Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation of Gold Nanoparticles (GNPs)
2.4. Optimization of Relevant Parameters of Lateral Flow Assay
2.5. Assembling of Lateral Flow Assay
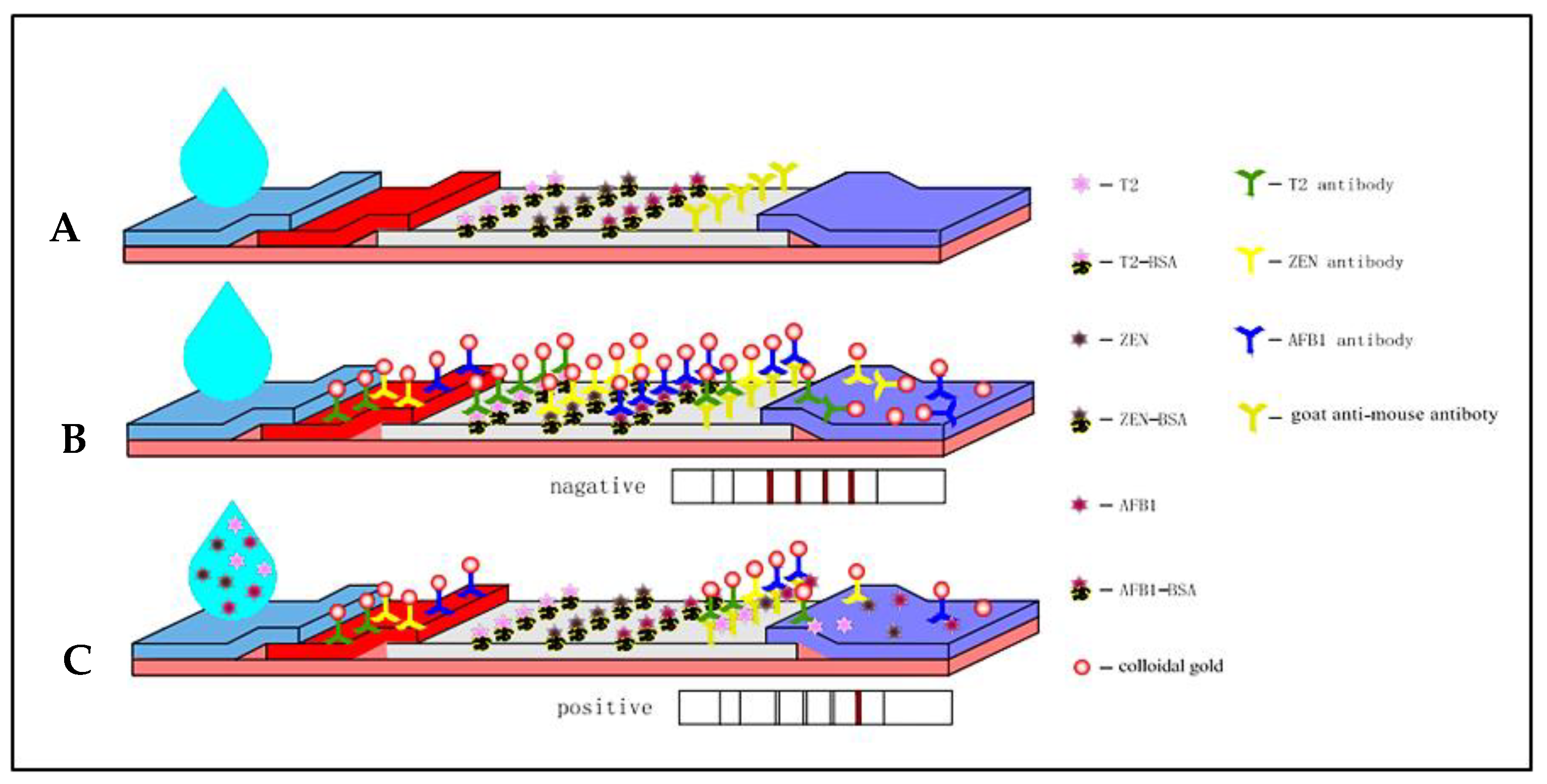
2.6. Principles of the GNP-Based ICS Assay
2.7. Preparation of Monoclonal Antibodies (mAbs) Labelled with GNPs
2.8. Determination of the Specificity of the Test Strips
2.9. Determination of the Visual Detection Limit of the Test Strips
2.10. Real Sample Detection
3. Results and Discussion
3.1. Preparation of GNPs
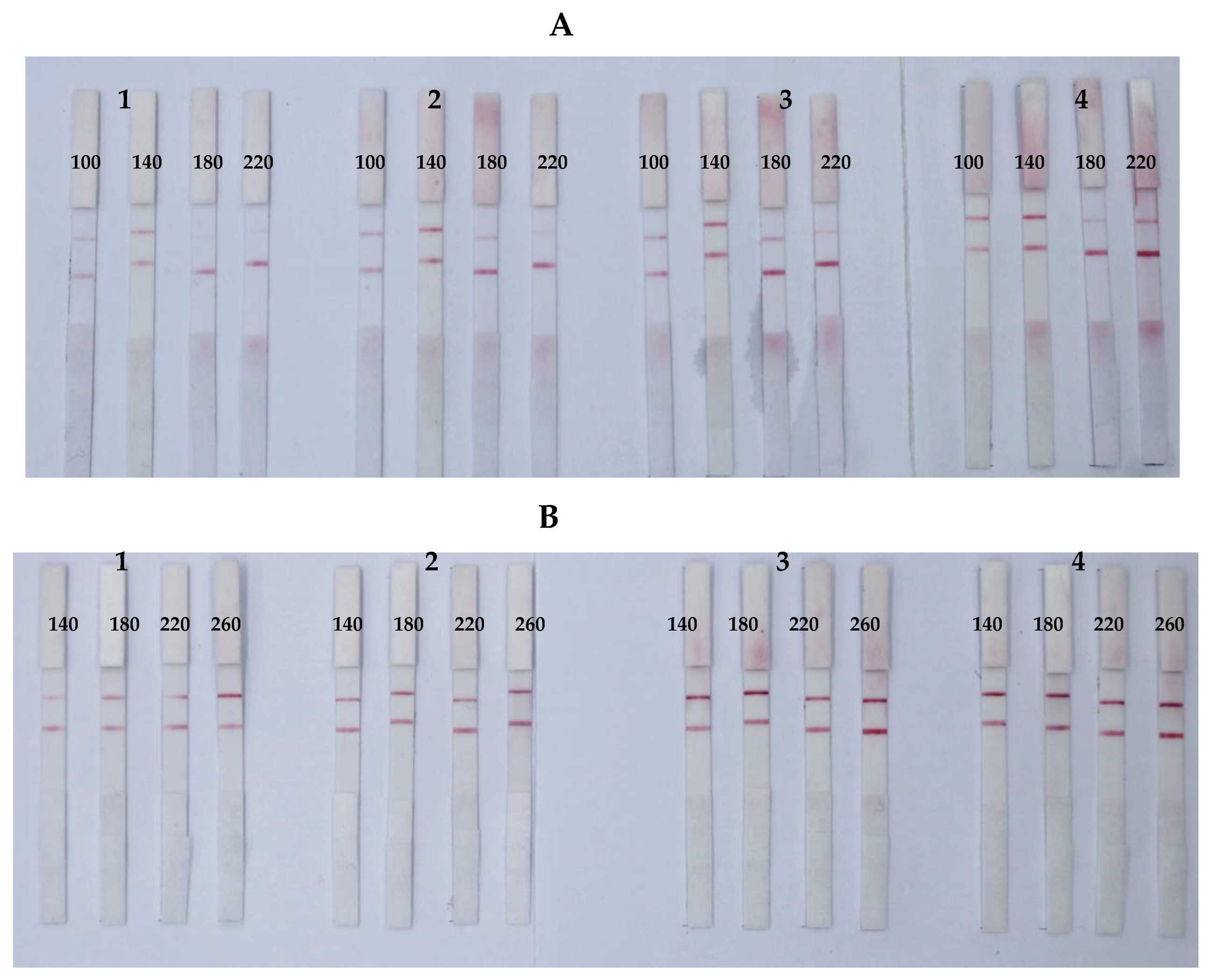
3.2. Optimization of Parameters
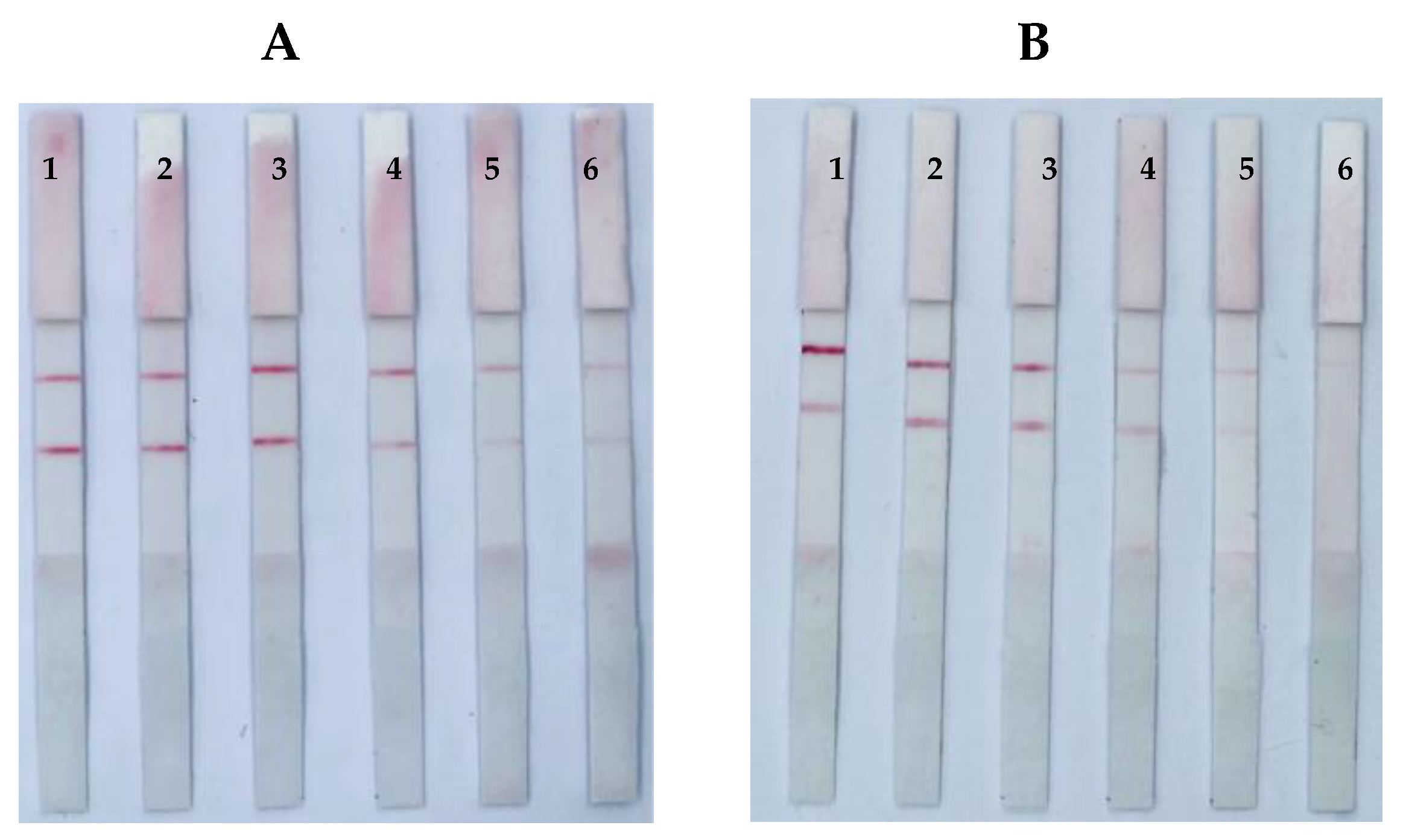
3.3. Optimization of Loading Solution of the ICS
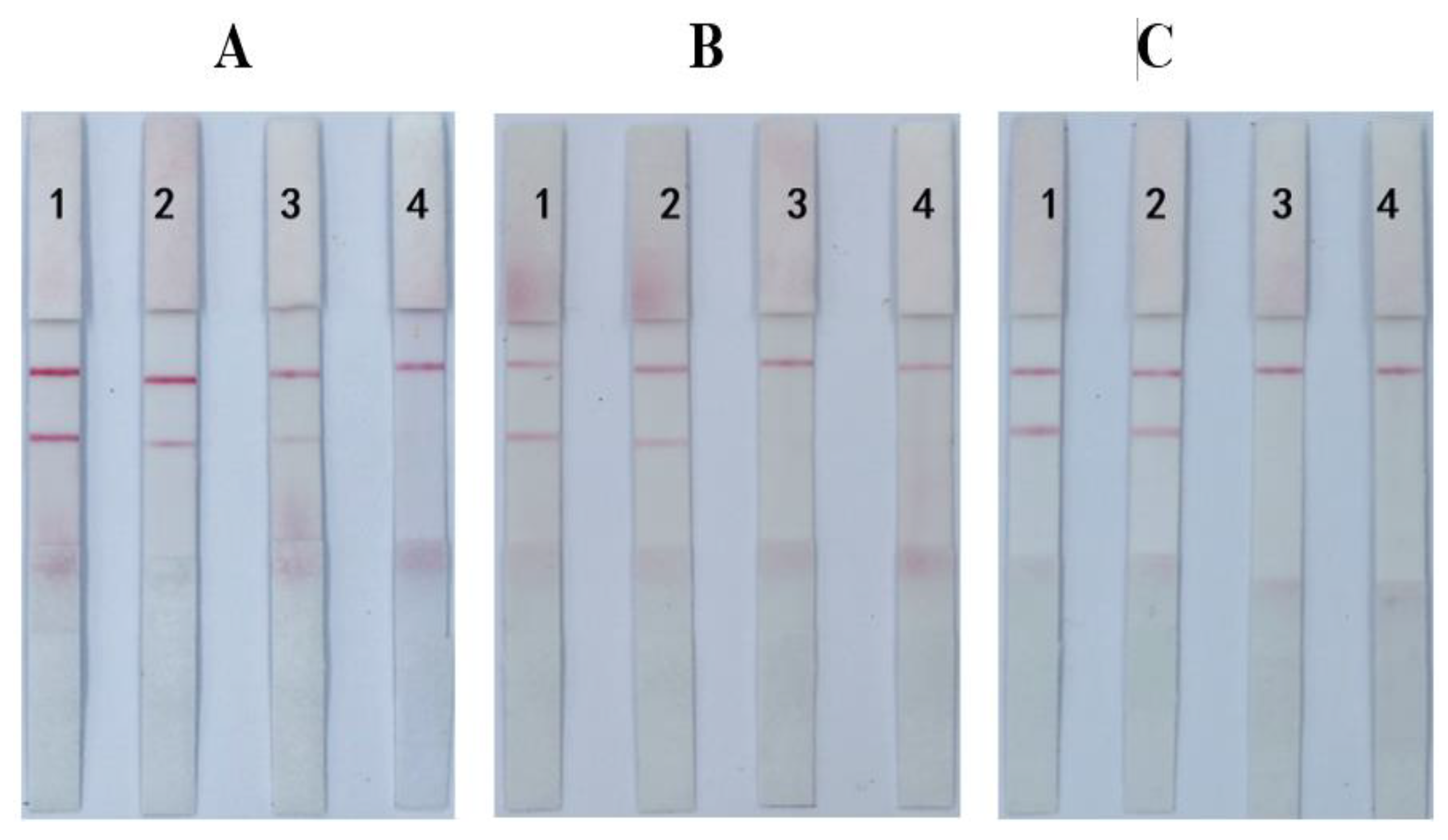
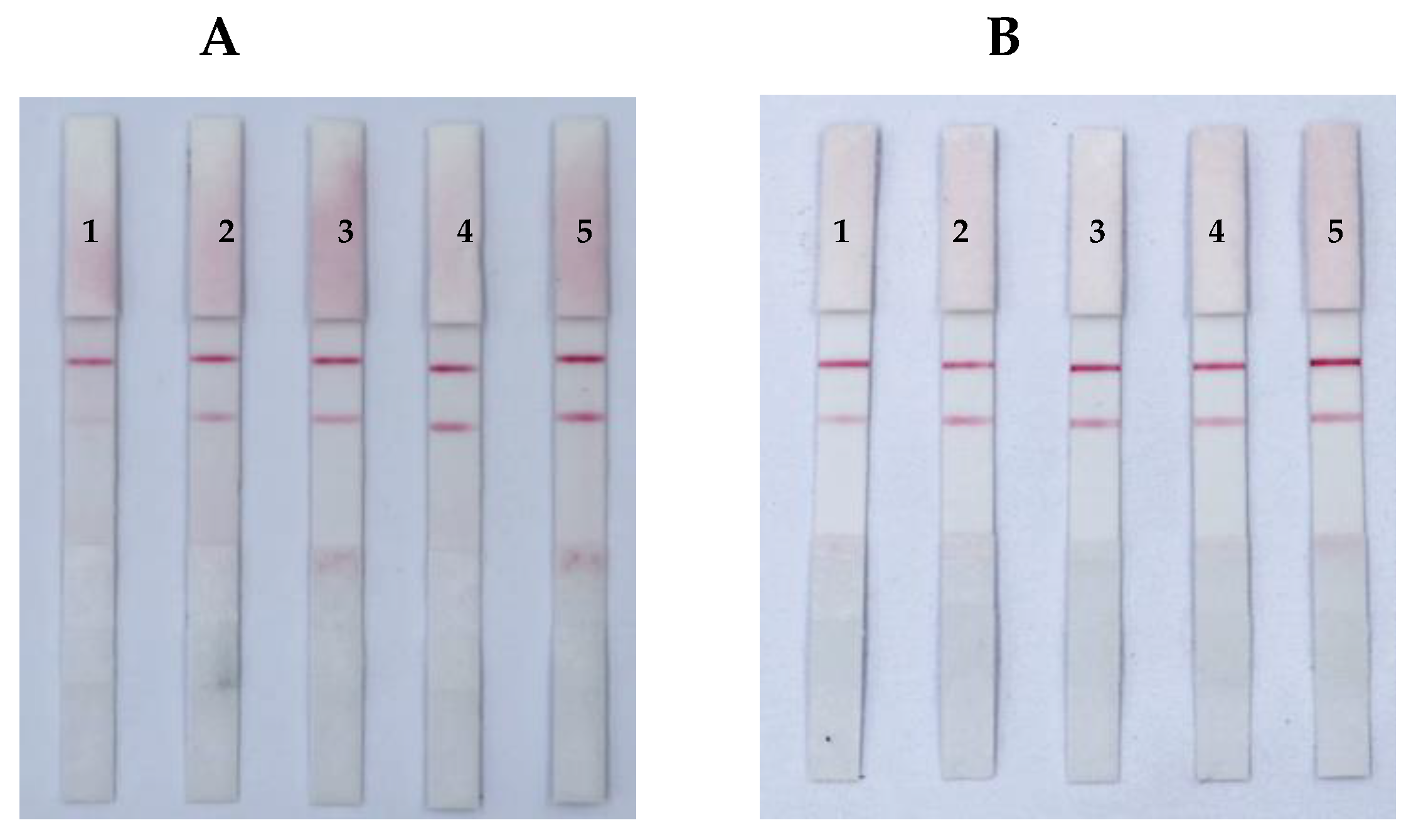
3.4. Sensitivity, Specificity, and Stability of the ICS
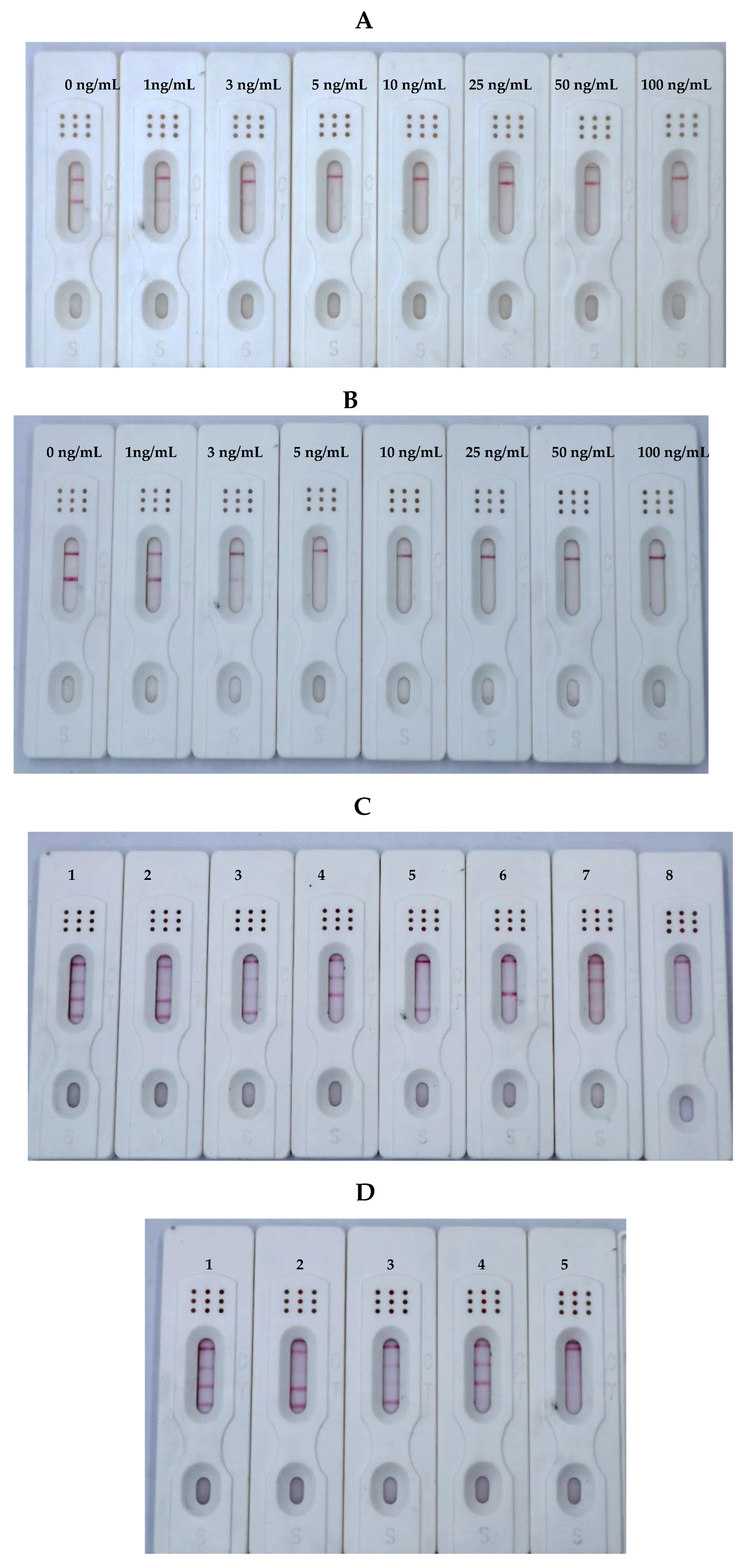
3.5. Analysis of Mycotoxins in Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO Traditional Medicine Strategy. Geneva Switzerland WHO, (2013) 2014–2023. Available online: https://apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf?ua=1 (accessed on 10 January 2020).
