Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tiger Nut Beverages Production and Processing
2.1.1. Tiger Nut Beverages Production
2.1.2. Beverage Treatments: UHPH, Homogenization-Pasteurization
2.2. Procedure of HP-SPME and GC-MS
2.2.1. HP-SPME Extractions
2.2.2. GC-MS Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of Treatments on the Volatile Composition of Beverages
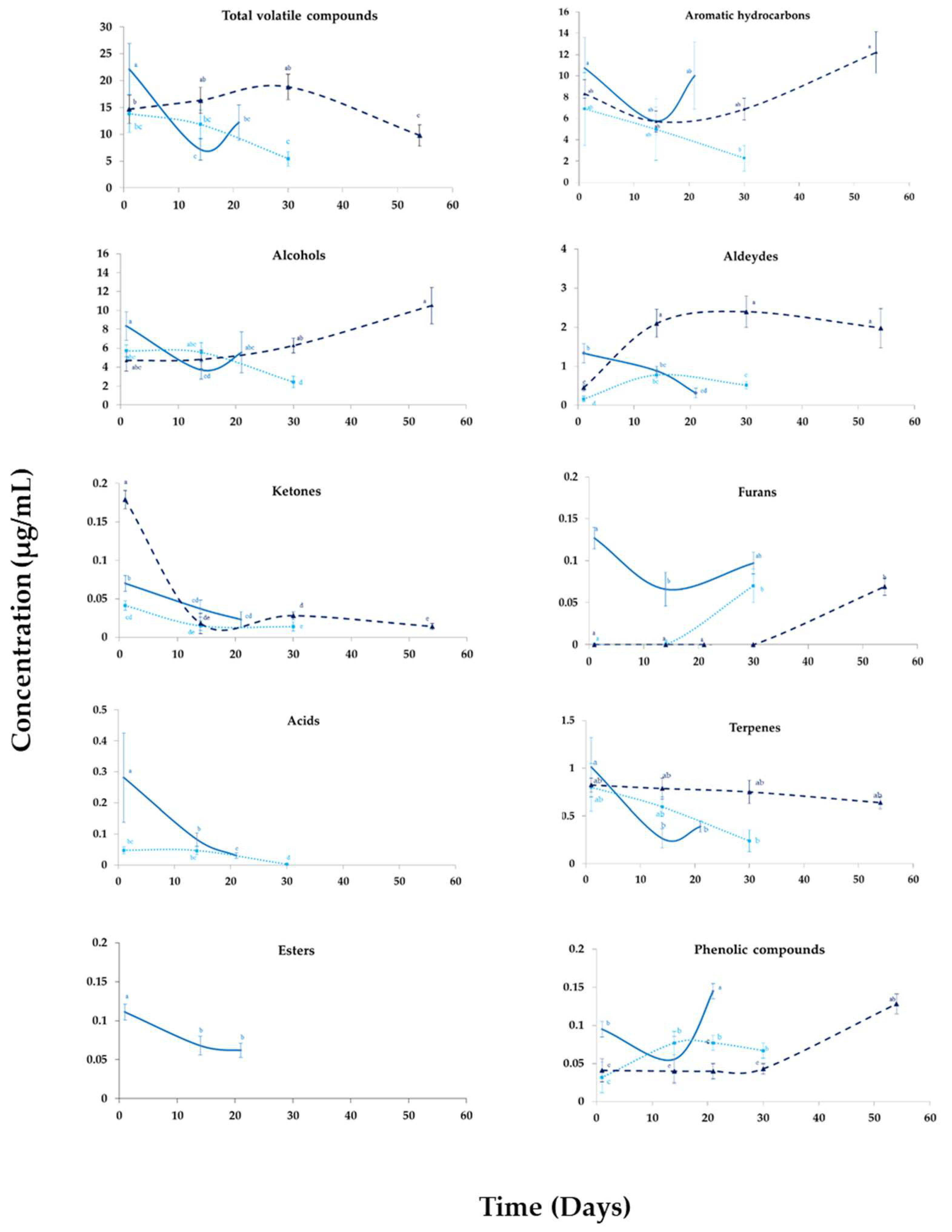
3.1.1. Main Groups of Volatile Patterns: Aromatic Hydrocarbons, Alcohols and Aldehydes
