Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cashew Nut Kernel Oil
2.2. Physicochemical Analysis of Cashew Nut Kernel Oil
2.3. Determination of Fatty Acid Composition of Cashew Nut Kernel Oil
2.4. Determination of Lipids of Cashew Nut Kernel Oil
2.5. Determination of Fourier Transform Infrared Spectroscopy of Cashew Nut Kernel Oil
2.6. Determination of Oxidative Stability of Cashew Nut Kernel Oil
2.7. Data Processing and Analysis
3. Results and Analysis
3.1. Analysis of Fatty Acid and Lipid Composition of Cashew Nut Kernel Oil
3.2. Physicochemical Properties of Cashew Nut Kernel Oil
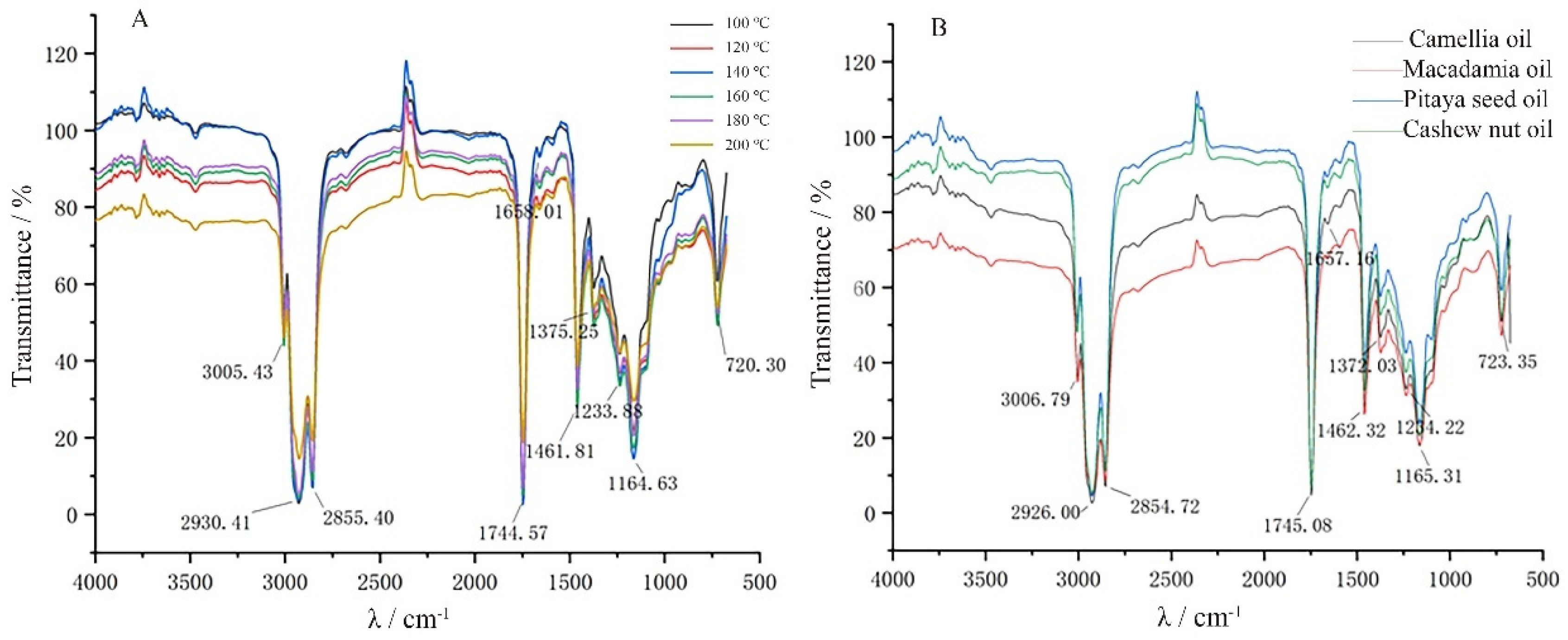
3.3. Near-Infrared Spectral Characteristics of Cashew Nut Kernel Oil
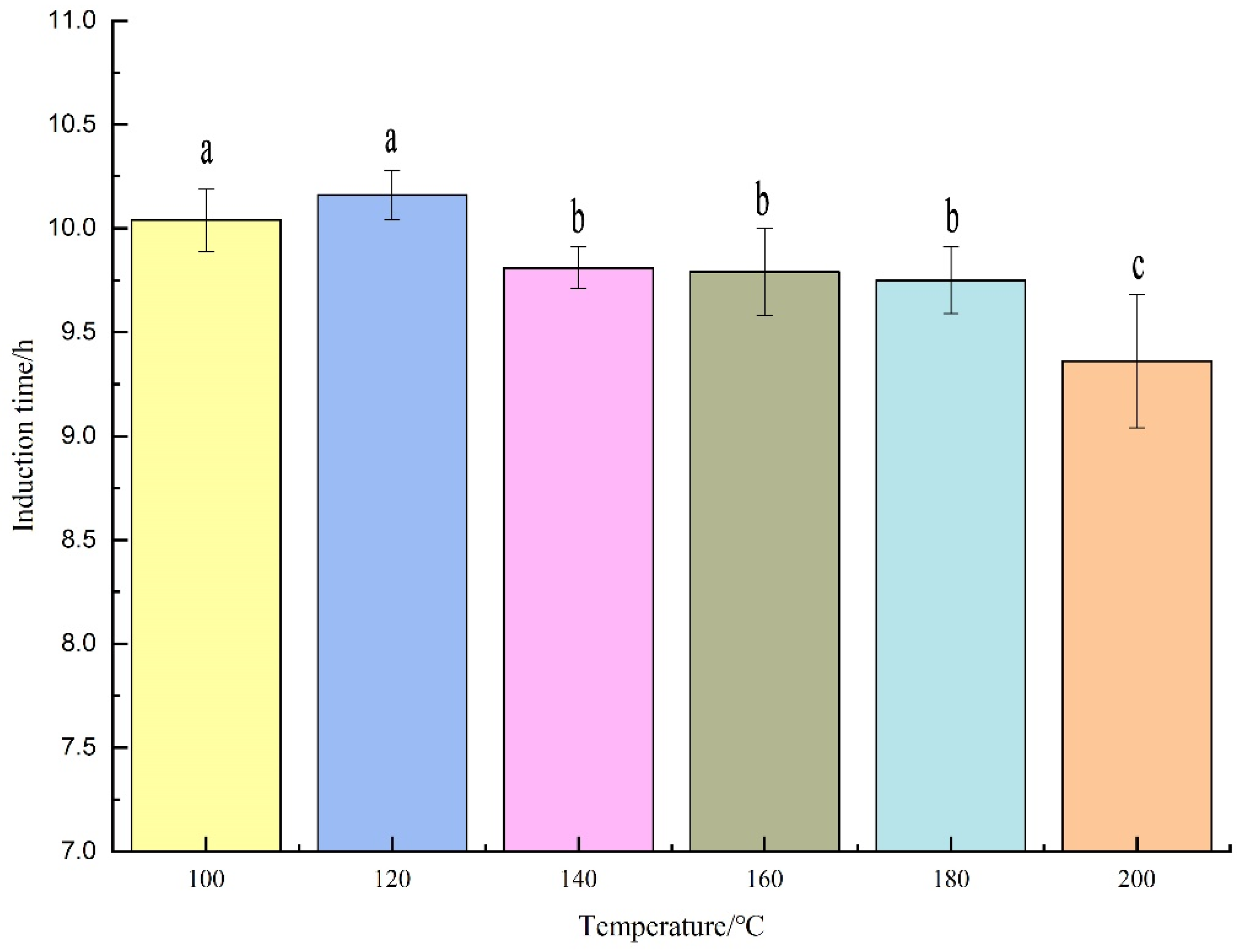
3.4. Analysis of Oxidative Stability of Cashew Nut Kernel Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.J.; Zhu, D.M.; Huang, M.F. Research progress on processing and utilization of cashew nuts. Acad. Period. Farm Prod. Process. 2013, 22, 43–45. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, G.L.; Cao, K.K. Study on the preparation process of cashew nut oil microcapsules. J. Chifeng Univ. 2014, 30, 66–67. [Google Scholar] [CrossRef]
- Mariana, S.B.; Cristina, D.S.D.; Luciana, D.C.F.; Fernando, M.S.; Maria, N.B.R.; Elisabete, C.A.A.; Teresa, B.P.M. Bioaccessibility of cashew nut kernel flour compounds released after simulated in vitro human gastrointestinal digestion. Food Res. Int. 2021, 139, 109906. [Google Scholar] [CrossRef]
- Cabral, R.M.; Passos, R.M.d.C.; Amorim, A.M.R. Optimization of the acceptance of prebiotic beverage made from cashew nut kernels and passion fruit juice. J. Food Sci. 2014, 79, 1393–1398. [Google Scholar] [CrossRef]
- Sravani, B.; Rajia, K.; Srinivas, K.; Chakravarthi, M. Growth performance and carcass characteristics of ram lambs fed concentrate mixture containing varying levels of cashew nut kernel meal. J. Anim. Res. 2021, 11, 471–476. [Google Scholar] [CrossRef]
- Anvo, M.P.M.; Sissao, R.; Aboua, B.R.D.; Kaboré, C.Y.Z.; Otchoumou, A.K.; Kouamelan, E.P.; Toguyéni, A. Preliminary use of cashew kernel oil in Clarias gariepinus fingerlings diet: Comparison with fish oil and palm oil. Int. Aquat. Res. 2017, 9, 129–139. [Google Scholar] [CrossRef]
- Emelike, N.J.T.; Barber, L.I. Effect of cashew kernel and soya bean oils on blood serum cholesterol and triglyceride of albino rats (Rattus rattus). Asian Food Sci. J. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Cao, Y.P.; Liu, Y.J.; Huang, H.H.; Zhang, F.; Fu, Y.F.; Zhu, D.M.; Huang, M.F. determination of oil content of cashew kernel by low field nuclear magnetic resonance technology. Sichuan Food Ferment. 2016, 52, 71–74. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Wang, L.S.; Liu, X.M. Analysis of fatty acids in cashew-nut kernel oil by GC-MS. Acta Nutr. Sinica. 2004, 26, 321–322. [Google Scholar] [CrossRef]
