The Disturbance of the Antioxidant System Results in Internal Blue Discoloration of Postharvest Cherry Radish (Raphanus sativus L. var. radculus pers) Roots
Abstract
1. Introduction
2. Results and Discussion
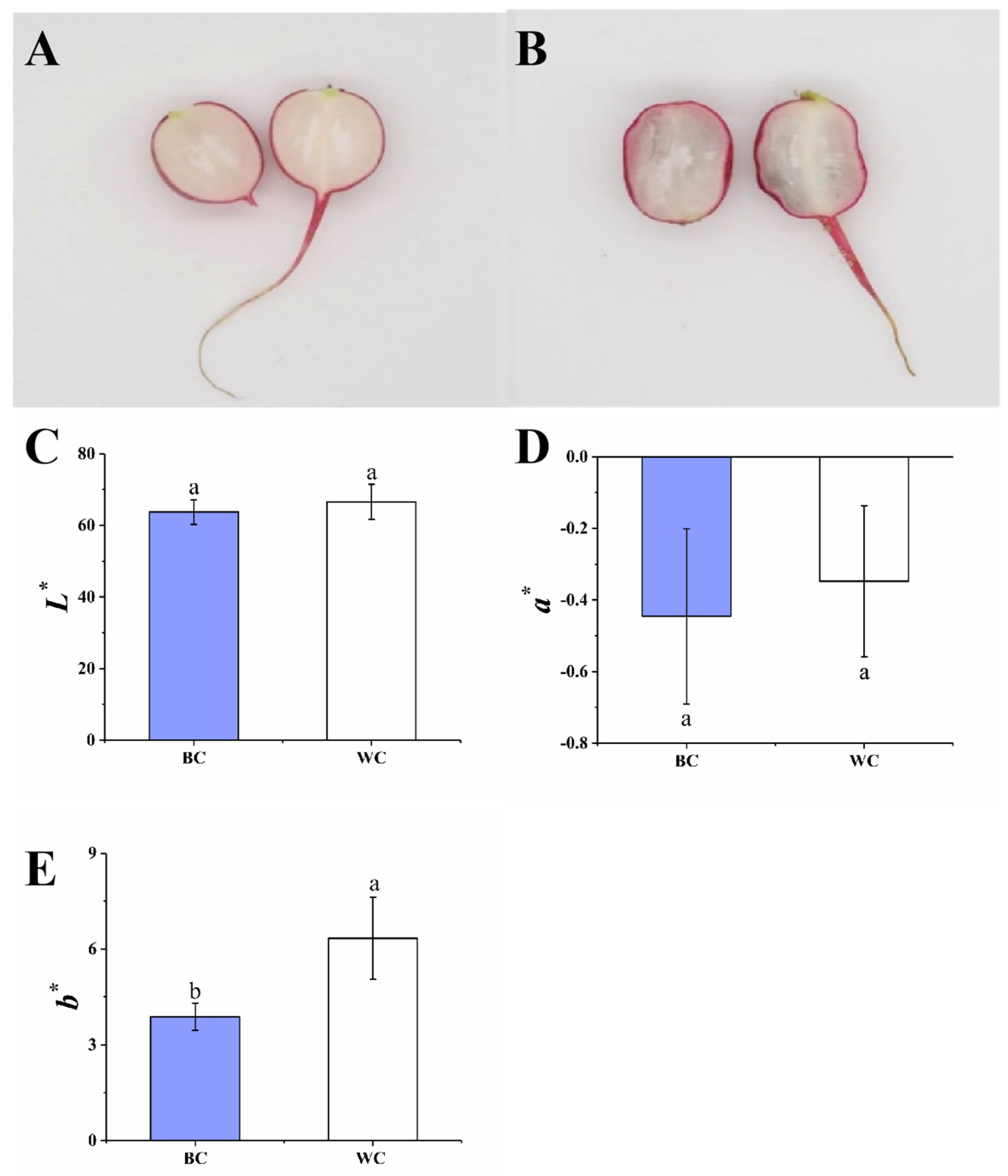
2.1. Appearance and Color
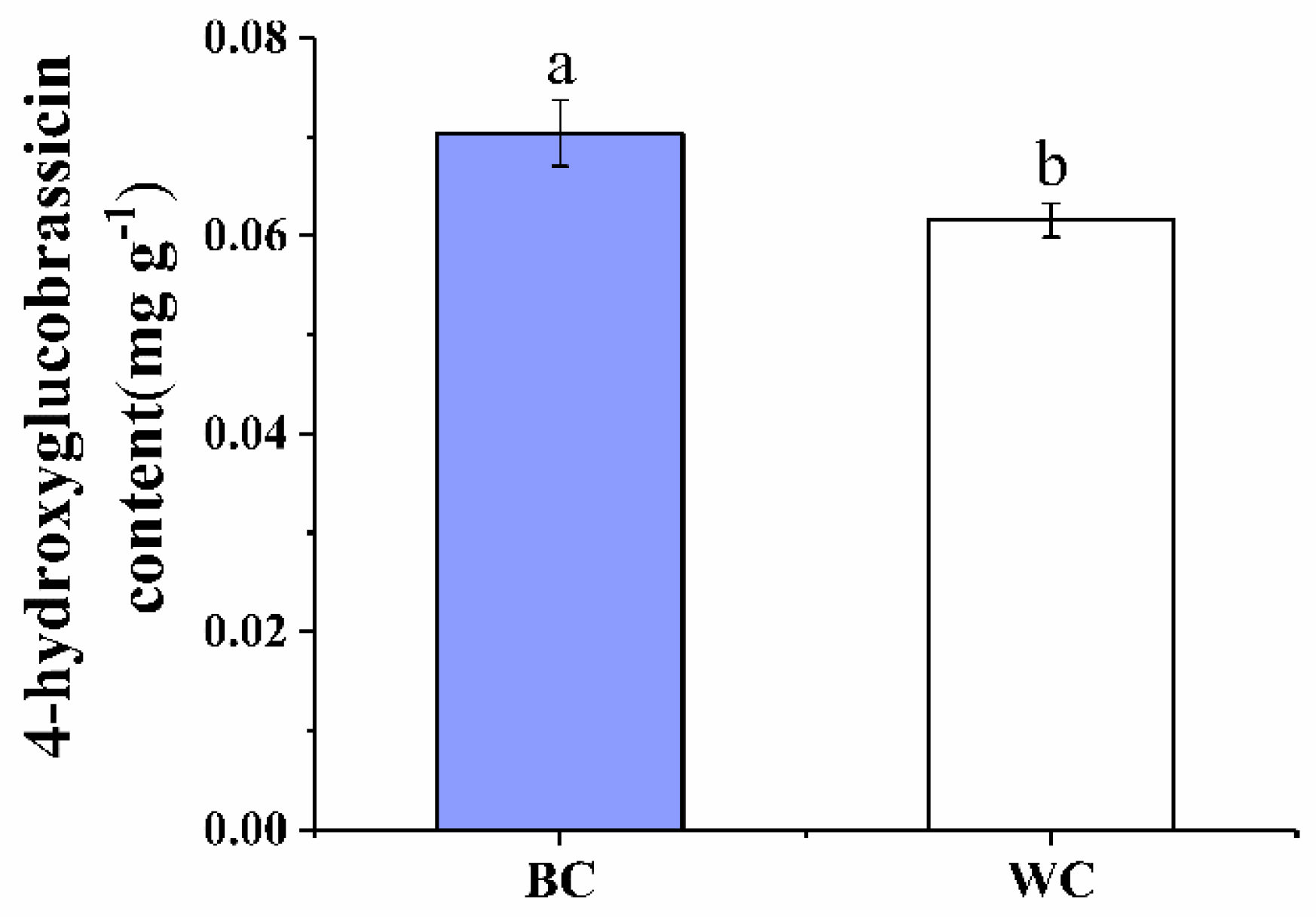
2.2. 4-Hydroxyglucobrassicin Content
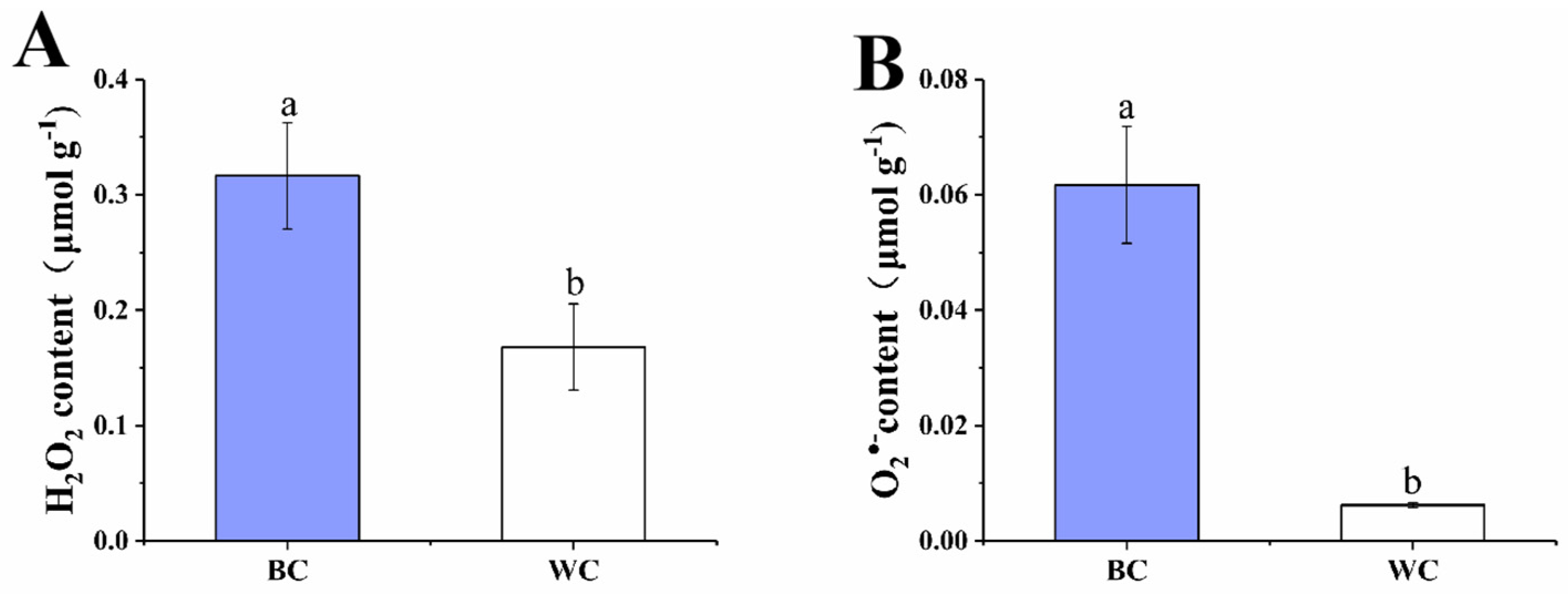
2.3. H2O2 and O2•− Content
2.4. The Antioxidant System
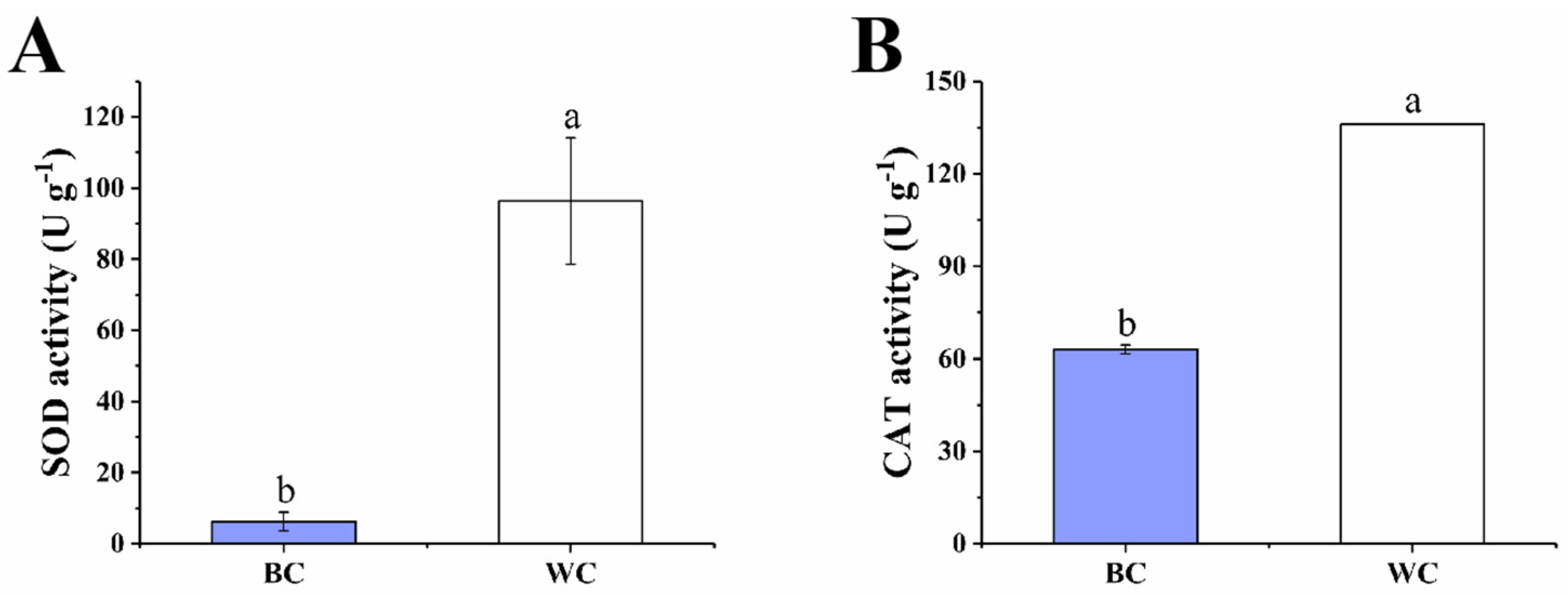
2.4.1. SOD and CAT Activity
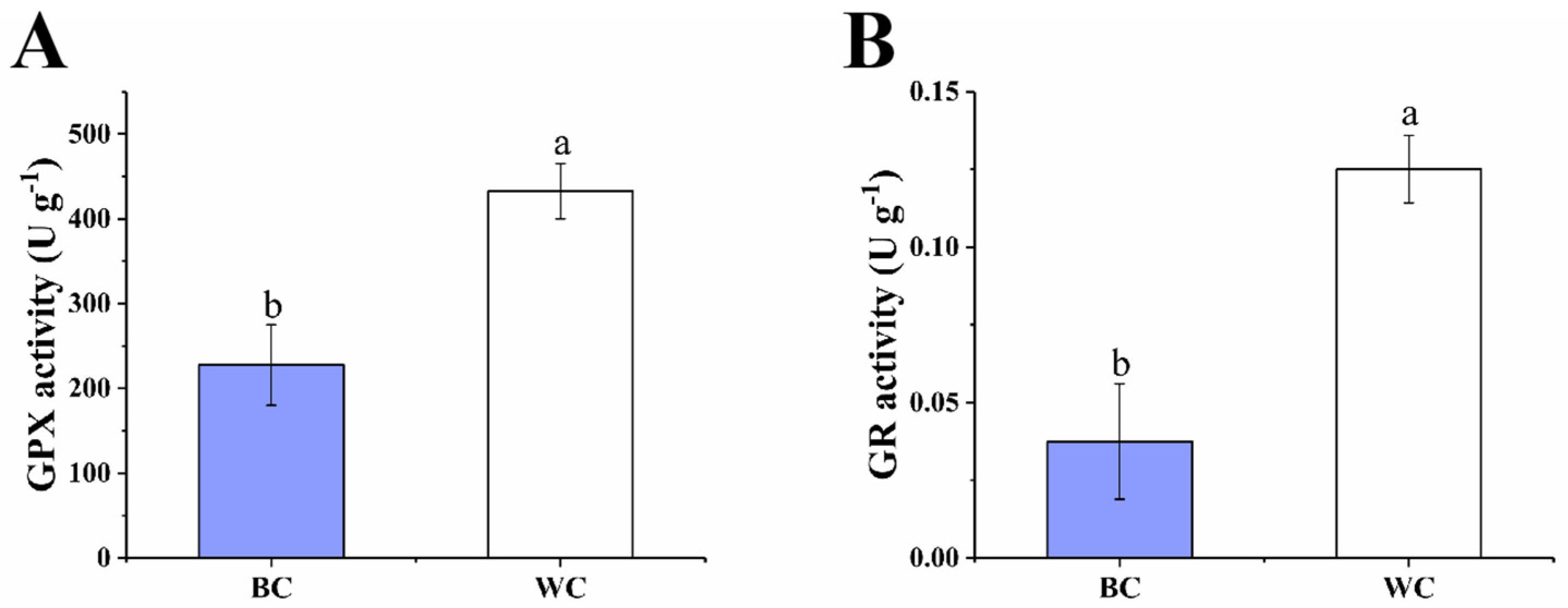
2.4.2. The GPX Cycle
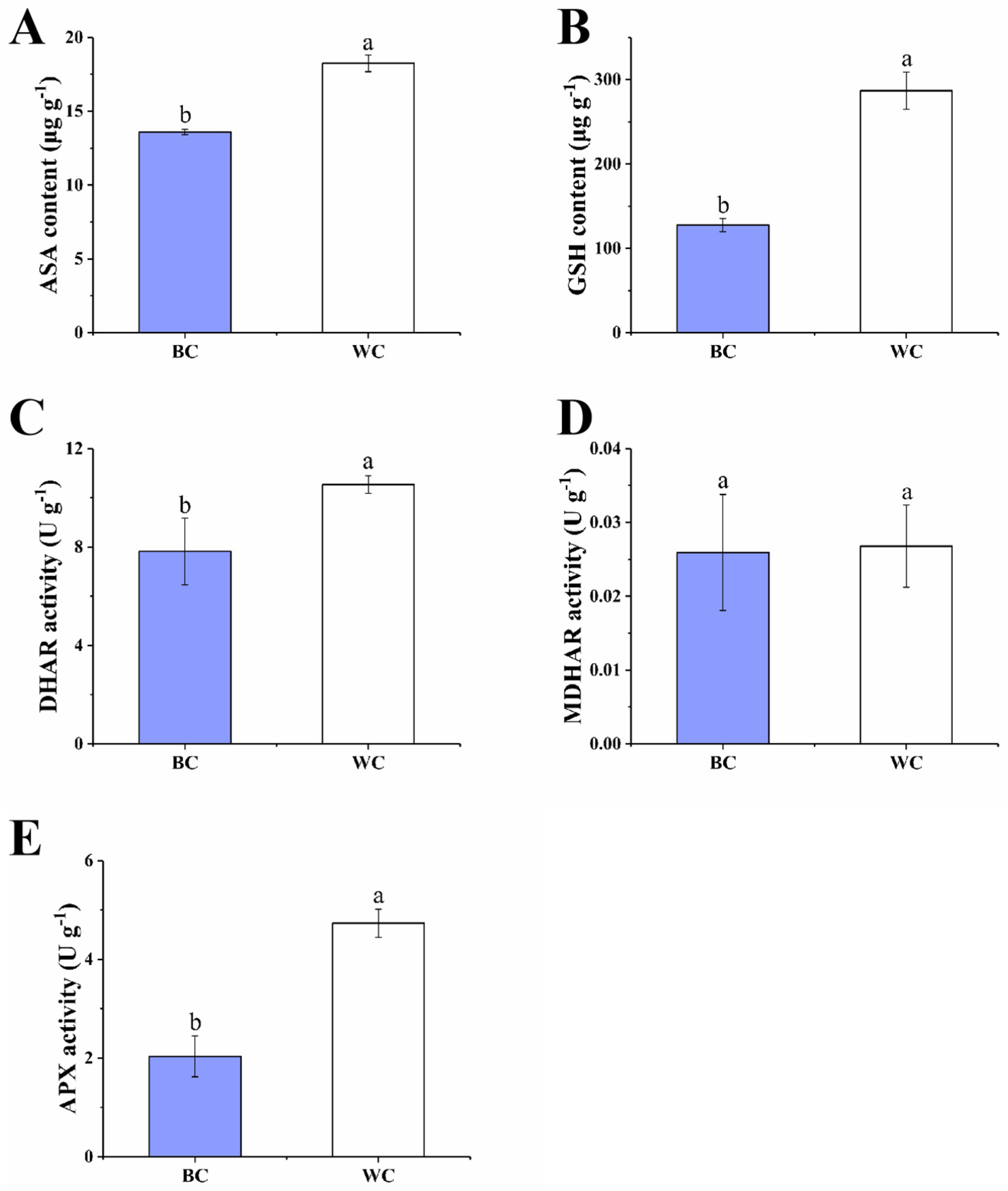
2.4.3. ASA–GSH Cycle
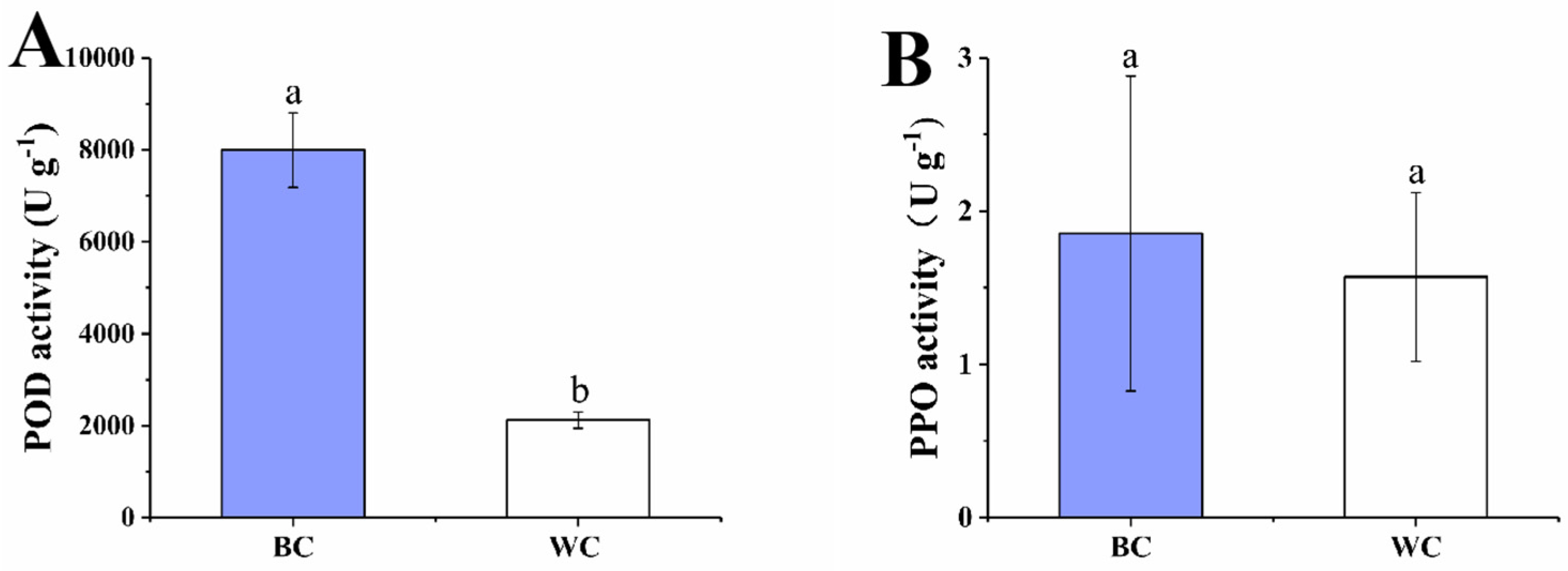
2.5. POD and PPO Activity
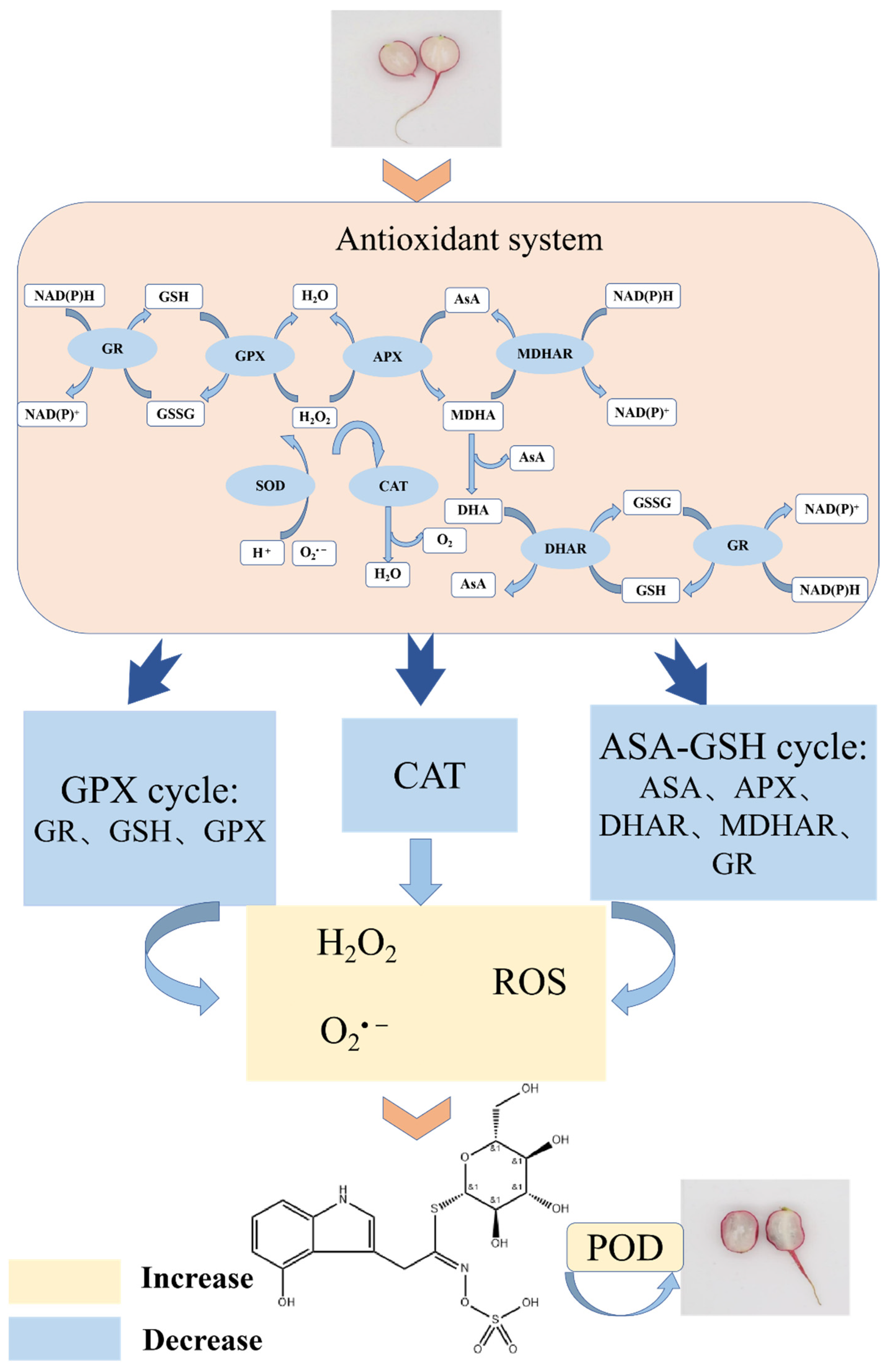
2.6. Mechanism Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Treatment
3.3. Color Measurements
3.4. 4-Hydroxyglucobrassicin Determination
3.5. H2O2 and Superoxide Anion (O2•−) Measurements
3.6. Determination of Enzyme Activities
3.7. Determination of ASA and Reduced Glutathione (GSH) Content
