proximity (Figure 3) between BPS and the conditions with tannins, and more specifically with BPQ conditions, suggested that these treatments gave quite similar colours, whereas the position of BP reveals a clear, perceptible difference in colour between BP on the one hand and the condition with antioxidant compounds on the other. These results are in accordance with the ΔE* values calculated (Table S2b) and the C* results. Moreover, BP samples showed low colour repeatability, which was already suggested by the high standard deviations on the L*, a*, b*, and C* parameters presented in Table S2a and the ΔE* values. These data underline that the use of bioprotection alone can impact rosé wine colour expression in an unpredictable way. On the other hand, the addition of oenological tannins allowed better colour reproducibility, as with SO2. Moreover, quebracho tannins induced a better colour expression than nut gall tannins at the doses studied. These first results obtained on rosé wines are in accordance with previous literature focused on white wine and the chemical properties of tannins [23,26].
4.4. Phenolic Composition of Wines
Spectrophotometric analyses showed similar concentrations of total anthocyanins in all conditions (Table 3). These results indicated that the total anthocyanin concentration in bioprotected wines was not impacted either by the addition of SO2 or oenological tannins. After storage, the main anthocyanins formed in wine were their combined forms in all treatments. In our experimental conditions, the addition of oenological tannins or SO2 did not impact the final combined anthocyanin concentrations (Table 3). The lack of differences for the ionisation index between conditions could be explained by the weak impact of the antioxidant compound tested on the proportion of anthocyanins, which contributed to wine colour, but the high standard deviation of the results did not allow further investigation into this result.
Concerning anthocyanin concentrations quantified by UHPLC methods, no significant difference was observed between wines. Only mono-glycoside anthocyanins were quantified, which are the only native anthocyanin form of Pinot Noir [47]. The concentration obtained (≈1 mg/L) corresponded to free anthocyanins and anthocyanins in weak co-pigmentation interaction with other wine compounds. This result could mean that oenological tannins, as well as SO2 addition, had no impact on the evolution of free or weakly bound anthocyanins. These concentrations were close to the concentrations of free anthocyanins obtained by spectrophotometric analyses. The colour differences observed in wines by CIELab analyses cannot therefore be explained by anthocyanin concentrations, by a different free/combined anthocyanin ratio, or by a coloured anthocyanin proportion.
Concerning the other phenolic compounds detected by the UHPLC method (Table 4), the higher gallic acid concentration in the BPG condition was explained by the addition of nut gall tannin in this treatment. It is a hydrolysable tannin composed of gallic acid units [22]. The gentisic and coumaric phenolic acids, which were in significantly different concentrations as a function of the conditions, are oxidation-sensitive wine compounds [48,49]. The concentration of these compounds was higher in the condition with antioxidant inputs (specifically SO2 or quebracho tannin) compared to the condition with bioprotection alone. This could suggest that the addition of an antioxidant compound (tannin or SO2) could protect certain oxidation-sensitive compounds during winemaking and ageing and explain their protective effect on wine colour.
5. Conclusions
The bioprotection of musts and grapes represents a strategy for limiting sulfite addition during winemaking. Although previous studies have demonstrated that bioprotectant non-Saccharomyces strains are able to protect musts and wines against microbial spoilage, they cannot protect must against oxidation. This fact can limit its practice, especially in rosé must, where it is crucial to preserve not only the freshness of aromatic grape varieties but also the colour after pressing.
This experiment carried out with the constraints of winemaking in the cellar led to a preliminary conclusion that the combined action of bioprotection and tannins could be an alternative to sulfites in pre-fermentative steps to guarantee microbiological protection and the colour of the wine, as shown by tristimulus coordinates and MDS analysis. In our experiments, it appeared preferable to favour quebracho tannins over gall nut tannins for better colour protection. The HPLC analysis showed that tannin addition protected oxidation-sensitive compounds (gentisic and coumaric acids) in a way comparable to the conditions with SO2. It will be interesting to carry out further experiments to study new pigments formed by interactions between anthocyanins, and between anthocyanins and other phenolic compounds to better understand the action of oenological tannins used as antioxidants in a bioprotected must.
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12040735/s1. Table S1: Must composition at the end of alcoholic fermentation; Table S2: CIELab tristimulus coordinates and colorimetric variation (ΔE*) of aged wines.