- Nguyen, P.H.N.; Dang, T.Q. Enzyme-assisted aqueous extraction of cashew nut (Anacardium occidentale L.) oil. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 175–179. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, G.L.; Cao, K.K. Study on the technology of water extraction process of cashew nut oil. J. Bengbu Univ. 2014, 3, 16–20. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission. China, GB 5009.229-2016 National Standard for Food Safety Determination of Acid Value in Food; National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016; p. 20. [Google Scholar]
- National Technical Committee for Grain and Oil Standardization. China, GBT5532-2008 Oil Iodine Valence Determination Method; Ministry of Chemical Industry of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Tu, X.H.; Lin, L.J.; Du, L.Q.; Feng, X.Q. Analysis of lipids in pitaya seed oil by ultra-performance liquid chromatography–time-of-flight tandem mass spectrometry. Foods 2022, 11, 2988. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, B.; Akanbi, T.O.; Li, R.; Yang, W.; Adhikari, B.; Barrow, C.J. Microencapsulation of lipase produced omega-3 concentrates resulted in complex coacervates with unexpectedly high oxidative stability. J. Funct. Foods 2017, 35, 499–506. [Google Scholar] [CrossRef]
- National Health Care Commission of the People’s Republic of China; State Administration of Market Supervision and Administration. GB 2716-2018 National Food Safety Standards Vegetable Oil, National Health Care Commission of the People’s Republic of China; State Administration of Market Supervision and Administration: Beijing, China, 2018; p. 8. [Google Scholar]
- Barbaro, A.; Cecchi, G.; Mazzinghi, P. Oil UV extinction coefficient measurement using a standard spectrophotometer. Appl. Opt. 1991, 30, 852–857. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Papadopoulou, P.; Kiritsakis, A. Effects of processing methods and commercial storage conditions on the extra virgin olive oil quality indexes. Grasas Aceites 2007, 58, 237. [Google Scholar] [CrossRef]
- He, Y. New Chemometric Algorithms in Analysis of Infrared Spectral Data for Several Edible Vegetable Oil Identification Studies; Jimei University: Xiamen, China, 2015. [Google Scholar]
- Ruan, L.; Lu, L.; Zhao, X.Y.; Xiong, W.W.; Xu, H.Y.; Wu, S.Z. Effects of natural antioxidants on the oxidative stability of Eucommia ulmoides seed oil: Experimental and molecular simulation investigations. Food Chem. 2022, 383, 132640. [Google Scholar] [CrossRef]
- Maja, J.Š.; Zlatko, L.M.; Cinzia, M.; Marilena, M.; Ivica, L.; Barbara, S.; Mirella, Ž.; Elda, V.; Olivera, P.; Dubravka, Š. Quantitatively unraveling hierarchy of factors impacting virgin olive oil phenolic profile and oxidative stability. Antioxidants 2022, 11, 594. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gong, X.; Jing, W.; Lin, L.J.; Zhou, W.; He, J.N.; Li, J.H. Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds. Open Chem. 2021, 19, 367–376. [Google Scholar] [CrossRef]
- Ouyang, H.J.; Liu, Y.J.; Yuan, Y.; Jing, W.; Zhang, L.; Li, J.H. HS-SPME-GC-MS coupled with OPLS-DA to analyze the effects of extraction methods on volatile aroma compounds of avocado oil. J. South. Agric. 2021, 52, 779–788. [Google Scholar] [CrossRef]