3.1.2. Secondary Compounds of Beverages: Ketones, Acids, Esters, Phenolic Compounds, and Furans
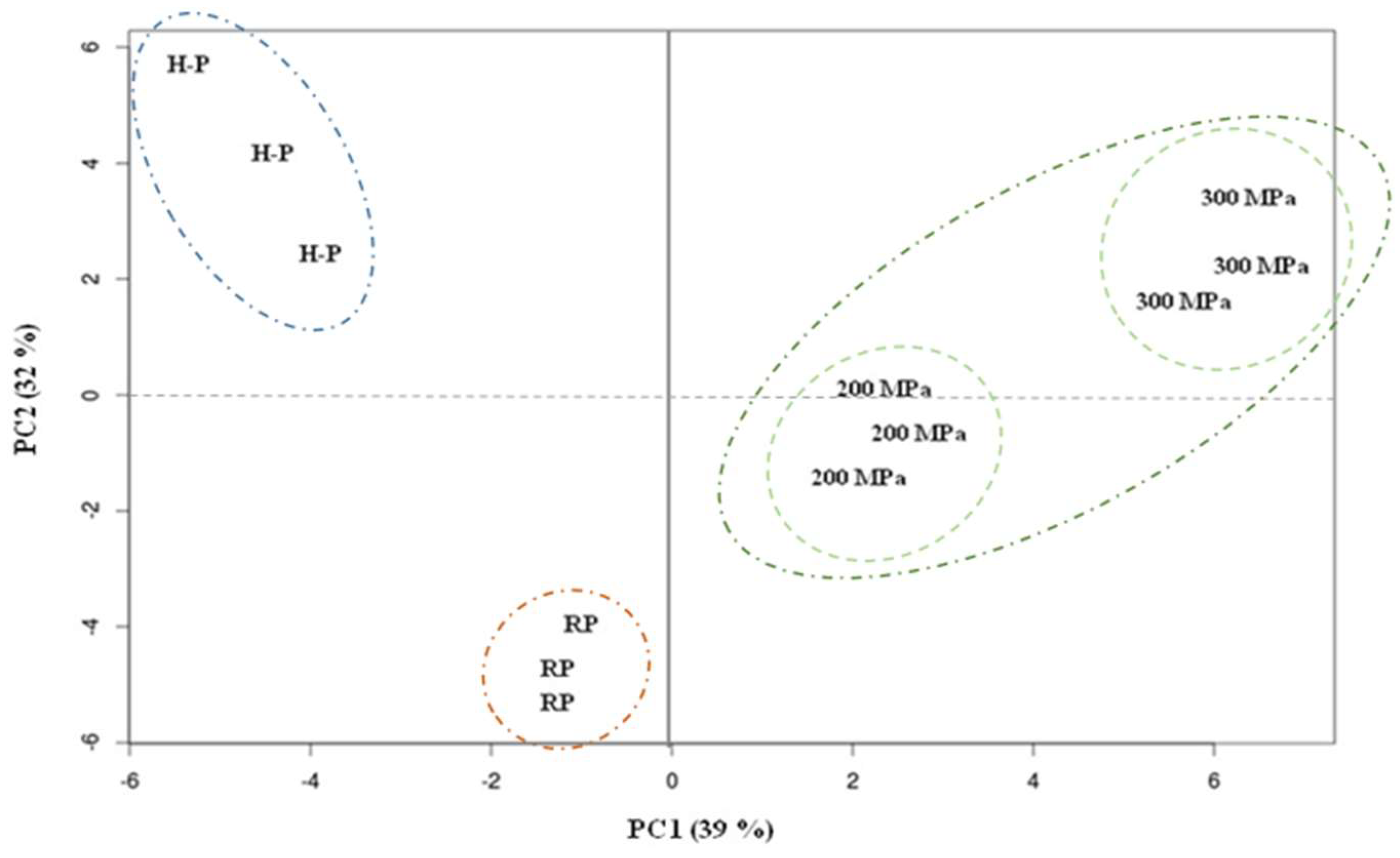
3.2. Principal Component Analysis (PCA)
3.3. Changes in the Volatile Profile of Beverages during Their Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CRDO Consejo Regulador de la, D.O. Horchata de Chufa de Valencia. 2022. Available online: http//www.chufadevalencia.org/ (accessed on 3 November 2022).