- Wu, C.; Wang, C.; Kennedy, J. Changes in herb and dietary supplement use in the U.S. adult population: A comparison of the 2002 and 2007 National Health Interview Surveys. Clin. Ther. 2011, 33, 749–1758. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Qiu, F.; Kong, W.; Wei, J.; Yang, M.; Luo, Z.; Ma, X. Co-occurrence of aflatoxin B1, B2, G1, G2 and OchrotoxinA in Glycyrrhiza uralensis analyzed by LC-MS/MS. Food Control 2013, 32, 216–221. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Tan, L.; Yu, B.; Cao, W. Analysis of aflatoxins in traditional Chinese medicines: Classification of analytical method on the basis of matrix variations. Sci. Rep. 2016, 6, 30822. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, P. Advanced synchrotron-based and globar-sourced molecular (micro) spectroscopy contributions to advances in food and feed research on molecular structure, mycotoxin determination, and molecular nutrition. Crit. Rev. Food Sci. 2018, 58, 2164–2175. [Google Scholar] [CrossRef]
- Shi, H.; Li, S.; Bai, Y.; Prates, L.; Lei, Y.; Yu, P. Mycotoxin contamination of food and feed in china: Occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control 2018, 91, 202–215. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, H.; Gou, T.; Zhou, Y.; Zhang, Q.; Zhang, Y.; Ma, L. Recent advances on formation, trans-formation, occurrence, and analytical strategy of modified mycotoxins in cereals and their products. Food Chem. 2023, 405, 134752. [Google Scholar] [CrossRef]