- Sonia, T.R.; Augusto, L.E.C.; María, P.V.J.; Mónica, C.S.; Feliciano, P.C. Metabolic patterns in the lipoxygenase pathway associated to fruitiness attributes of extra virgin olive oil. J. Food Compos. Anal. 2022, 109, 104478. [Google Scholar] [CrossRef]
- Ye, M.Q.; Zhou, H.F.; Hao, J.R.; Chen, T.; He, Z.P.; Wu, F.H.; Liu, X.Q. Microwave pretreatment on microstructure, characteristic compounds and oxidative stability of Camellia seeds. Ind. Crops Prod. 2021, 161, 113193. [Google Scholar] [CrossRef]
- Shuai, X.X.; Dai, T.T.; Chen, M.S.; Liu, C.M.; Ruan, R.; Liu, Y.H.; Chen, J. Characterization of lipid compositions, minor components and antioxidant capacities in macadamia (Macadamia integrifolia) oil from four major areas in China. Food Biosci. 2022, 50, 102009. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, X.F.; Liang, Y.E.; Liang, J.M.; Deng, D.Y.; Li, J.H. Comparative study on the physicochemical characteristics and fatty acid composition of cashew nuts and other three tropical fruits, iop conference series: Earth and environmental science. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052011. [Google Scholar] [CrossRef]
- Hu, A.P.; Wei, F.; Huang, F.H.; Xie, Y.; Wu, B.F.; Lv, X.; Chen, H. Comprehensive and high-coverage lipidomic analysis of oilseeds based on ultrahigh-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2021, 69, 8964–8980. [Google Scholar] [CrossRef]
- Yang, Y.H.; Pan, H.Z.; Song, B.; Zhu, X.J.; Sun, Y.W. Anti-fatigue and anti-oxidation effects of soybean lecithin in mice. Prog. Mod. Biomed. 2011, 11, 4024–4026. [Google Scholar] [CrossRef]
- Cooke, R.F.; Río, N.S.D.; Caraviello, D.Z.; Bertics, S.J.; Ramos, M.H.; Grummer, R.R. Supplemental choline for prevention and alleviation of fatty liver in dairy cattle. J. Dairy Sci. 2007, 90, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; He, Z.Y.; Ma, N.; Chen, Z.Y. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef]
- Gray, M.J.; Gong, J.; Parks, R.N.; Hatch, M.M.S.; Nguyen, V.; Hughes, C.C.W.; Hutchins, J.T.; Freimark, B.D. Abstract 5116: Phosphatidylserine-targeting antibodies augment anti-tumor activity of PD-1 antibodies and alter immuno-profiles in murine triple negative breast cancers. Cancer Res. 2016, 76, 5116. [Google Scholar] [CrossRef]
- Ma, T.J.; Qin, X.J.; Jia, C.X. Enhancement effect of soybean lecithin on immune activity. Chin. Agric. Sci. Bull. 2010, 26, 97–99. [Google Scholar]
- Li, Y.H.; Wang, X.D.; Li, X.D.; Wang, N.N.; Xu, Y.H. Effect of pressing process on the oxidation stability of oil and the quality of cake meal. J. Henan Univ. Technol. Nat. Sci. Ed. 2017, 38, 26–31. [Google Scholar] [CrossRef]
- Gordon, M.H.; Mursi, E.; Rossell, J.B. Assessment of thin-film oxidation with ultraviolet irradiation for predicting the oxidative stability of edible oils. J. Am. Oil Chem. Soc. 1994, 71, 1309–1313. [Google Scholar] [CrossRef]
- Niu, F.H.; Liang, J.M.; Zhang, Y.Q.; Jiang, Y.R.; Wang, M.J.; Shang, P.L. Preliminary study on the oxidation mechanism of lipid under the accelerated conditions of the osi method. J. Chin. Cereals Oils Assoc. 2014, 29, 67–71. [Google Scholar] [CrossRef]
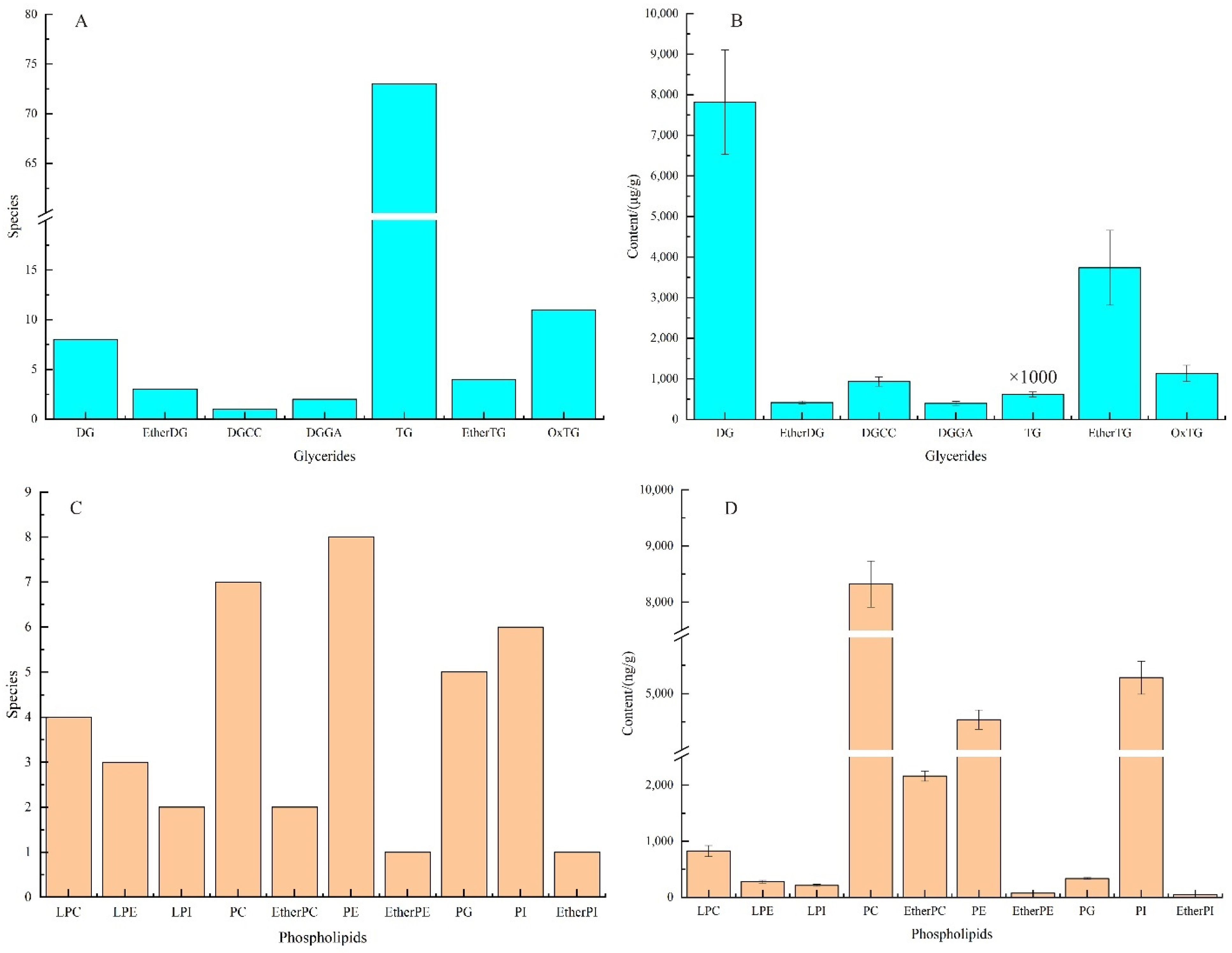
