The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. The Characterization of Scorias Spongiosa Polysaccharides
2.2. The Formulation of Scorias Spongiosa Polysaccharide-Based Microcapsules
2.3. Animals, Treatment and Diet
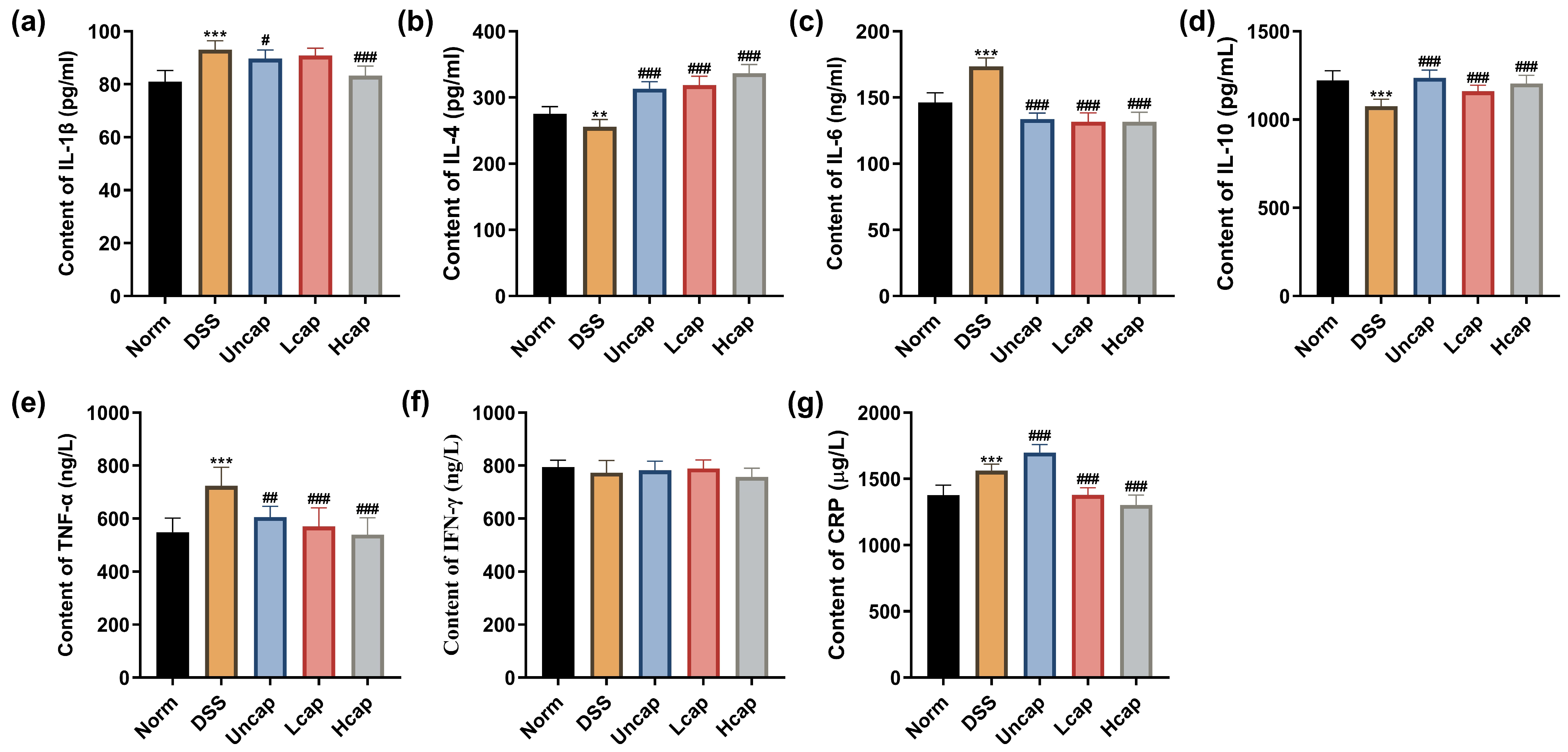
2.4. Determination of Serum Cytokines
2.5. Western Blotting Analysis
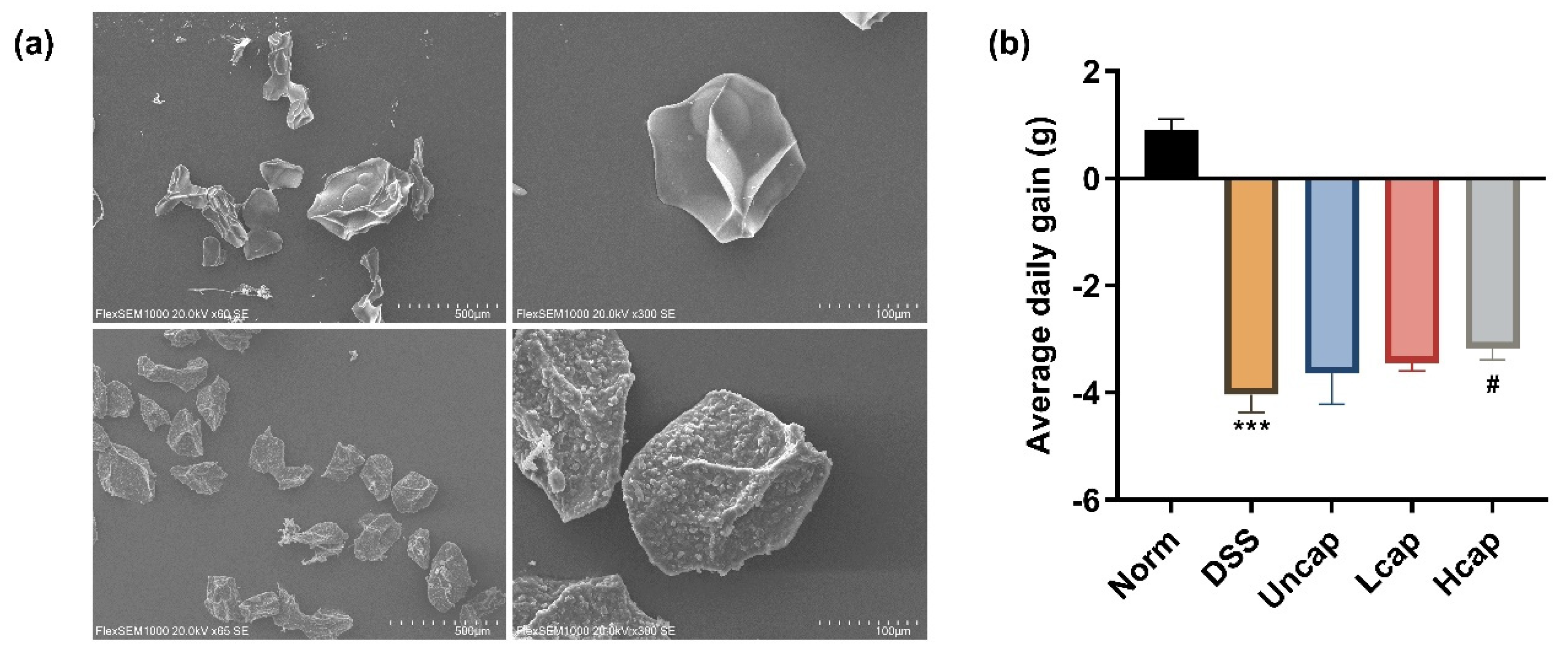
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. 16S rDNA Analysis
2.8. Statistical Analysis
3. Results
3.1. S. spongiosa Polysaccharides Attenuated Weight Loss in Colitis Mice
3.2. S. spongiosa Polysaccharides Reduced Disruption of Intestinal Barrier Integrity in Colitis Mice
3.3. S. spongiosa Polysaccharides Improved Antioxidant Capacity in Colitis Mice
3.4. S. spongiosa Polysaccharides Alleviated Inflammatory Response in Colitis Mice
3.5. S. spongiosa Polysaccharides Modulate the NF-κB Signaling in Colitis Mice
3.6. S. spongiosa Polysaccharides Modulate the JAK/STAT Signaling in Colitis Mice
3.7. S. spongiosa Polysaccharides Modulate the MAPK/ERK Pathway in Colitis Mice
3.8. S. spongiosa Polysaccharides Remold the Richness and Diversity of Intestinal Microbiota
3.9. S. spongiosa Polysaccharides Remold the Composition of Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.A.-O. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Gitter, A.H.; Wullstein, F.; Fromm, M.; Schulzke, J.D. Epithelial barrier defects in ulcerative colitis: Characterization and quantification by electrophysiological imaging. Gastroenterology 2001, 121, 1320–1328. [Google Scholar] [CrossRef]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. J. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Gancarcikova, S.; Lauko, S.; Hrckova, G.; Andrejcakova, Z.; Hajduckova, V.; Madar, M.; Kolesar Fecskeova, L.; Mudronova, D.; Mravcova, K.; Strkolcova, G.; et al. Innovative Animal Model of DSS-Induced Ulcerative Colitis in Pseudo Germ-Free Mice. Cells 2020, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch. Arztebl. Int. 2020, 117, 564–574. [Google Scholar] [CrossRef]
- Nakov, R. New markers in ulcerative colitis. Clin. Chim. Acta 2019, 497, 141–146. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, L.; Liu, Q.; Huang, X. Genome assembly of Scorias spongiosa and comparative genomics provide insights into ecological adaptation of honeydew-dependent sooty mould fungi. Genomics 2021, 113, 2189–2198. [Google Scholar] [CrossRef]
