Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
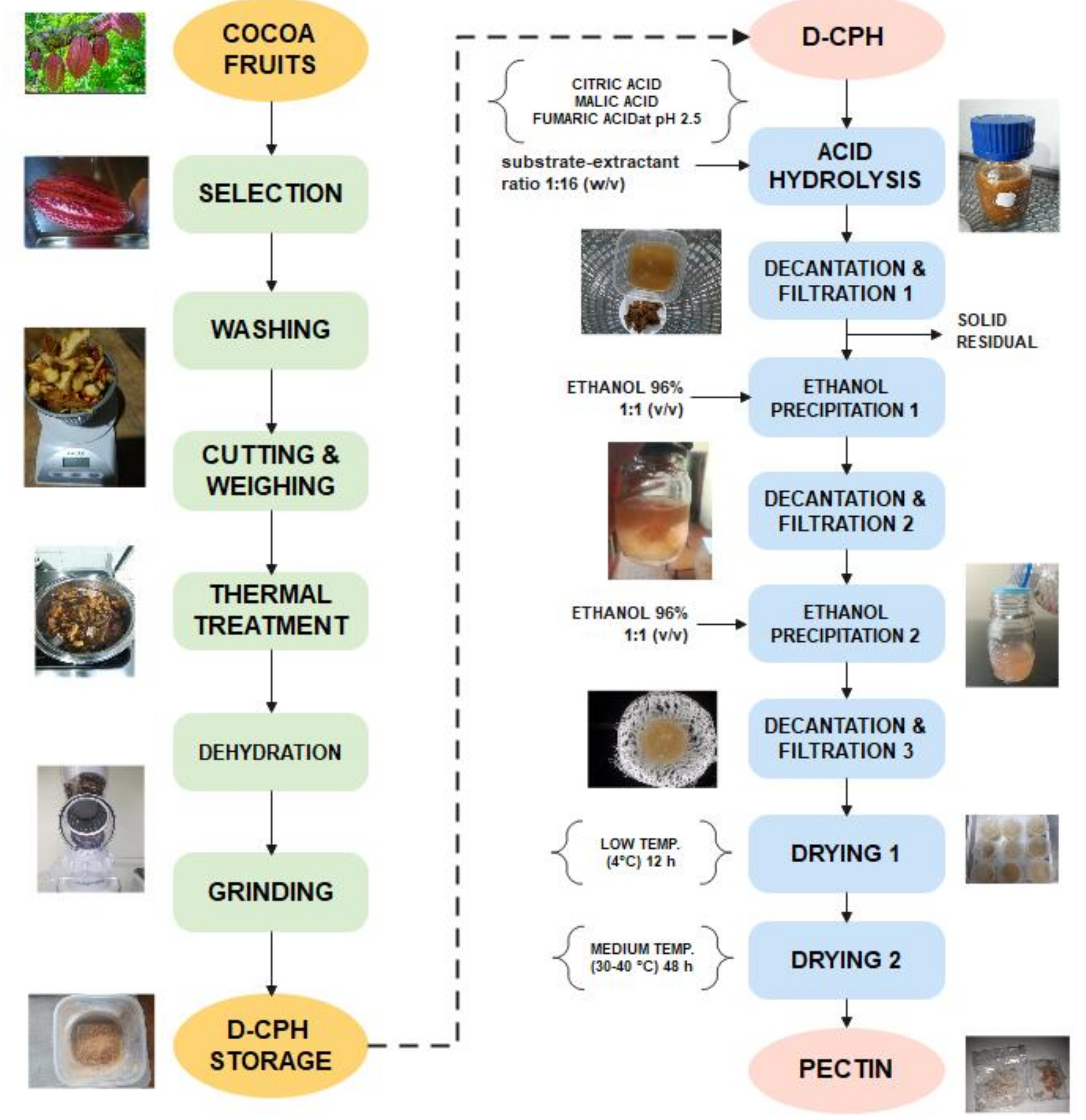
2.3. Preparation of Dehydrated Cocoa Pod Husk
2.4. Pectin Extraction Experiments from Dehydrated CPH
2.5. Central Composite Design of Experiments Using Response Surface Methodology
2.6. Characterisation of CHP-Pectins
2.6.1. FTIR Analysis of CHP-Pectins