- Alegría-Torán, A.; Farré-Rovira, R. Horchata y Salud: Aspectosnutricionales y Dietéticos; Jornada Chufa y Horchata: Tradición y Salud; Fundación Valenciana de Estudios Avanzados, Ed.; Consellería de Agricultura, Pesca y Alimentación: Valencia, Spain, 2003; pp. 55–70. [Google Scholar]
- Clemente-Villalba, J.; Cano-Lamadrid, M.; Issa-Issa, H.; Hurtado, P.; Hernández, F.; Carbonell-Barrachina, A.A.; López-Lluch, D. Comparison on sensory profile, volatile composition and consumer’s acceptance for PDO or non PDO tigernut (Cyperus esculentus L.) milk. LWT 2021, 140, 110606. [Google Scholar] [CrossRef]
- Klein, B.; Gallardo-Chacón, J.J.; Codina-Torrella, I.; Trujillo, A.J.; Juan, B. Evaluation of volatile compounds of “Tiger nut beverage” (Orxata de xufla) headspace by optimized solid-phase micro-extraction. OALib J. 2014, 1, 1–15. [Google Scholar] [CrossRef]
- Ezeh, O.; Gordon, M.H.; Niranjan, K. Tiger nut oil (Cyperus esculentus L.): A review of its composition and physico-chemical properties. Eur. J Lipid Sci Technol. 2014, 116, 783–794. [Google Scholar] [CrossRef]
- Morell, J.; Barber, S. Chufa y Horchata: Características Físicas, Químicas y Nutritivas; Valencia: C.S.I.C. Instituto de Agroquímica y Tecnología de Alimentos: Valencia, Spain, 1983. [Google Scholar]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2012, 31, 13–26. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Guamis, B.; Ferragut, V.; Trujillo, A.J. Potential application of ultra-high pressure homogenization in the physico-chemical stabilization of tiger nuts’ milk beverage. Innov. Food Sci. Emerg. Technol. 2017, 40, 42–51. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Guamis, B.; Zamora, A.; Quevedo, J.M.; Trujillo, A.J. Microbiological stabilization of tiger nuts’ milk beverage using ultra-high pressure homogenization. A preliminary study on microbial shelf-life extension. Food Microbiol. 2018, 69, 143–150. [Google Scholar] [CrossRef]
- Ferragut, V.; Valencia-Flores, D.C.; Pérez-González, M.; Gallardo, J.; Hernández-Herrero, M. Quality characteristics and shelf-life of ultra-high pressure homogenized (UHPH) almond beverage. Foods 2015, 4, 159–172. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Gallardo-Chacón, J.J.; Juan, B.; Guamis, B.; Ferragut, V. Characterisation of volatile profile in soymilk treated by ultra high pressure homogenisation. Food Chem. 2013, 141, 2541–2548. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Johnson, A.E.; Nursten, H.E.; Self, R. Aromatic hydrocarbons in foodstuffs and related materials. Chemy Ind. 1969, 1, 10–12. [Google Scholar]
- Mackiewicz-Walec, E.; Krzebietke, S.J.; Sienkiewicz, S. The Influence of Crops on the Content of Polycyclic Aromatic Hydrocarbons in Soil Fertilized with Manure and Mineral Fertilizers. Int. J. Environ. Res. Public Health 2022, 19, 13627. [Google Scholar] [CrossRef]