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| GPX | Glutathione peroxidase |
| GSH | Glutathione |
| ASA–GSH | Ascorbic acid–Glutathione |
| O2•− | Superoxide anions |
| H2O2 | Hydrogen peroxide |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSSG | Glutathione disulfide |
| GR | Glutathione reductase |
| NADPH | Triphosphopyridine nucleotide |
| DHAR | Dehydroascorbate reductase |
| MDHAR | Monodehydroascorbate reductase |
| APX | Ascorbate peroxidase |
| DHA | Docosahexaenoic acid |
References
- Xie, Y.; Ying, J.; Tang, M.; Wang, Y.; Xu, L.; Liu, M.; Liu, L. Genome–wide identification of AUX/IAA in radish and functional characterization of RsIAA33 gene during taproot thickening. Gene 2021, 795, 145782. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Wang, J.; Wang, P.; Qiu, Y.; Zhao, W.; Pang, S.; Li, X.; Wang, H.; Song, J.; et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol. Plant 2021, 14, 2032–2055. [Google Scholar] [CrossRef]
- Teranishi, K.; Masayasu, N. Structure of a Precursor to the Blue Components Produced in the Blue Discoloration in Japanese Radish (Raphanus sativus) Roots. J. Nat. Prod. 2016, 79, 1381–1387. [Google Scholar] [CrossRef]