- Hussein, H.; Brasel, S.J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Cimbalo, A.; Frangiamone, M.; Font, G.; Manyes, L. The importance of transcriptomics and proteomics for studying molecular mechanisms of mycotoxin exposure: A review. Food Chem. Toxicol. 2022, 169, 113396. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.H.; Cai, Q.Q.; Fu, Y.C.; Lei, C.Y.; Yang, W.G. Visual and quantitative detection of E. coli O157:H7 by coupling immunomagnetic separation and quantum dot-based paper strip. Anal. Bioanal. Chem. 2021, 413, 4417–4426. [Google Scholar] [CrossRef]
- Duarte, S.C.; Salvador, N.; Machado, F.; Costa, E.; Almeida, A.; Silva, L.J.; Pena, A. Mycotoxins in teas and medicinal plants destined to prepare infusions in Portugal. Food Control 2020, 115, 107290. [Google Scholar] [CrossRef]
- Reinholds, I.; Bogdanova, E.; Pugajeva, I.; Bartkevics, V. Mycotoxins in herbal teas marketed in Latvia and dietary exposure assessment. Food Addit. Contam. Part B 2019, 12, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Oketch-Rabah, H.; Kim, C.; Monagas, M.; Bzhelyansky, A.; Sarma, N.; Giancaspro, G. Quality specifications for articles of botanical origin from the United States Pharmacopeia. Phytomedicine 2018, 45, 105–119. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. USP Herbal Medicines Compendium; United States Pharmacopeial Convention: Rockville, MD, USA, 2017. [Google Scholar]
- Japanese Pharmacopoeia Commentary Editorial Committee. The Japanese Pharmacopoeia, 17th ed.; Analytical Methods for Aflatoxins in Crude Drug and Crude Drug Preparations; Japanese Pharmacopoeia Commentary Editorial Committee: Tokyo, Japan, 2016; pp. 2513–2515.
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia 2015 Edition; Chinese Medicine Science and Technology Press: Beijing, China, 2015; Volume IV. [Google Scholar]
- Korean Food, Drug. Korean Pharmacopoeia, 10th ed.; General Tests, Processes and Apparatu; Korean Food & Drug: Chungcheongbuk-do, Republic of Korea, 2012; pp. 1673–1675. [Google Scholar]
- Han, Z.; Zheng, Y.; Luan, L.; Cai, Z.; Ren, Y.; Wu, Y. An ultra-high-performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal. Chim. Acta 2010, 664, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, L.; Ji, S.; Wang, K. Simultaneous determination of seventeen mycotoxins residues in Puerariaelobatae radix by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 98, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Humphrey, C.; King, R.S.; Richard, J.L. Validation of an ELISA test kit for the detection of total af latoxins in grain and grain products by comparison with HPLC. Mycopathologia 2005, 159, 255–263. [Google Scholar] [CrossRef]
- Zang, X.; Kong, K.; Tang, H.; Tang, Y.; Tang, H.; Jiao, X.; Huang, J. AGICA strip for Campylobacter jejuni real-time monitoring at meat production site. LWT-Food Sci. Technol. 2018, 98, 500–505. [Google Scholar] [CrossRef]
- Hu, S.; Dou, X.; Zhang, L.; Xie, Y.; Yang, S.; Yang, M. Rapid detection of aflatoxin B1 in medicinal materials of radix and rhizome by gold immunochromatographic assay. Toxicon 2018, 150, 144–150. [Google Scholar] [CrossRef]
- Di Nardo, F.; Baggiani, C.; Giovannoli, C.; Spano, G.; Anfossi, L. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim. Acta 2017, 184, 51295–51304. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Zhang, Z.; Ding, X.; Tang, X. Multi-component immunochromatog-raphic assay for simultaneous detection of aflatoxin B1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013, 49, 426–432. [Google Scholar] [CrossRef]