| No. | Average Rt (min) | Average Mz | Lipid Name | Adduct Type | Formula | Ontology | Content (μg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 6.007 | 586.53 | DG 32:0|DG 16:0_16:0 | [M+NH4]+ | C35H68O5 | DG | 43.81 ± 7.84 |
| 2 | 6.562 | 614.562 | DG 34:0|DG 16:0_18:0 | [M+NH4]+ | C37H72O5 | DG | 48.39 ± 6.18 |
| 3 | 6.069 | 612.548 | DG 34:1|DG 16:0_18:1 | [M+NH4]+ | C37H70O5 | DG | 900.65 ± 178.65 |
| 4 | 6.627 | 640.5787 | DG 36:1|DG 18:0_18:1 | [M+NH4]+ | C39H74O5 | DG | 632.07 ± 114.33 |
| 5 | 6.154 | 638.5631 | DG 36:2|DG 18:1_18:1 | [M+NH4]+ | C39H72O5 | DG | 3291.57 ± 497.25 |
| 6 | 5.732 | 636.5476 | DG 36:3|DG 18:1_18:2 | [M+NH4]+ | C39H70O5 | DG | 2286.09 ± 346.24 |
| 7 | 5.351 | 634.5338 | DG 36:4|DG 18:2_18:2 | [M+NH4]+ | C39H68O5 | DG | 529.40 ± 122.34 |
| 8 | 7.188 | 668.6134 | DG 38:1|DG 20:0_18:1 | [M+NH4]+ | C41H78O5 | DG | 88.25 ± 12.06 |
| 9 | 7.175 | 596.5529 | DG O-34:2|DG O-19:1_15:1 | [M+NH4]+ | C37H70O4 | EtherDG | 48.04 ± 4.95 |
| 10 | 7.241 | 622.5684 | DG O-36:3|DG O-19:1_17:2 | [M+NH4]+ | C39H72O4 | EtherDG | 282.66 ± 26.48 |
| 11 | 6.786 | 620.5507 | DG O-36:4|DG O-19:2_17:2 | [M+NH4]+ | C39H70O4 | EtherDG | 85.64 ± 7.88 |
| 12 | 5.612 | 780.6304 | DGCC 36:2 | [M+H]+ | C46H85NO8 | DGCC | 935.46 ± 116.33 |
| 13 | 3.89 | 788.5853 | DGGA 34:1|DGGA 16:0_18:1 | [M+NH4]+ | C43H78O11 | DGGA | 67.60 ± 9.66 |
| 14 | 3.924 | 814.6013 | DGGA 36:2|DGGA 18:1_18:1 | [M+NH4]+ | C45H80O11 | DGGA | 329.67 ± 40.97 |
| 15 | 6.381 | 628.5412 | TG 34:0|TG 8:0_10:0_16:0 | [M+NH4]+ | C37H70O6 | TG | 34.85 ± 1.66 |
| 16 | 6.943 | 656.5758 | TG 36:0|TG 10:0_12:0_14:0 | [M+NH4]+ | C39H74O6 | TG | 40.62 ± 1.74 |
| 17 | 6.51 | 654.5604 | TG 36:1|TG 8:0_10:0_18:1 | [M+NH4]+ | C39H72O6 | TG | 38.25 ± 4.56 |
| 18 | 7.482 | 684.6063 | TG 38:0|TG 10:0_12:0_16:0 | [M+NH4]+ | C41H78O6 | TG | 32.71 ± 1.81 |
| 19 | 7.08 | 682.5913 | TG 38:1|TG 10:0_10:0_18:1 | [M+NH4]+ | C41H76O6 | TG | 47.68 ± 5.67 |
| 20 | 8.019 | 712.6378 | TG 40:0|TG 10:0_14:0_16:0 | [M+NH4]+ | C43H82O6 | TG | 27.08 ± 0.39 |
| 21 | 7.578 | 710.6234 | TG 40:1|TG 10:0_12:0_18:1 | [M+NH4]+ | C43H80O6 | TG | 23.59 ± 2.48 |
| 22 | 8.527 | 740.6711 | TG 42:0|TG 10:0_16:0_16:0 | [M+NH4]+ | C45H86O6 | TG | 25.50 ± 1.40 |
| 23 | 8.098 | 738.6581 | TG 42:1|TG 8:0_16:0_18:1 | [M+NH4]+ | C45H84O6 | TG | 23.49 ± 1.11 |
| 24 | 9.016 | 768.703 | TG 44:0|TG 14:0_14:0_16:0 | [M+NH4]+ | C47H90O6 | TG | 21.62 ± 2.27 |
| 25 | 8.602 | 766.6914 | TG 44:1|TG 10:0_16:0_18:1 | [M+NH4]+ | C47H88O6 | TG | 27.25 ± 2.60 |
| 26 | 9.252 | 782.7144 | TG 45:0|TG 14:0_15:0_16:0 | [M+NH4]+ | C48H92O6 | TG | 18.69 ± 2.46 |
| 27 | 9.503 | 796.7383 | TG 46:0|TG 14:0_16:0_16:0 | [M+NH4]+ | C49H94O6 | TG | 30.36 ± 4.03 |
| 28 | 9.09 | 794.7215 | TG 46:1|TG 12:0_16:0_18:1 | [M+NH4]+ | C49H92O6 | TG | 26.84 ± 4.20 |
| 29 | 8.665 | 792.7044 | TG 46:2|TG 10:0_18:1_18:1 | [M+NH4]+ | C49H90O6 | TG | 21.53 ± 3.18 |
| 30 | 9.328 | 808.7332 | TG 47:1|TG 15:0_16:0_16:1 | [M+NH4]+ | C50H94O6 | TG | 21.45 ± 3.22 |
| 31 | 9.919 | 824.7706 | TG 48:0|TG 16:0_16:0_16:0 | [M+NH4]+ | C51H98O6 | TG | 173.58 ± 19.77 |
| 32 | 9.533 | 822.7522 | TG 48:1|TG 14:0_16:0_18:1/TG 16:0_16:0_16:1 | [M+NH4]+ | C51H96O6 | TG | 134.10 ± 17.14 |