- Cantalejo, J.M. Analysis of Volatile Components Derived from Raw and Roasted Earth-Almond (Cyperus esculentus L.). J Agric. Food Chem. 1997, 45, 1853–1860. [Google Scholar] [CrossRef]
- Lozano, P.R.; Drake, M.; Benitez, D.; Cadwallader, K.R. Instrumental and sensory characterization of heat-induced odorants in aseptically packaged soy milk. J. Agric. Food Chem. 2007, 55, 3018–3026. [Google Scholar] [CrossRef]
- Mazza, G.; Pietrzak, E.M. Headspace Volatiles and Sensory Characteristics of Earthy, Musty Flavored Potatoes. Food Chem. 1990, 36, 97–112. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Wang, C.; Pei, D. Identification of volatile oxidation compounds as potential markers of walnut oil quality. Food Chem. 2018, 83, 2745–2752. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Liu, Y.; Li, J.; Song, H.-L. Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments. Foods 2020, 9, 227. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Aromagrams-Aromatic profiles in the appreciation of food quality. Food Chem. 2007, 101, 845–872. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.; Buxaderas, S.; López-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Modifications induced by oxidation and suitable markers of oxidative status. J. Agric. Food Chem. 2003, 51, 6564–6571. [Google Scholar] [CrossRef]
- Yuan, S.H.; Chang, K.C. Selected odor compounds in cooked soymik as affected by soybean materials and direct steam injection. J. Food Sci. 2007, 72, 481–486. [Google Scholar] [CrossRef]
- Gohtani, S.; Sirendi, M.; Yamamoto, N.; Kajikawa, K.; Yamano, Y. Effect of droplet size on oxidation of docosahexanoic acid in emulsion system. J. Dispers. Sci. Technol. 1999, 20, 1319–1325. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Guamis, B.; Blasco-Moreno, A.; Trujillo, A.J. Physical and oxidative stability of whey protein oil-in-water emulsions produced by conventional and ultra high-pressure homogenization: Effects of pressure and protein concentration on emulsion characteristics. Innov. Food Sci. Emerg. Technol. 2015, 32, 79–90. [Google Scholar] [CrossRef]
- Pontin, M.; Bottini, R.; Burba, J.L.; Piccoli, P. Allium sativum produces terpenes with fungistatic properties in response to infection with Sclerotium cepivorum. Phytochemistry 2007, 115, 152–160. [Google Scholar] [CrossRef]
- Badui, S. Química de los Alimentos, 4th ed.; Pearson Educación: Mexico City, Mexico, 2006. [Google Scholar]
- Ayala-Zavala, J.F.; González-Aguilar, G.A.; Del-Toro-Sánchez, L. Enhancing safety and aroma appealing of fresh-cut fruits and vegetables using the antimicrobial and aromatic power of essential oils. J. Food Sci. 2009, 74, 84–90. [Google Scholar] [CrossRef]