Author Contributions
Conceptualization, R.T.-M.; data curation, M.P. and R.T.-M.; formal analysis, M.P.; funding acquisition, R.T.-M.; investigation, M.P., S.S. and V.D.-V.; methodology, R.T.-M.; project administration, R.T.-M.; supervision, R.T.-M.; validation, R.T.-M.; visualization, M.P. and R.T.-M.; writing—original draft, M.P.; writing—review & editing, S.S., G.K., N.Q.-M., H.A. and R.T.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AEB group (Grant CIFRE n°2019/1162), Conseil Régional de Bourgogne through ENVERGURE BIOVI, The European Funds for Regional Development (FEDER) through the FEDER-FSE Bourgogne 2014–2020, and Bourgogne-Franche-Comté (n°17312) programme for providing financial support for this work.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We also thank Laurence Noret for his technical contribution and Julie Thomas for her participation in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora Delbrueckii as a Bio-Protection Agent in Winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Simonin, S.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Peyron, D.; Alexandre, H.; Tourdot-Maréchal, R. Bio-Protection As an Alternative to Sulphites: Impact on Chemical and Microbial Characteristics of Red Wines. Front. Microbiol. 2020, 11, 1308. [Google Scholar] [CrossRef]
- Windholtz, S.; Dutilh, L.; Lucas, M.; Maupeu, J.; Vallet-Courbin, A.; Farris, L.; Coulon, J.; Masneuf-Pomarède, I. Population Dynamics and Yeast Diversity in Early Winemaking Stages without Sulfites Revealed by Three Complementary Approaches. Appl. Sci. 2021, 11, 2494. [Google Scholar] [CrossRef]
- Windholtz, S.; Vinsonneau, E.; Farris, L.; Thibon, C.; Masneuf-Pomarède, I. Yeast and Filamentous Fungi Microbial Communities in Organic Red Grape Juice: Effect of Vintage, Maturity Stage, SO2, and Bioprotection. Front. Microbiol. 2021, 12, 748416. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Yang, H.; Tian, T.; Gu, H.; Li, X.; Cai, G.; Sun, J.; Wu, D.; Lu, J. Analysis of Factors Related to Browning of Dangshan Pear (Pyrus Spp.) Wine. Food Chem. 2020, 308, 125665. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Coetzee, C. Oxidation Treatments Affecting Sauvignon Blanc Wine Sensory and Chemical Composition. PhD Thesis, Stellenbosch University, Institute for Wine Biotechnology, Faculty of AgriSciences, South Africa, 2014. [Google Scholar]
- Hoenicke, K.; Simat, T.J.; Steinhart, H.; Christoph, N.; Geßner, M.; Köhler, H.-J. ‘Untypical Aging off-Flavor’ in Wine: Formation of 2-Aminoacetophenone and Evaluation of Its Influencing Factors. Anal. Chim. Acta 2002, 458, 29–37. [Google Scholar] [CrossRef]
- Mayr, C.M.; Capone, D.L.; Pardon, K.H.; Black, C.A.; Pomeroy, D.; Francis, I.L. Quantitative Analysis by GC-MS/MS of 18 Aroma Compounds Related to Oxidative Off-Flavor in Wines. J. Agric. Food Chem. 2015, 63, 3394–3401. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen Contribution to Wine Aroma Evolution during Bottle Aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Simonin, S. Etude de la Bio-Protection en œnologie. Univresité de Bourgogne Franche-Compte, Dijon, France, 2019. [Google Scholar]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of Interaction of Polyphenols, Oxygen, and Sulfur Dioxide in Model Wine and Wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar] [CrossRef]
- Carrascón, V.; Bueno, M.; Fernandez-Zurbano, P.; Ferreira, V. Oxygen and SO2 Consumption Rates in White and Rosé Wines: Relationship with and Effects on Wine Chemical Composition. J. Agric. Food Chem. 2017, 65, 9488–9495. [Google Scholar] [CrossRef]
- Carrascón, V.; Vallverdú-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The Kinetics of Oxygen and SO2 Consumption by Red Wines. What Do They Tell about Oxidation Mechanisms and about Changes in Wine Composition? Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C.; Standing, M.J. Reaction Mechanisms of Oxygen and Sulfite in Red Wine. Am. J. Enol. Vitic. 2018, 69, 189–195. [Google Scholar] [CrossRef]
- Ugliano, M.; Slaghenaufi, D.; Picariello, L.; Olivieri, G. Oxygen and SO2 Consumption of Different Enological Tannins in Relationship to Their Chemical and Electrochemical Characteristics. J. Agric. Food Chem. 2020, 68, 13418–13425. [Google Scholar] [CrossRef] [PubMed]
- OIV Code International Des Pratiques OEnologiques. Available online: https://www.oiv.int/fr/standards/code-international-des-pratiques-oenologiques (accessed on 12 December 2022).