| 33 | 9.147 | 820.7355 | TG 48:2|TG 14:0_16:0_18:2 | [M+NH4]+ | C51H94O6 | TG | 70.43 ± 13.58 |
| 34 | 10.135 | 838.7834 | TG 49:0|TG 15:0_16:0_18:0/TG 16:0_16:0_17:0 | [M+NH4]+ | C52H100O6 | TG | 14.64 ± 3.10 |
| 35 | 9.739 | 836.7692 | TG 49:1|TG 15:0_16:0_18:1 | [M+NH4]+ | C52H98O6 | TG | 49.24 ± 3.61 |
| 36 | 10.341 | 852.8005 | TG 50:0|TG 16:0_16:0_18:0 | [M+NH4]+ | C53H102O6 | TG | 244.63 ± 22.24 |
| 37 | 9.934 | 850.7884 | TG 50:1|TG 16:0_16:0_18:1 | [M+NH4]+ | C53H100O6 | TG | 10,906.80 ± 725.50 |
| 38 | 9.579 | 848.7698 | TG 50:2|TG 16:0_16:0_18:2 | [M+NH4]+ | C53H98O6 | TG | 6620.33 ± 739.40 |
| 39 | 9.19 | 846.7538 | TG 50:3|TG 16:0_16:1_18:2/TG 14:0_18:1_18:2 | [M+NH4]+ | C53H96O6 | TG | 691.78 ± 129.37 |
| 40 | 8.797 | 844.7354 | TG 50:4|TG 14:0_18:2_18:2/TG 16:1_16:1_18:2 | [M+NH4]+ | C53H94O6 | TG | 77.47 ± 14.40 |
| 41 | 10.159 | 864.7983 | TG 51:1|TG 16:0_17:0_18:1 | [M+NH4]+ | C54H102O6 | TG | 234.84 ± 34.49 |
| 42 | 9.785 | 862.7827 | TG 51:2|TG 16:0_17:1_18:1 | [M+NH4]+ | C54H100O6 | TG | 316.62 ± 58.60 |
| 43 | 9.409 | 860.7709 | TG 51:3|TG 15:0_18:1_18:2/TG 16:0_17:1_18:2 | [M+NH4]+ | C54H98O6 | TG | 135.56 ± 28.82 |
| 44 | 9.052 | 858.7533 | TG 51:4|TG 15:1_18:1_18:2 | [M+NH4]+ | C54H96O6 | TG | 37.49 ± 8.61 |
| 45 | 10.728 | 880.8356 | TG 52:0|TG 16:0_18:0_18:0 | [M+NH4]+ | C55H106O6 | TG | 245.29 ± 27.13 |
| 46 | 10.356 | 878.8196 | TG 52:1|TG 16:0_18:0_18:1 | [M+NH4]+ | C55H104O6 | TG | 19,799.4 ± 1726.82 |
| 47 | 9.981 | 876.8026 | TG 52:2|TG 16:0_18:1_18:1 | [M+NH4]+ | C55H102O6 | TG | 77,017.74 ± 6597.30 |
| 48 | 9.621 | 874.7887 | TG 52:3|TG 16:0_18:1_18:2 | [M+NH4]+ | C55H100O6 | TG | 52,885.54 ± 4331.86 |
| 49 | 9.248 | 872.7718 | TG 52:4|TG 16:0_18:2_18:2 | [M+NH4]+ | C55H98O6 | TG | 16,682.36 ± 2329.85 |
| 50 | 8.858 | 870.7551 | TG 52:5|TG 16:1_18:2_18:2 | [M+NH4]+ | C55H96O6 | TG | 698.56 ± 161.73 |
| 51 | 8.465 | 868.7395 | TG 52:6|TG 16:1_18:2_18:3 | [M+NH4]+ | C55H94O6 | TG | 24.58 ± 4.44 |
| 52 | 10.557 | 892.8341 | TG 53:1|TG 17:0_18:0_18:1 | [M+NH4]+ | C56H106O6 | TG | 222.64 ± 42.24 |
| 53 | 10.198 | 890.8204 | TG 53:2|TG 17:0_18:1_18:1 | [M+NH4]+ | C56H104O6 | TG | 978.34 ± 240.31 |
| 54 | 9.834 | 888.8024 | TG 53:3|TG 17:0_18:1_18:2 | [M+NH4]+ | C56H102O6 | TG | 955.72 ± 182.39 |
| 55 | 9.469 | 886.7875 | TG 53:4|TG 17:1_18:1_18:2 | [M+NH4]+ | C56H100O6 | TG | 345.10 ± 70.18 |
| 56 | 9.071 | 884.7697 | TG 53:5|TG 17:1_18:2_18:2 | [M+NH4]+ | C56H98O6 | TG | 65.36 ± 15.02 |
| 57 | 11.079 | 908.8664 | TG 54:0|TG 18:0_18:0_18:0 | [M+NH4]+ | C57H110O6 | TG | 166.27 ± 26.64 |
| 58 | 10.747 | 906.8533 | TG 54:1|TG 18:0_18:0_18:1 | [M+NH4]+ | C57H108O6 | TG | 14,911.87 ± 2064.66 |
| 59 | 10.389 | 904.837 | TG 54:2|TG 18:0_18:1_18:1 | [M+NH4]+ | C57H106O6 | TG | 71,670.86 ± 6993.73 |
| 60 | 10.023 | 902.82 | TG 54:3|TG 18:1_18:1_18:1 | [M+NH4]+ | C57H104O6 | TG | 158,176.36 ± 14,506.08 |
| 61 | 9.664 | 900.8034 | TG 54:4|TG 18:1_18:1_18:2 | [M+NH4]+ | C57H102O6 | TG | 113,315.23 ± 9538.59 |
| 62 | 9.291 | 898.7886 | TG 54:5|TG 18:1_18:2_18:2 | [M+NH4]+ | C57H100O6 | TG | 46,748.99 ± 4593.31 |