- Molinelli, A.; Grossalber, K.; Führer, M.; Baumgartner, S.; Sulyok, M.; Krska, R. Development of qualitative and semiquantitative immunoassay-based rapid strip tests for the detection of T-2 toxin in wheat and oat. J. Agric. Food Chem. 2008, 56, 2589–2594. [Google Scholar] [CrossRef]
- Imourana, K.; Gerd, S.; Ionelia, T.; Daniela, M.; Olivier, P.; Isabelle Paule, O. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 349–3507. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold spensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, X.; Xie, J.; Li, X.; Huang, Z. Rapid simultaneous detection of fumonisin B1 and deoxynivalenol in grain by immunochromatographic test strip. Anal. Biochem. 2020, 606, 113878. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, T.; Li, X.; Huang, Z. Flower-like goldnanoparticles-based immunochromatographic test strip for rapid simultaneous detection of fumonisin B1 and deoxynivalenol in Chinese traditional medicine. J. Pharm. Biomed. Anal. 2020, 177, 112895. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Xie, J.; Huang, Z. Development of two immunochromatographic test strips based on gold nanospheres and gold nanoflowers for the rapid and simultaneous detection of aflatoxin B1 and aristolochic acid A in dual-use medicinal and food ingredients. Microchem. J. 2022, 186, 108307. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; Li, L.; Li, Y.; He, Q. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chem. 2010, 119, 834–839. [Google Scholar] [CrossRef]