- Herath, K.; Jayasuriya, H.; Zink, D.L.; Sigmund, J.; Vicente, F.; de la Cruz, M.; Basilio, A.; Bills, G.F.; Polishook, J.D.; Donald, R.; et al. Isolation, structure elucidation, and antibacterial activity of methiosetin, a tetramic acid from a tropical sooty mold (Capnodium sp.). J. Nat. Prod. 2012, 75, 420–424. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.; Jia, X.; Liu, H.; Li, Z.; Huang, D.; Nong, L.; Ning, Z.; Hu, Z.; Xu, C.-P. Effect of Tween 80 and Chloroform on the Secretion, Structure and Cytotoxic Activities of Exopolysaccharides from Scorias spongiosa. J. Biol. Act. Prod. Nat. 2018, 8, 312–318. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Feng, H.; Tang, J.; Peng, W.; Chen, Y.; Zhou, J.; Wang, Y. Scorias spongiosa Polysaccharides Promote the Antioxidant and Anti-Inflammatory Capacity and Its Effect on Intestinal Microbiota in Mice. Front. Microbiol. 2022, 13, 865396. [Google Scholar] [CrossRef]
- Schumacher, R.W.; Waters, A.L.; Peng, J.; Schumacher, R.A.; Bateman, A.; Thiele, J.; Mitchell, A.J.; Miller, S.G.; Goldberg, A.; Tripathi, S.K.; et al. Structure and Antimicrobial Activity of Rare Lactone Lipids from the Sooty Mold (Scorias spongiosa). J. Nat. Prod. 2022, 85, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Kim, S.R.; Ruiz, D.R.; Farmer, J.R. Microencapsulation for Food Applications: A Review. ACS Appl. Bio Mater. 2022, 5, 5497–5512. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Orlando, P.; Fratianni, F.; Coppola, R. Microencapsulation in food science and biotechnology. Curr. Opin. Biotechnol. 2012, 23, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free. Radic. Biol. Med. 2019, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tse, H.; Yuan, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F.; Epidemiology; et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.J.; Jackson, A.A. Function of the human colon. Br. J. Surg. 1992, 79, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Eizuka, M.; Torisu, T.; Sugai, T.; Matsumoto, T. Unclassified serrated lesion of the colon observed by magnifying endoscopy. Gastrointest. Endosc. 2021, 94, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.N.; Wang, H.; Ma, J.Y.; Zhai, S.S.; Duan, J. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-κB signaling pathway. Mol. Immunol. 2020, 120, 13–22. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Peng, H.; Liu, J.; Cai, M.; Wu, H.; Zhang, Z.; Bai, J.; Yao, Y.; Dong, X.; Yin, X.; et al. Catalpol Protects ARPE-19 Cells against Oxidative Stress via Activation of the Keap1/Nrf2/ARE Pathway. Cells 2021, 10, 2635. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, W.; Wang, J.; Yan, J.; Shi, Y.; Zhang, C.; Ge, W.; Wu, J.; Du, P.; Chen, Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-kappaB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef]