| 63 | 8.915 | 896.7748 | TG 54:6|TG 18:2_18:2_18:2 | [M+NH4]+ | C57H98O6 | TG | 9332.70 ± 2221.89 |
| 64 | 8.552 | 894.7576 | TG 54:7|TG 18:2_18:2_18:3 | [M+NH4]+ | C57H96O6 | TG | 162.36 ± 28.41 |
| 65 | 10.588 | 918.8489 | TG 55:2|TG 18:0_18:1_19:1 | [M+NH4]+ | C58H108O6 | TG | 120.82 ± 29.04 |
| 66 | 10.232 | 916.8322 | TG 55:3|TG 18:1_18:1_19:1 | [M+NH4]+ | C58H106O6 | TG | 204.50 ± 48.06 |
| 67 | 11.098 | 934.887 | TG 56:1|TG 18:0_20:0_18:1 | [M+NH4]+ | C59H112O6 | TG | 1453.44 ± 314.39 |
| 68 | 10.772 | 932.8681 | TG 56:2|TG 20:0_18:1_18:1 | [M+NH4]+ | C59H110O6 | TG | 4643.38 ± 855.3 |
| 69 | 10.45 | 930.8524 | TG 56:3|TG 20:0_18:1_18:2 | [M+NH4]+ | C59H108O6 | TG | 2809.19 ± 381.97 |
| 70 | 10.098 | 928.8333 | TG 56:4|TG 18:1_20:1_18:2 | [M+NH4]+ | C59H106O6 | TG | 895.92 ± 118.64 |
| 71 | 9.725 | 926.8198 | TG 56:5|TG 20:1_18:2_18:2 | [M+NH4]+ | C59H104O6 | TG | 169.66 ± 22.44 |
| 72 | 10.964 | 946.8829 | TG 57:2|TG 21:0_18:1_18:1 | [M+NH4]+ | C60H112O6 | TG | 39.59 ± 7.56 |
| 73 | 11.444 | 962.9172 | TG 58:1|TG 16:0_24:0_18:1 | [M+NH4]+ | C61H116O6 | TG | 366.37 ± 76.17 |
| 74 | 11.135 | 960.9021 | TG 58:2|TG 22:0_18:1_18:1 | [M+NH4]+ | C61H114O6 | TG | 819.05 ± 196.76 |
| 75 | 10.826 | 958.882 | TG 58:3|TG 22:0_18:1_18:2 | [M+NH4]+ | C61H112O6 | TG | 372.88 ± 73.15 |
| 76 | 10.506 | 956.8675 | TG 58:4|TG 22:0_18:2_18:2 | [M+NH4]+ | C61H110O6 | TG | 95.71 ± 17.54 |
| 77 | 11.3 | 974.9174 | TG 59:2|TG 23:0_18:1_18:1 | [M+NH4]+ | C62H116O6 | TG | 77.38 ± 17.82 |
| 78 | 11.002 | 972.8971 | TG 59:3|TG 23:0_18:1_18:2 | [M+NH4]+ | C62H114O6 | TG | 45.41 ± 10.59 |
| 79 | 11.757 | 990.9497 | TG 60:1|TG 18:0_24:0_18:1 | [M+NH4]+ | C63H120O6 | TG | 131.71 ± 26.08 |
| 80 | 11.463 | 988.9363 | TG 60:2|TG 24:0_18:1_18:1 | [M+NH4]+ | C63H118O6 | TG | 541.34 ± 129.81 |
| 81 | 11.173 | 986.9159 | TG 60:3|TG 24:0_18:1_18:2 | [M+NH4]+ | C63H116O6 | TG | 338.33 ± 80.07 |
| 82 | 10.875 | 984.9028 | TG 60:4|TG 24:0_18:2_18:2 | [M+NH4]+ | C63H114O6 | TG | 90.47 ± 19.19 |
| 83 | 11.625 | 1002.948 | TG 61:2|TG 25:0_18:1_18:1 | [M+NH4]+ | C64H120O6 | TG | 43.33 ± 8.71 |
| 84 | 11.345 | 1000.933 | TG 61:3|TG 25:0_18:1_18:2 | [M+NH4]+ | C64H118O6 | TG | 30.67 ± 6.49 |
| 85 | 12.069 | 1018.979 | TG 62:1|TG 18:0_26:0_18:1/TG 20:0_24:0_18:1 | [M+NH4]+ | C65H124O6 | TG | 16.10 ± 2.92 |
| 86 | 11.783 | 1016.962 | TG 62:2|TG 26:0_18:1_18:1 | [M+NH4]+ | C65H122O6 | TG | 51.33 ± 9.60 |
| 87 | 11.507 | 1014.945 | TG 62:3|TG 26:0_18:1_18:2 | [M+NH4]+ | C65H120O6 | TG | 35.72 ± 8.31 |
| 88 | 9.98 | 876.8322 | TG O-53:2|TG O-17:0_18:1_18:1 | [M+NH4]+ | C56H106O5 | EtherTG | 3103.79 ± 829.83 |
| 89 | 9.687 | 874.8325 | TG O-53:3|TG O-19:2_16:0_18:1 | [M+NH4]+ | C56H104O5 | EtherTG | 128.76 ± 40.46 |
| 90 | 9.791 | 888.8312 | TG O-54:3|TG O-19:2_17:0_18:1 | [M+NH4]+ | C57H106O5 | EtherTG | 127.67 ± 14.35 |
| 91 | 9.317 | 898.8256 | TG O-55:5|TG O-19:1_18:2_18:2/TG O-19:2_18:1_18:2 | [M+NH4]+ | C58H104O5 | EtherTG | 377.49 ± 38.47 |
| 92 | 8.259 | 864.7666 | TG 50:2;1O|TG 16:0_18:1_16:1;1O | [M+NH4]+ | C53H98O7 | OxTG | 41.54 ± 6.31 |
| 93 | 7.841 | 862.7492 | TG 50:3;1O|TG 16:0_18:2_16:1;1O | [M+NH4]+ | C53H96O7 | OxTG | 18.86 ± 1.96 |