- Ho, C.T.; Carlin, J.T. Formation and aroma characteristics of heterocyclic compounds in foods. Am. Chem. Soc. USA 1989, 388, 92–104. [Google Scholar]
- Wilkens, W.F.; Lin, F.M. Gas chromatographic and mass spectral analyses of soybean milk volatiles. J. Agric. Food Chem. 1970, 18, 333–336. [Google Scholar] [CrossRef]
- Lasekan, O. Volatile constituents of roasted tigernut oil (Cyperus esculentus L.). J. Sci. Food Agric. 2012, 93, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Boye, J.I.; Zamami, Y. Soybean variety and storage effects on soymilk flavor and quality. J. Food Qual. 2008, 30, 731–744. [Google Scholar] [CrossRef]
- Wang, Z.H.; Dou, J.; Macura, D.; Durance, T.D.; Nakai, S. Solid phase extraction for GC analysis of beany flavors in soymilk. Food Res. Int. 1997, 30, 503–511. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC-MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef]
- Neffat, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity Impact on Fruit Yield, Essential Oil Composition and Antioxidant Activities of Coriandrum sativum Fruit Extracts. Food Chem. 2009, 124, 221–225. [Google Scholar] [CrossRef]
- Lee, B.M.; Shim, G.A. Dietary exposure estimation of benzo[a]pyrene and cancer risk assessment. J. Toxicol. Environ. Health 2007, 70, 1391–1394. [Google Scholar] [CrossRef]
| Family Group | Compound | ID 1 | KI 2 | KI lit. 3 | Treatment 4 | |||
|---|---|---|---|---|---|---|---|---|
| RP | H-P | 200 MPa | 300 MPa | |||||
| Aromatic Hydrocarbons | Toluene | MS, IR, S | 1053.0 | 1042 | 3.419 ± 0.961 b | 9.835 ± 1.567 ab | 6.182 ± 1.949 ab | 7.424 ± 0.738 ab |
| Ethylbenzene | MS, IR, S | 1132.2 | 1124 | 0.028 ± 0.002 b | 0.048 ± 0.009 ab | 0.086 ± 0.018 a | 0.089 ± 0.007 a | |
| p-xylene | MS, IR, S | 1141.4 | 1150 | 0.013 ± 0.001 c | 0.199 ± 0.050 a | 0.036 ± 0.003 b | 0.068 ± 0.003 b | |
| m-xylene | MS, IR | 1146.3 | 1150 | 0.071 ± 0.006 b | 0.216 ± 0.039 a | 0.247 ± 0.048 a | 0.289 ± 0.018 a | |
| o-xylene | MS, IR | 1191.9 | 1182 | 0.036 ± 0.002 c | 0.167 ± 0.030 b | 0.114 ± 0.020 b | 0.213 ± 0.017 a | |
| Styrene | MS, IR | 1256.5 | 1273 | 0.024 ± 0.012 c | 0.061 ± 0.011 ab | 0.076 ± 0.024 a | 0.030 ± 0.003 b | |
| m-cymene | MS, IR, S | 1288.4 | 1267 | <LOQ | <LOQ | 0.096 ± 0.025 a | 0.085 ± 0.010 a | |
| 1,2,4-trimethylbenzene | MS, IR | 1295.3 | 1293 | 0.002 ± 0.000 c | 0.183 ± 0.032 a | <LOQ | 0.092 ± 0.005 b | |
| Naftalene | MS, IR | 1795.7 | 1825 | <LOQ | 0.022 ± 0.004 ab | 0.029 ± 0.003 a | 0.018 ± 0.003 b | |
| Total | 3.593 ± 1.961 b | 10.731 ± 2.805 a | 6.866 ± 3.424 ab | 8.308 ± 1.354 ab | ||||
| Alcohols | Ethanol | MS, RI | 941.4 | 936 | 0.523 ± 0.181 c | 1.924 ± 0.572 a | 0.823 ± 0.243 b | 0.490 ± 0.127 c |
| 1-hexanol | MS, RI, S | 1357.6 | 1355 | 0.105 ± 0.022 c | 0.192 ± 0.013 b | 0.355 ± 0.027 a | 0.295 ± 0.031 a | |
| 2-octanol | MS | 1417.8 | 1421 | <LOQ | 0.034 ± 0.005 a | <LOQ | <LOQ | |