- Ogata, H.; Hibi, T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Neurath, M.F. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Franze, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Puleston, J.; Cooper, M.; Murch, S.; Bid, K.; Makh, S.; Ashwood, P.; Bingham, A.H.; Green, H.; Moss, P.; Dhillon, A.; et al. A distinct subset of chemokines dominates the mucosal chemokine response in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2005, 21, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Pao, L.I.; Dhanji, S.; Murakami, K.; Ohashi, P.S.; Neel, B.G. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J. Exp. Med. 2013, 210, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-Y.; Fan, Y.-M.; Ga, Y.; Zhang, Y.-N.; Han, J.-C.; Hao, Z.-H. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 2021, 92, 153743. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2019, 16, 38–51. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef]
- Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; Vallance, B.A. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am. J. Physiol. Liver Physiol. 2018, 314, G360–G377. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, L.; Stefanucci, A.; Scioli, G.; Della Valle, A.; Zengin, G.; Cichelli, A.; Mollica, A. Peptide Human Neutrophil Elastase Inhibitors from Natural Sources: An Overview. Int. J. Mol. Sci. 2022, 23, 2924. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Feng, H.; Zhang, Z.; Zhang, Q.; Tang, J.; Zhou, J.; Wang, Y.; Peng, W. The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice. Foods 2023, 12, 669. https://doi.org/10.3390/foods12030669
Xu Y, Feng H, Zhang Z, Zhang Q, Tang J, Zhou J, Wang Y, Peng W. The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice. Foods. 2023; 12(3):669. https://doi.org/10.3390/foods12030669
Chicago/Turabian StyleXu, Yingyin, Huiyu Feng, Zhiyuan Zhang, Qian Zhang, Jie Tang, Jie Zhou, Yong Wang, and Weihong Peng. 2023. "The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice" Foods 12, no. 3: 669. https://doi.org/10.3390/foods12030669
APA StyleXu, Y., Feng, H., Zhang, Z., Zhang, Q., Tang, J., Zhou, J., Wang, Y., & Peng, W. (2023). The Protective Role of Scorias spongiosa Polysaccharide-Based Microcapsules on Intestinal Barrier Integrity in DSS-Induced Colitis in Mice. Foods, 12(3), 669. https://doi.org/10.3390/foods12030669