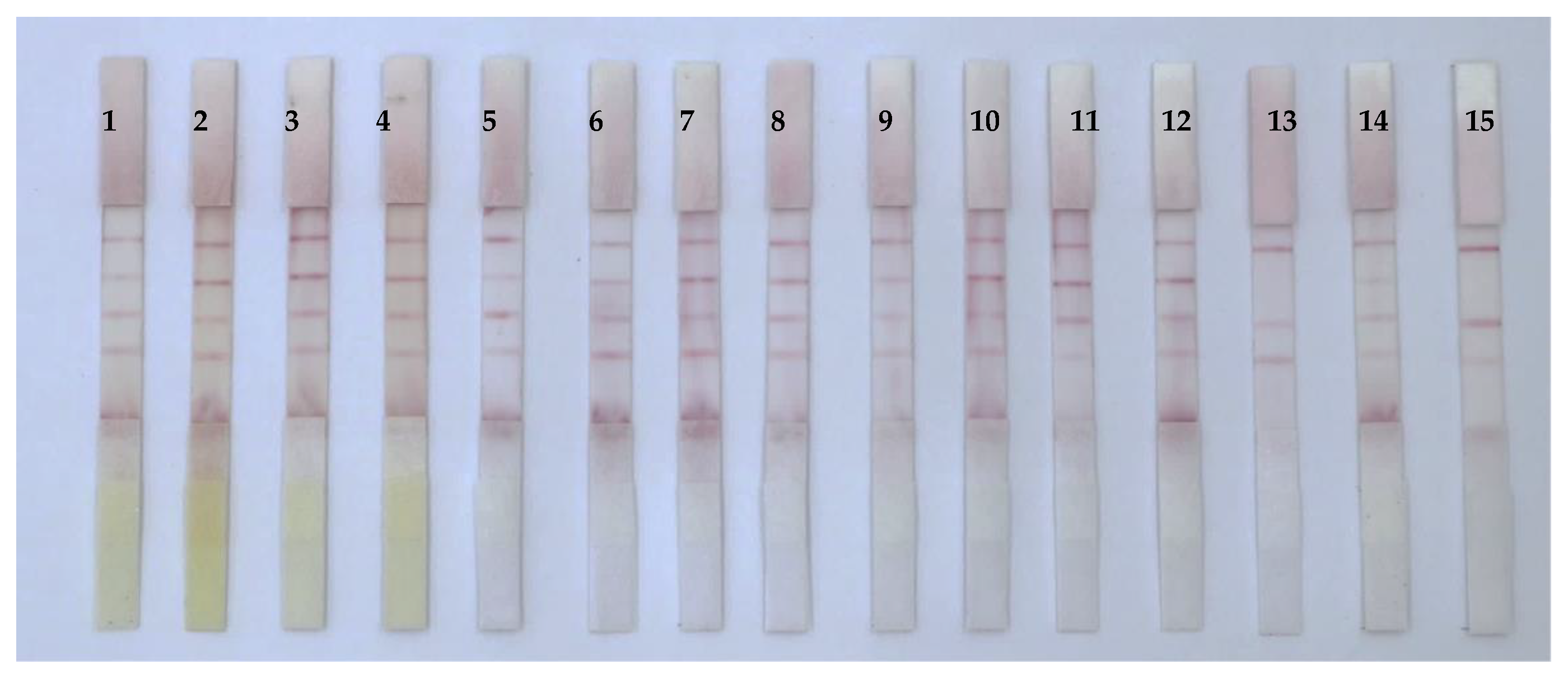
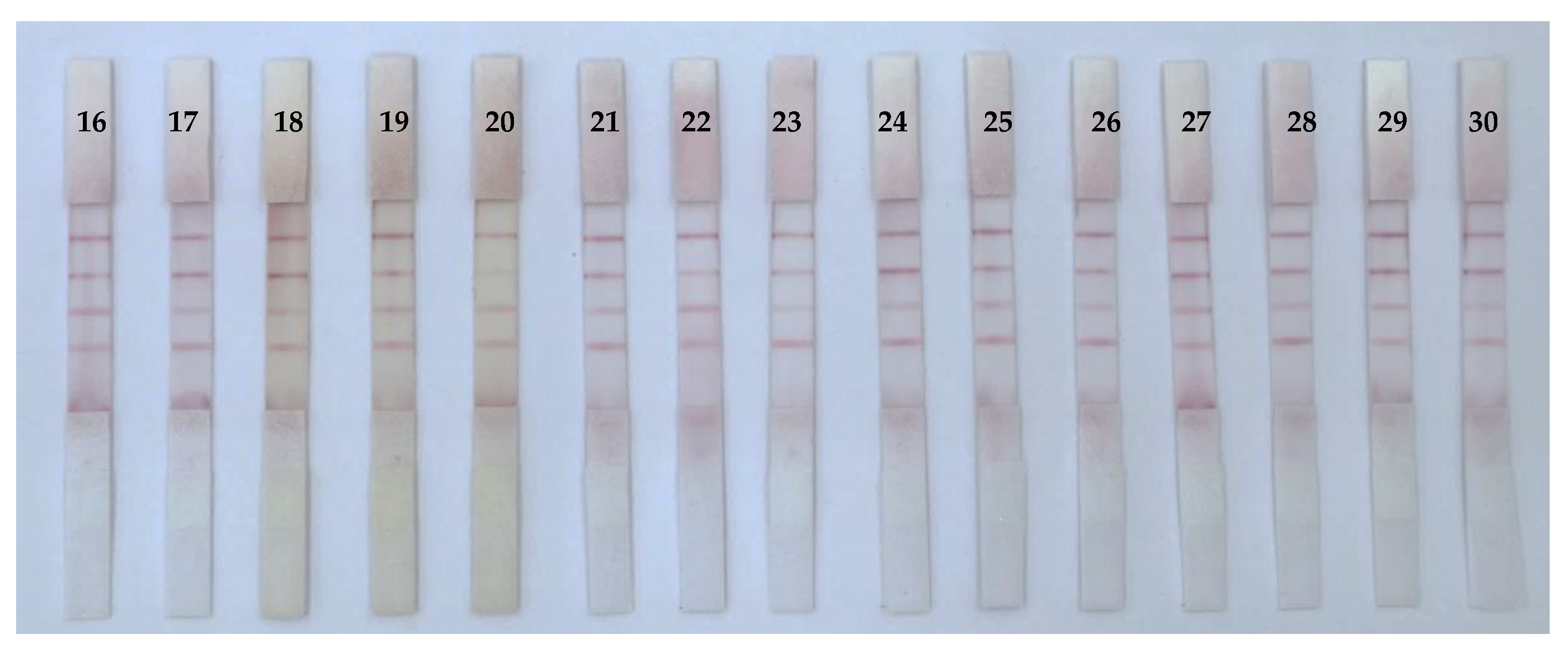
| Chinese Medicine | ICS | HPLC | Chinese Medicine | ICS | HPLC |
|---|---|---|---|---|---|
| (n = 3) | (μg/kg) | (n = 3) | (μg/kg) | ||
| 1 | -,-,-, | ND | 16 | -,-,-, | ND |
| 2 | -,-,-, | ND | 17 | -,-,-, | ND |
| 3 | -,-,-, | ND | 18 | -,-,-, | ND |
| 4 | -,-,-, | ND | 19 | -,-,-, | ND |
| 5 | -,-,-, | ND | 20 | -,-,-, | ND |
| 6 | -,-,-, | ND | 21 | -,-,-, | ND |
| 7 | -,-,-, | ND | 22 | -,-,-, | ND |
| 8 | -,-,-, | ND | 23 | -,-,-, | ND |
| 9 | -,-,-, | ND | 24 | -,-,-, | ND |
| 10 | -,-,-, | ND | 25 | -,-,-, | ND |
| 11 | -,-,-, | ND | 26 | -,-,-, | ND |
| 12 | -,-,-, | ND | 27 | -,-,-, | ND |
| 13 | -,-,-, | ND | 28 | -,-,-, | ND |
| 14 | -,-,-, | ND | 29 | -,-,-, | ND |
| 15 | -,-,-, | ND | 30 | -,-,-, | ND |
| Chinese Medicine | ICS | ELISA | Chinese Medicine | ICS | ELISA |
|---|---|---|---|---|---|
| (n = 3) | (μg/kg) | (n = 3) | (μg/kg) | ||
| 1 | -,-,-, | 33.49 | 16 | -,-,-, | 40.09 |
| 2 | -,-,-, | ND | 17 | -,-,-, | 31.28 |
| 3 | -,-,-, | ND | 18 | -,-,-, | 36.07 |
| 4 | -,-,-, | 17.16 | 19 | -,-,-, | 41.53 |
| 5 | -,-,-, | 29.26 | 20 | -,-,-, | 38.14 |