- Lee, J.G.; Lim, S.; Kim, J.; Lee, E.J. The mechanism of deterioration of the glucosinolate-myrosynase system in radish roots during cold storage after harvest. Food Chem. 2017, 233, 60–68. [Google Scholar] [CrossRef]
- Teranishi, K.; Masayasu, N.; Masuda, D. Mechanism Underlying the Onset of Internal Blue Discoloration in Japanese Radish (Raphanus sativus) Roots. J. Agric. Food Chem. 2016, 64, 6745–6751. [Google Scholar] [CrossRef]
- Teranishi, K.; Nagata, M. Prediction and suppression of internal blue discoloration in roots of daikon, the Japanese radish (Raphanus sativus L.). Food Sci. Nutr. 2018, 6, 2134–2140. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Ma, Y.; Zhang, L.; Jiang, Y.; Liang, H.; Wang, D. Transcriptome and metabolome profiling to elucidate mechanisms underlying the blue discoloration of radish roots during storage. Food Chem. 2021, 362, 130076. [Google Scholar] [CrossRef]
- Guo, R.; Li, W.; Wang, X.; Chen, B.; Huang, Z.; Liu, T.; Chen, X.; XuHan, X.; Lai, Z. Effect of photoperiod on the formation of cherry radish root. Sci. Hortic. 2019, 244, 193–199. [Google Scholar] [CrossRef]
- Foyer, C.H. Making sense of hydrogen peroxide signals. Nature 2020, 578, 518–519. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, Q.; Cheng, S.; Zhou, X.; Wei, B.; Zhao, Y.; Ji, S. 24-epibrassinolide alleviates postharvest yellowing of broccoli via improving its antioxidant capacity. Food Chem. 2021, 365, 130529. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Chen, M.; Lin, Y. The role of active oxygen metabolism in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, R.K.; Rajpoot, R.; Rani, A.; Pandey, A.K.; Dubey, R.S. Water deficit and aluminum interactive effects on generation of reactive oxygen species and responses of antioxidative enzymes in the seedlings of two rice cultivars differing in stress tolerance. Environ. Sci. Pollut. Res. 2016, 23, 1516–1528. [Google Scholar] [CrossRef]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotox. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Z.; Shen, W.; Shao, Y.; Zeng, J.; Li, W. Polyphenol treatment delays the browning of litchi pericarps and promotes the total antioxidant capacity of litchi fruit. Sci. Hortic. 2022, 291, 110563. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Nadafzadeh, M.; Abdanan Mehdizadeh, S.; Soltanikazemi, M. Development of computer vision system to predict peroxidase and polyphenol oxidase enzymes to evaluate the process of banana peel browning using genetic programming modeling. Sci. Hortic. 2018, 231, 201–209. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Ma, Y.; Zhang, L.; Jiang, Y.; Liang, H.; Wang, D. Inhibitory mechanism of low-oxygen-storage treatment in postharvest internal bluing of radish (Raphanus sativus) roots. Food Chem. 2021, 364, 130423. [Google Scholar] [CrossRef]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot water treatment to alleviate chilling injury and enhance ascorbate-glutathione cycle in sweet pepper fruit during postharvest cold storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Anjum, N.A.; Amreen; Tantray, A.Y.; Khan, N.A.; Ahmad, A. Reactive oxygen species detection-approaches in plants: Insights into genetically encoded FRET-based sensors. J. Biotechnol. 2020, 308, 108–117. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free. Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free. Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Bhuyan, M.H.M.B.; Hasanuzzaman, M.; Mahmud, J.A.; Hossain, M.S.; Bhuiyan, T.F.; Fujita, M. Unraveling Morphophysiological and Biochemical Responses of Triticum aestivum L. to Extreme pH: Coordinated Actions of Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 24. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Ahmad, P.; Alam, P.; Balawi, T.H.; Altalayan, F.H.; Ahanger, M.A.; Ashraf, M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480. [Google Scholar] [CrossRef]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of action, sources, and application of peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef]
- Panadare, D.; Rathod, V.K. Extraction and purification of polyphenol oxidase: A review. Biocatal. Agric. Biotechnol. 2018, 14, 431–437. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Ma, Y.; Jiang, Y.; Wang, D.; Liang, H. Comparison of blue discoloration in radish root among different varieties and blue pigment stability analysis. Food Chem. 2021, 340, 128164. [Google Scholar] [CrossRef]
- Cocetta, G.; Baldassarre, V.; Spinardi, A.; Ferrante, A. Effect of cutting on ascorbic acid oxidation and recycling in fresh-cut baby spinach (Spinacia oleracea L.) leaves. Postharvest Biol. Technol. 2014, 88, 8–16. [Google Scholar] [CrossRef]
- Kuijpers, T.F.M.; Narváez-Cuenca, C.-E.; Vincken, J.-P.; Verloop, A.J.W.; van Berkel, W.J.H.; Gruppen, H. Inhibition of Enzymatic Browning of Chlorogenic Acid by Sulfur-Containing Compounds. J. Agric. Food Chem. 2012, 60, 3507–3514. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yan, L.; Zheng, H.; Liu, C.; Zheng, L. Effects of postharvest cysteine treatment on sensory quality and contents of bioactive compounds in goji fruit. Food Chem. 2022, 366, 130546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Zhao, W.; Wang, P.; Zhao, S.; Zhao, X.; Wang, D. The Disturbance of the Antioxidant System Results in Internal Blue Discoloration of Postharvest Cherry Radish (Raphanus sativus L. var. radculus pers) Roots. Foods 2023, 12, 677. https://doi.org/10.3390/foods12030677
Wang X, Liu Y, Zhao W, Wang P, Zhao S, Zhao X, Wang D. The Disturbance of the Antioxidant System Results in Internal Blue Discoloration of Postharvest Cherry Radish (Raphanus sativus L. var. radculus pers) Roots. Foods. 2023; 12(3):677. https://doi.org/10.3390/foods12030677
Chicago/Turabian StyleWang, Xingyu, Yu Liu, Wenting Zhao, Pan Wang, Shuang Zhao, Xiaoyan Zhao, and Dan Wang. 2023. "The Disturbance of the Antioxidant System Results in Internal Blue Discoloration of Postharvest Cherry Radish (Raphanus sativus L. var. radculus pers) Roots" Foods 12, no. 3: 677. https://doi.org/10.3390/foods12030677
APA StyleWang, X., Liu, Y., Zhao, W., Wang, P., Zhao, S., Zhao, X., & Wang, D. (2023). The Disturbance of the Antioxidant System Results in Internal Blue Discoloration of Postharvest Cherry Radish (Raphanus sativus L. var. radculus pers) Roots. Foods, 12(3), 677. https://doi.org/10.3390/foods12030677