| 1-octen-3-ol | MS, RI, S | 1451.9 | 1451 | <LOQ | <LOQ | 0.272 ± 0.117 a | <LOQ | |
| 1-heptanol | MS | 1454.8 | 1455 | 0.096 ± 0.010 c | 0.273 ± 0.023 b | <LOQ | 1.295 ± 0.184 a | |
| 1-octanol | MS, RI. S | 1549.2 | 1565 | 0.421 ± 0.025 b | 1.285 ± 0.306 a | 1.167 ± 0.081 a | 0.013 ± 0.001 c | |
| 1-nonanol | MS, RI | 1659.2 | 1661 | 1.339 ± 0.020 c | 4.560 ± 0.652 a | 3.067 ± 0.151 b | 2.570 ± 0.314 bc | |
| Total | 2.484 ± 0.109 b | 8.268 ± 1.522 a | 5.684 ± 0.632 a | 4.664 ± 1.111 ab | ||||
| Phenolic Compounds | Phenol-2-metoxy | MS, RI | 1889.8 | 1883 | <LOQ | 0.007 ± 0.002 a | <LOQ | <LOQ |
| Phenol | MS, RI, S | 2024.8 | 2209 | <LOQ | <LOQ | 0.018 ± 0.002 b | 0.037 ± 0.015 a | |
| 4-vinil-2-metoxyphenol | MS, RI | 2227.7 | 2223 | 0.034 ± 0.003 b | 0.088 ± 0.022 a | 0.014 ± 0.001 c | 0.004 ± 0.001 d | |
| Total | 0.034 ± 0.003 b | 0.095 ± 0.005 a | 0.032 ± 0.002 b | 0.041 ± 0.007 b | ||||
| Aldehydes | Hexanal | MS, RI, S | 1089.4 | 1098.0 | 0.011 ± 0.001 c | 0.028 ± 0.003 bc | 0.045 ± 0.012 b | 0.212 ± 0.0253 a |
| Octanal | MS, RI, S | 1296.9 | 1299 | 0.037 ± 0.004 bc | <LOQ | 0.047 ± 0.007 b | 0.139 ± 0.009 a | |
| Nonanal | MS, RI, S | 1406.2 | 1394 | 0.389 ± 0.400 b | 1.303 ± 0.192 a | 0.071 ± 0.022 c | 0.093 ± 0.015 c | |
| Total | 0.437 ± 0.079 b | 1.331 ± 0.341 a | 0.163 ± 0.072 c | 0.444 ± 0.082 b | ||||
| Terpenes | α- pinene | MS, RI | 1028.7 | 1032 | 0.030 ± 0.003 b | <LOQ | 0.040 ± 0.008 a | 0.037 ± 0.005 a |
| β- pinene | MS, RI | 1104 | 1113 | <LOQ | <LOQ | 0.009 ± 0.002 a | 0.006 ± 0.001 a | |
| Limonene | MS, RI, S | 1195.9 | 1203 | 0.166 ± 0.011 b | 0.975 ± 0.185 a | 0.722 ± 0.136 a | 0.750 ± 0.022 a | |
| γ-terpiene | MS, RI | 1246.1 | 1178 | 0.059 ± 0.016 a | <LOQ | 0.014 ± 0.002 b | 0.018 ± 0.003 b | |
| l-α-terpineol | MS, RI | 1708.8 | 1719 | <LOQ | 0.036 ± 0.007 a | 0.016 ± 0.004 b | 0.012 ± 0.001 b | |
| Total | 0.255 ± 0.018 c | 1.011 ± 0.311 a | 0.801 ± 0.251 ab | 0.823 ± 0.073 ab | ||||
| Ketones | 3-octanone | MS, RI | 1266.1 | 1265.5 | <LOQ | <LOQ | <LOQ | 0.005 ± 0.000 a |
| 6-methyl-5-hepten-2-one | MS, RI, S | 1351.0 | 1342 | <LOQ | 0.017 ± 0.005 a | 0.022 ± 0.001 a | 0.027 ± 0.001 a | |
| 2-nonanone | MS, RI, S | 1399.3 | 1436 | <LOQ | 0.012 ± 0.001 b | <LOQ | 0.123 ± 0.007 a | |
| 1-phenylethanone | MS, RI | 1684.7 | 1650 | <LOQ | 0.025 ± 0.002 a | 0.019 ± 0.003 a | 0.024 ± 0.002 a | |
| 1-ethanone | MS | 1903.2 | <LOQ | 0.016 ± 0.004 a | <LOQ | <LOQ | ||
| Total | <LOQ | 0.070 ± 0.021 b | 0.041 ± 0.015 b | 0.179 ± 0.012 a | ||||
| Acids | Hexanoic | MS, RI | 1236.3 | 1244 | 0.003 ± 0.000 a | <LOQ | <LOQ | <LOQ |
| Butanoic | MS, RI | 1681.6 | 1638 | 0.014 ± 0.005 b | 0.246 ± 0.059 a | 0.048 ± 0.020 b | <LOQ | |
| Benzoic | MS | 1821.1 | 0.015 ± 0.002 b | 0.036 ± 0.006 a | <LOQ | <LOQ | ||