| 94 | 8.734 | 892.7969 | TG 52:2;1O|TG 16:0_18:1_18:1;1O | [M+NH4]+ | C55H102O7 | OxTG | 101.76 ± 17.18 |
| 95 | 8.334 | 890.7819 | TG 52:3;1O|TG 18:1_18:1_16:1;1O | [M+NH4]+ | C55H100O7 | OxTG | 188.32 ± 35.24 |
| 96 | 7.926 | 888.7655 | TG 52:4;1O|TG 18:1_18:2_16:1;1O | [M+NH4]+ | C55H98O7 | OxTG | 71.32 ± 17.76 |
| 97 | 9.243 | 920.8271 | TG 54:2;1O|TG 18:0_18:1_18:1;1O | [M+NH4]+ | C57H106O7 | OxTG | 84.42 ± 12.31 |
| 98 | 8.784 | 918.8133 | TG 54:3;1O|TG 18:1_18:1_18:1;1O | [M+NH4]+ | C57H104O7 | OxTG | 206.75 ± 41.87 |
| 99 | 8.465 | 916.7983 | TG 54:4;1O|TG 18:1_18:1_18:2;1O | [M+NH4]+ | C57H102O7 | OxTG | 223.56 ± 29.44 |
| 100 | 8.099 | 914.7819 | TG 54:5;1O|TG 18:1_18:2_18:2;1O | [M+NH4]+ | C57H100O7 | OxTG | 131.72 ± 23.86 |
| 101 | 7.706 | 912.7652 | TG 54:6;1O|TG 18:2_18:2_18:2;1O | [M+NH4]+ | C57H98O7 | OxTG | 41.80 ± 6.28 |
| 102 | 9.702 | 948.8707 | TG 56:2;1O|TG 18:1_18:1_20:0;1O | [M+NH4]+ | C59H110O7 | OxTG | 25.57 ± 9.29 |
| No | Average Rt (min) | Average Mz | Lipid Name | Adduct Type | Formula | Ontology | Content (ng/g) |
|---|---|---|---|---|---|---|---|
| 1 | 2.2 | 554.3408 | LPC 16:0 | [M+CH3COO]− | C24H50NO7P | LPC | 71.72 ± 44.14 |
| 2 | 2.766 | 582.3713 | LPC 18:0 | [M+CH3COO]− | C26H54NO7P | LPC | 59.52 ± 7.31 |
| 3 | 2.247 | 580.3578 | LPC 18:1 | [M+CH3COO]− | C26H52NO7P | LPC | 452.94 ± 17.71 |
| 4 | 1.862 | 578.3447 | LPC 18:2 | [M+CH3COO]− | C26H50NO7P | LPC | 241.56 ± 29.44 |
| 5 | 1.979 | 452.2763 | LPE 16:0 | [M−H]− | C21H44NO7P | LPE | 55.49 ± 1.64 |
| 6 | 2.182 | 478.2912 | LPE 18:1 | [M−H]− | C23H46NO7P | LPE | 171.82 ± 5.68 |
| 7 | 1.705 | 476.2744 | LPE 18:2 | [M−H]− | C23H44NO7P | LPE | 51.41 ± 17.18 |
| 8 | 1.133 | 571.2898 | LPI 16:0 | [M−H]− | C25H49O12P | LPI | 85.04 ± 8.21 |
| 9 | 1.242 | 597.294 | LPI 18:1 | [M−H]− | C27H51O12P | LPI | 130.13 ± 10.53 |
| 10 | 5.58 | 792.5732 | PC 32:0|PC 16:0_16:0 | [M+CH3COO]− | C40H80NO8P | PC | 174.80 ± 54.47 |
| 11 | 5.761 | 760.5845 | PC 34:1|PC 16:0_18:1 | [M−H]- | C42H82NO8P | PC | 1947.39 ± 81.42 |
| 12 | 5.134 | 816.5735 | PC 34:2|PC 16:0_18:2 | [M+CH3COO]− | C42H80NO8P | PC | 505.54 ± 10.96 |
| 13 | 6.348 | 846.618 | PC 36:1|PC 18:0_18:1 | [M+CH3COO]− | C44H86NO8P | PC | 820.53 ± 70.57 |
| 14 | 5.619 | 844.6074 | PC 36:2|PC 18:1_18:1 | [M+CH3COO]− | C44H84NO8P | PC | 3165.79 ± 125.22 |
| 15 | 5.173 | 842.5898 | PC 36:3|PC 18:1_18:2 | [M+CH3COO]− | C44H82NO8P | PC | 1505.05 ± 52.29 |
| 16 | 4.781 | 782.5712 | PC 36:4|PC 18:2_18:2 | [M−H]− | C44H80NO8P | PC | 201.99 ± 16.96 |
| 17 | 5.595 | 818.5919 | PC O-34:2;1O|PC O-17:0_17:2;1O | [M+CH3COO]− | C42H82NO8P | EtherPC | 2095.91 ± 87.63 |
| 18 | 6.953 | 846.6531 | PC O-37:1|PC O-21:1_16:0 | [M+CH3COO]− | C45H90NO7P | EtherPC | 63.43 ± 1.90 |
| 19 | 4.901 | 690.5027 | PE 32:0|PE 16:0_16:0 | [M−H]− | C37H74NO8P | PE | 31.97 ± 7.02 |
| 20 | 5.417 | 718.5353 | PE 34:0|PE 16:0_18:0 | [M−H]− | C39H78NO8P | PE | 69.89 ± 19.96 |
| 21 | 4.955 | 716.5236 | PE 34:1|PE 16:0_18:1 | [M−H]− | C39H76NO8P | PE | 1298.75 ± 15.45 |
| 22 | 4.621 | 714.5069 | PE 34:2|PE 16:0_18:2 | [M−H]− | C39H74NO8P | PE | 248.80 ± 7.56 |