- Amarowicz, R.; Janiak, M. Hydrolysable Tannins. Ref. Modul. Food Sci. 2018, 3, 7. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- Jourdes, M.; Pouységu, L.; Deffieux, D.; Teissedre, P.-L.; Quideau, S. Hydrolyzable Tannins: Gallotannins and Ellagitannins. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1975–2010. ISBN 978-3-642-22143-9. [Google Scholar]
- Versari, A.; du Toit, W.; Parpinello, G.P. Oenological Tannins: A Review: Oenological Tannins. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Ramos, I.I.; Reis, S.; Segundo, M.A. Antioxidant Profile of Commercial Oenological Tannins Determined by Multiple Chemical Assays. Aust. J. Grape Wine Res. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.-P. Biological Activity of Ellagitannins: Effects as Anti-Oxidants, pro-Oxidants and Metal Chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef]
- Vignault, A.; Pascual, O.; Jourdes, M.; Moine, V.; Fermaud, M.; Roudet, J.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Impact of Enological Tannins on Laccase Activity. OENO One 2019, 53, 27–38. [Google Scholar] [CrossRef]
- Gombau, J.; Vignault, A.; Pascual, O.; Gómez-Alonso, S.; Gracía-Romero, E.; Hermosín, I.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Influence of Oenological Tannins on Malvidin-3-O-Monoglucoside Copigmentation in a Model Wine Solution. OENO One 2019, 53, 531–547. [Google Scholar] [CrossRef]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First Confirmation in Red Wine of Products Resulting from Direct Anthocyanin-Tannin Reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Vignault, A.; González-Centeno, M.R.; Pascual, O.; Gombau, J.; Jourdes, M.; Moine, V.; Iturmendi, N.; Canals, J.M.; Zamora, F.; Teissedre, P.-L. Chemical Characterization, Antioxidant Properties and Oxygen Consumption Rate of 36 Commercial Oenological Tannins in a Model Wine Solution. Food Chem. 2018, 268, 210–219. [Google Scholar] [CrossRef]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL Medium to Profile Native Flora Fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Kluyver, A.J.; van der Walt, J.P.; van Triet, A.J. Pulcherrimin, The Pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 1953, 39, 583–593. [Google Scholar] [CrossRef]
- MacDonald, J. Biosynthesis of Pulcherriminic Acid. Biochem. J. 1965, 96, 533–538. [Google Scholar] [CrossRef]
- Gerbaux, V.; Briffox, C.; Dumont, A.; Krieger, S. Influence of Inoculation with Malolactic Bacteria on Volatile Phenols in Wines. Am. J. Enol. Vitic. 2009, 60, 233–235. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Le Dosage Des Anthocyanes Dans Le Vin Rouge [Determination of Anthocyanins in Red Wine]. Bull Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- Glories, Y. Recherche Sur La Matière Colorante Des Vins Rouges. Ph.D. Thesis, Université de Bordeaux II, Nouvelle-Aquitaine, France, 1978. [Google Scholar]
- Chris Somers, T.; Evans, M.E. Spectral Evaluation of Young Red Wines: Anthocyanin Equilibria, Total Phenolics, Free and Molecular SO2, “Chemical Age”. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Popîrdă, A.; Luchian, C.E.; Colibaba, L.C.; Focea, E.C.; Nicolas, S.; Noret, L.; Cioroiu, I.B.; Gougeon, R.; Cotea, V.V. Carbon-Isotope Ratio (Δ13C) and Phenolic-Compounds Analysis in Authenticity Studies of Wines from Dealu Mare and Cotnari Regions (Romania). Agronomy 2022, 12, 2286. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Guillamón, J.M.; Mas, A. Acetic Acid Bacteria. In Acetic Acid Bacteria; König, H., Unden, G., Fröhlich, J., Eds.; Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 10.1007/978-3-540-85463-0_2. [Google Scholar]
- Park, H.; Hwang, Y.-S. Genome-Wide Transcriptional Responses to Sulfite in Saccharomyces Cerevisiae. J. Microbiol. 2008, 46, 542–548. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; Mira de Orduña, R. Multifactorial Analysis of Acetaldehyde Kinetics during Alcoholic Fermentation by Saccharomyces Cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. The Role of Sulfur Dioxide in Wine; Principles and Practices of Winemaking; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Liu, Y.-X.; Liang, N.-N.; Wang, J.; Pan, Q.-H.; Duan, C.-Q. Effect of the Prefermentative Addition of Five Enological Tannins on Anthocyanins and Color in Red Wines. J. Food Sci. 2013, 78, C25–C30. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.; Morata, A.; Tesfaye, W.; Calderon, F.; Benito, S.; Suárez-Lepe, J. The Effects of Pre-Fermentative Addition of Oenological Tannins on Wine Components and Sensorial Qualities of Red Wine. Molecules 2016, 21, 1445. [Google Scholar] [CrossRef] [PubMed]
- Gombau, J.; Vignault, A.; Pascual, O.; Canals, J.M.; Teissedre, P.-L.; Zamora, F. Influence of Supplementation with Different Oenological Tannins on Malvidin-3-Monoglucoside Copigmentation. In Proceedings of the BIO Web of Conferences, 39th World Congress of Vine and Wine, online conferences, Brazil, 26 October 2016; Volume 7. [Google Scholar] [CrossRef]
- García-Estévez, I.; Alcalde-Eon, C.; Puente, V.; Escribano-Bailón, M. Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products. Molecules 2017, 22, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zaffalon, P.-L.; Dienes-Nagy, Á.; Nardone, D.; Vuichard, F.; Koestel, C.; Rösti, J. Anthocyanes libres des vins, une analyse pour différencier des cépages suisses. Rev. Suisse Vitic. Arboric. Hortic. 2014, 46, 310–316. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourbieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wines and Vinification; John Wiley and Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2016; ISBN 978-1-118-73072-0. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).