| Total | 0.032 ± 0.005 b | 0.282 ± 0.143 a | 0.048 ± 0.011 b | <LOQ | ||||
| Esters | Etilcaprilate | MS, RI | 1444.3 | 1444 | <LOQ | 0.111 ± 0.010 a | <LOQ | <LOQ |
| Furans | 2-pentil furan | MS, RI, S | 1234.2 | 1244 | 0.028 ± 0.002 b | 0.127 ± 0.013 a | <LOQ | <LOQ |
| TOTAL | 6.86 ± 0.25 c | 22.03 ± 1.52 a | 13.63 ± 3.73 b | 14.46 ± 2.30 b | ||||
| Name | Principal Components 1 | |
|---|---|---|
| PC1 | PC2 | |
| Aromatic Hydrocarbons | ||
| Toluene | 0.045 | 0.248 |
| Ethylbenzene | 0.189 | 0.148 |
| p-xylene | −0.062 | 0.236 |
| m-xylene | 0.178 | 0.182 |
| o-xylene | 0.152 | 0.204 |
| Styrene | −0.081 | 0.070 |
| m-cymene | −0.165 | 0.135 |
| 1,2,4-trimethylbenzene | 0.130 | 0.071 |
| Naftalene | 0.065 | 0.196 |
| Aldehydes | ||
| Hexanal | 0.205 | 0.122 |
| Octanal | 0.123 | 0.196 |
| Nonanal | −0.207 | 0.117 |
| Alcohols | ||
| Ethanol | −0.169 | 0.127 |
| 1-hexanol | 0.122 | 0.216 |
| 2-octanol | −0.184 | 0.100 |
| 1-octen-3-ol | 0.184 | 0.180 |
| 1-heptanol | 0.052 | −0.036 |
| 1-octanol | −0.165 | 0.085 |
| 1-nonanol | −0.016 | 0.271 |
| Phenolic Compounds | ||
| Phenol-2-metoxy | −0.164 | 0.154 |
| Phenol | 0.196 | 0.063 |
| 4-vinil-2-metoxyphenol | −0.221 | 0.106 |
| Ketones | ||
| 3-octanone | 0.205 | 0.127 |
| 6-methyl-5-hepten-2-one | −0.108 | 0.070 |
| 2-nonanone | 0.221 | 0.096 |
| 1-phenylethanone | 0.014 | 0.244 |
| 1-etanone | −0.157 | 0.162 |
| Terpenes | ||
| α- pinene | 0.232 | −0.056 |
| β- pinene | 0.208 | 0.067 |
| Limonene | 0.080 | 0.243 |
| γ-terpiene | 0.046 | −0.215 |
| l-α–terpineol | −0.023 | 0.219 |
| Acids | ||
| Hexanoic | −0.061 | −0.209 |
| Benzoic | −0.219 | 0.076 |
| Butanoic | −0.161 | 0.171 |
| Furans | ||
| 2-pentil furan | −0.206 | 0.121 |
| Esters | ||
| Etilcaprilate | −0.148 | 0.178 |
| Variance explained (%) | 39 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codina-Torrella, I.; Gallardo-Chacón, J.J.; Juan, B.; Guamis, B.; Trujillo, A.J. Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage. Foods 2023, 12, 683. https://doi.org/10.3390/foods12040683
Codina-Torrella I, Gallardo-Chacón JJ, Juan B, Guamis B, Trujillo AJ. Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage. Foods. 2023; 12(4):683. https://doi.org/10.3390/foods12040683
Chicago/Turabian StyleCodina-Torrella, Idoia, Joan Josep Gallardo-Chacón, Bibiana Juan, Buenaventura Guamis, and Antonio José Trujillo. 2023. "Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage" Foods 12, no. 4: 683. https://doi.org/10.3390/foods12040683
APA StyleCodina-Torrella, I., Gallardo-Chacón, J. J., Juan, B., Guamis, B., & Trujillo, A. J. (2023). Effect of Ultra-High Pressure Homogenization (UHPH) and Conventional Thermal Pasteurization on the Volatile Composition of Tiger Nut Beverage. Foods, 12(4), 683. https://doi.org/10.3390/foods12040683