| 23 | 5.434 | 744.5578 | PE 36:1|PE 18:0_18:1 | [M−H]− | C41H80NO8P | PE | 577.32 ± 74.51 |
| 24 | 5.024 | 742.5381 | PE 36:2|PE 18:1_18:1 | [M−H]− | C41H78NO8P | PE | 1298.54 ± 12.70 |
| 25 | 4.675 | 740.5211 | PE 36:3|PE 18:1_18:2 | [M−H]− | C41H76NO8P | PE | 792.68 ± 31.88 |
| 26 | 4.367 | 738.5042 | PE 36:4|PE 18:2_18:2 | [M−H]− | C41H74NO8P | PE | 221.45 ± 2.94 |
| 27 | 5.01 | 824.541 | PE 40:5;2O|PE 18:1_22:4;2O | [M−H]− | C45H80NO10P | EtherPE | 74.15 ± 1.59 |
| 28 | 3.844 | 721.4987 | PG 32:0|PG 16:0_16:0 | [M−H]− | C38H75O10P | PG | 109.15 ± 3.55 |
| 29 | 4.112 | 749.5281 | PG 34:0|PG 16:0_18:0 | [M−H]− | C40H79O10P | PG | 67.33 ± 1.65 |
| 30 | 3.884 | 747.514 | PG 34:1|PG 16:0_18:1 | [M−H]− | C40H77O10P | PG | 113.30 ± 3.92 |
| 31 | 3.672 | 745.4976 | PG 34:2|PG 16:0_18:2 | [M−H]− | C40H75O10P | PG | 28.71 ± 1.90 |
| 32 | 3.938 | 773.5289 | PG 36:2|PG 18:1_18:1 | [M−H]− | C42H79O10P | PG | 16.96 ± 4.35 |
| 33 | 3.805 | 835.5365 | PI 34:1|PI 16:0_18:1 | [M−H]− | C43H81O13P | PI | 2347.70 ± 153.09 |
| 34 | 3.59 | 833.5206 | PI 34:2|PI 16:0_18:2 | [M−H]− | C43H79O13P | PI | 974.35 ± 9.66 |
| 35 | 4.082 | 863.5654 | PI 36:1|PI 18:0_18:1 | [M−H]− | C45H85O13P | PI | 587.39 ± 43.87 |
| 36 | 3.859 | 861.5489 | PI 36:2|PI 18:1_18:1 | [M−H]− | C45H83O13P | PI | 854.96 ± 67.01 |
| 37 | 3.638 | 859.5302 | PI 36:3|PI 18:1_18:2 | [M−H]− | C45H81O13P | PI | 401.45 ± 9.99 |
| 38 | 3.42 | 857.5183 | PI 36:4|PI 18:2_18:2 | [M−H]− | C45H79O13P | PI | 116.70 ± 7.24 |
| 39 | 1.591 | 599.3121 | PI O-18:0 | [M−H]− | C27H53O12P | EtherPI | 49.67 ± 5.94 |
| Squeezing Temperature | Acid Value (mgNaOH/g) | Iodine Value (g/100 g) | Peroxide Value (meq/kg) | Refractive Index | Specific Extinction Coefficient | |
|---|---|---|---|---|---|---|
| K232 | K270 | |||||
| 100 °C | 0.526 ± 0.86 a | 78.196 ± 17.56 b | 0.288 ± 0.04 c | 1.4612 ± 0.07 a | 1.027 ± 0.64 c | 0.114 ± 3.49 a |
| 120 °C | 0.457 ± 1.46 b | 79.550 ± 4.34 a | 0.325 ± 0.11 b | 1.4605 ± 0.13 a | 1.051 ± 0.12 c | 0.117 ± 0.40 a |
| 140 °C | 0.415 ± 0.25 b | 79.736 ± 29.55 a | 0.135 ± 0.11 d | 1.4623 ± 0.08 a | 1.007 ± 0.79 c | 0.053 ± 0.50 b |
| 160 °C | 0.416 ± 0.00 b | 76.546 ± 24.19 c | 0.116 ± 0.13 d | 1.4559 ± 0.33 b | 1.102 ± 0.12 b | 0.049 ± 0.47 b |
| 180 °C | 0.428 ± 0.99 b | 78.186 ± 41.35 b | 0.384 ± 0.09 b | 1.4578 ± 0.16 b | 1.154 ± 0.62 b | 0.080 ± 0.62 b |
| 200 °C | 0.421 ± 1.55 b | 75.214 ± 1.44 d | 0.419 ± 0.11 a | 1.4611 ± 0.31 a | 1.212 ± 1.70 a | 0.117 ± 0.64 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, L.; Xia, Q.; Lin, L. Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil. Foods 2023, 12, 693. https://doi.org/10.3390/foods12040693
Liu Y, Li L, Xia Q, Lin L. Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil. Foods. 2023; 12(4):693. https://doi.org/10.3390/foods12040693
Chicago/Turabian StyleLiu, Yijun, Leshi Li, Qiuyu Xia, and Lijing Lin. 2023. "Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil" Foods 12, no. 4: 693. https://doi.org/10.3390/foods12040693
APA StyleLiu, Y., Li, L., Xia, Q., & Lin, L. (2023). Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil. Foods, 12(4), 693. https://doi.org/10.3390/foods12040693