| 6 | -,-,-, | 29.41 | 21 | -,-,-, | ND |
| 7 | -,-,-, | 40.97 | 22 | -,-,-, | 26.27 |
| 8 | -,-,-, | 12.13 | 23 | -,-,-, | 4.41 |
| 9 | -,-,-, | 10.12 | 24 | -,-,-, | 6.93 |
| 10 | -,-,-, | 19.55 | 25 | -,-,-, | 3.40 |
| 11 | -,-,-, | 16.09 | 26 | -,-,-, | 2.75 |
| 12 | -,-,-, | 12.07 | 27 | -,-,-, | 3.87 |
| 13 | -,-,-, | 10.88 | 28 | -,-,-, | 36.76 |
| 14 | -,-,-, | 13.71 | 29 | -,-,-, | 34.31 |
| 15 | -,-,-, | 28.15 | 30 | -,-,-, | 25.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Xie, J.; Huang, Z. Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip. Foods 2023, 12, 633. https://doi.org/10.3390/foods12030633
Zhang J, Li X, Xie J, Huang Z. Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip. Foods. 2023; 12(3):633. https://doi.org/10.3390/foods12030633
Chicago/Turabian StyleZhang, Jiaying, Xiujiang Li, Jianhua Xie, and Zhibing Huang. 2023. "Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip" Foods 12, no. 3: 633. https://doi.org/10.3390/foods12030633
APA StyleZhang, J., Li, X., Xie, J., & Huang, Z. (2023). Rapid and Simultaneous Detection of Aflatoxin B1, Zearalenone, and T-2 Toxin in Medicinal and Edible Food Using Gold Immunochromatographic Test Strip. Foods, 12(3), 633. https://doi.org/10.3390/foods12030633