2.6.2. Determination of Equivalent Weight, Methoxyl Content (MeO), Anhydrouronic Acid Content (AUA) and Degree of Esterification (DE) of CHP-Pectins
3. Results
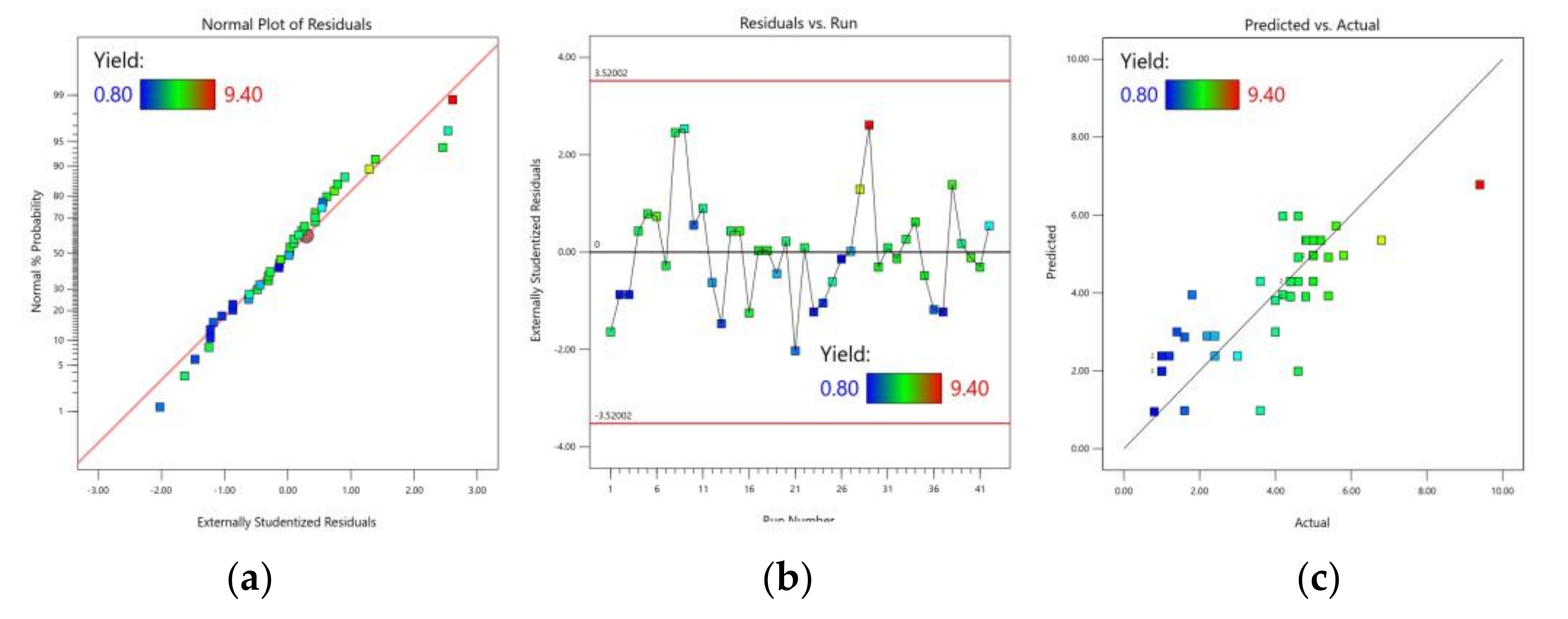
3.1. CCD Experiments and the Model Analysis
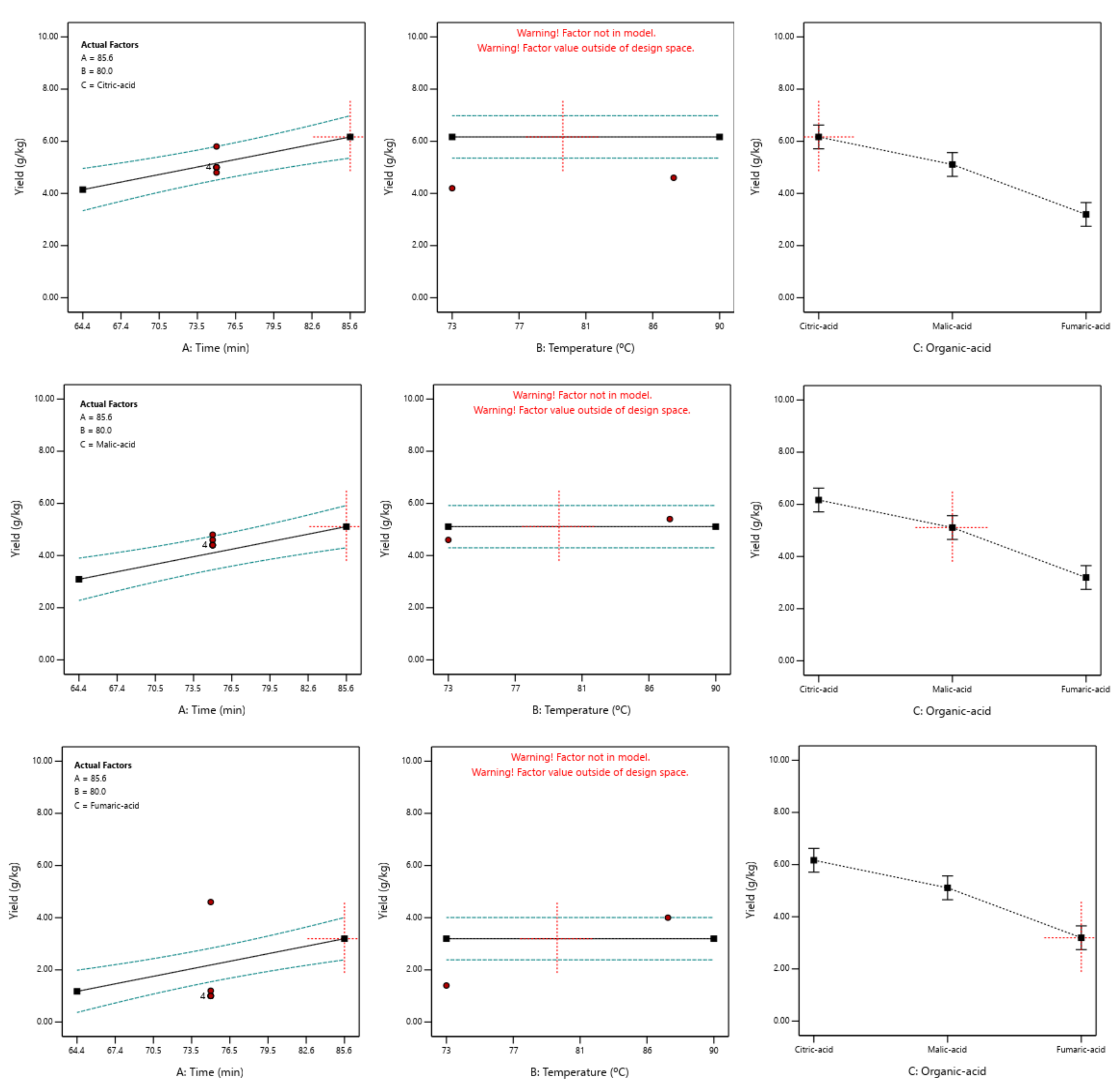
3.2. Optimisation of Pectin Yield Model
3.3. Confirmation Experiments for the Pectin Yield Model
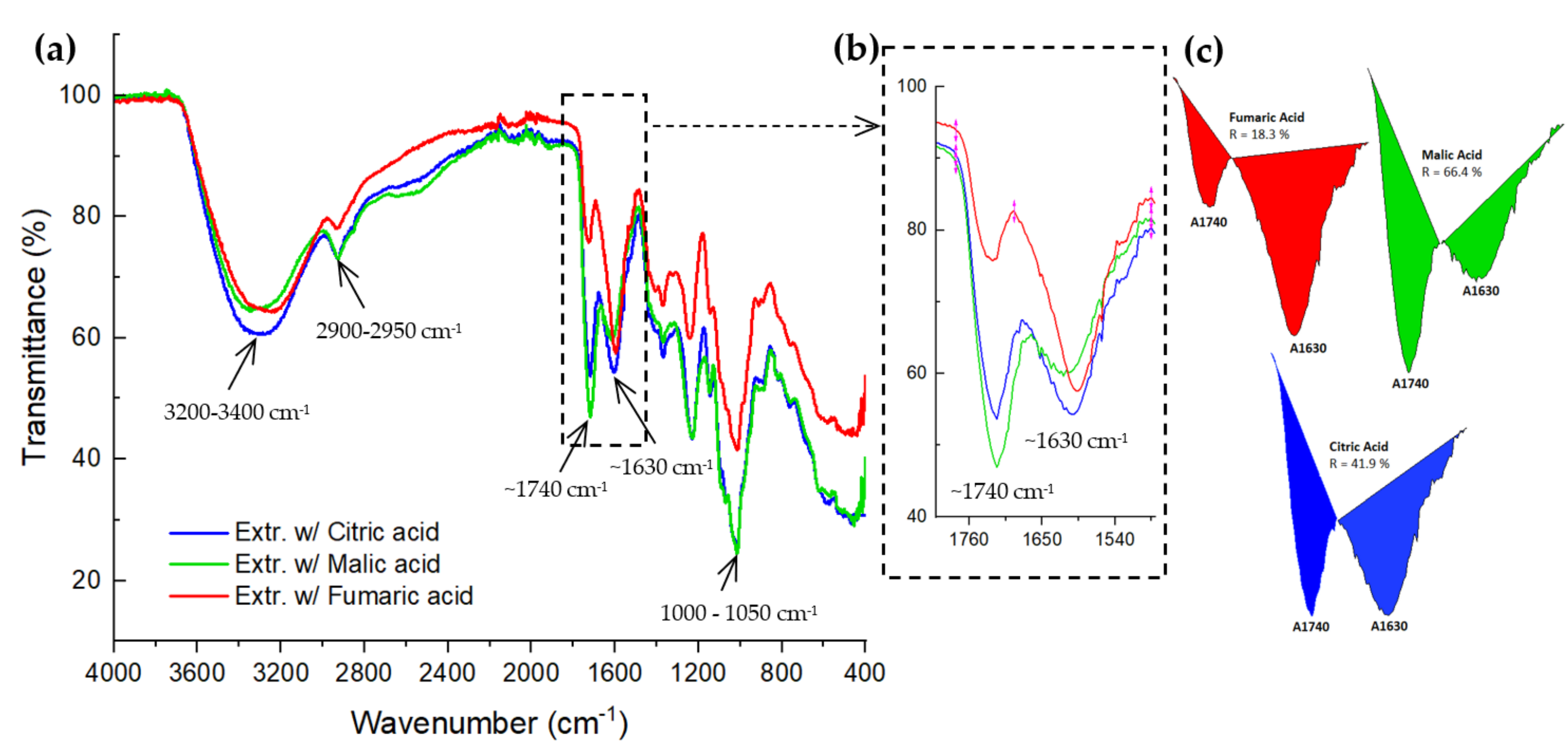
3.4. Characterisation of the Pectin from d-CPH Using Three Organic Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations—Statistics Division. 2022. Available online: http://www.fao.org/faostat/en/#data/qc (accessed on 8 March 2022).
- Díaz-Montenegro, J.; Varela, E.; Gil, J.M. Livelihood Strategies of Cacao Producers in Ecuador: Effects of National Policies to Support Cacao Farmers and Specialty Cacao Landraces. J. Rural Stud. 2018, 63, 141–156. [Google Scholar] [CrossRef]
- Ketema, M.W.; Tennhardt, L.; Cramon-Taubadel, S.V. Profitability of Small-Scale Cocoa Production in Ecuador. In Proceedings of the Tropentag, Conference on International Research on Food Security, Natural Resource Management and Rural Development, Stuttgart, Germany, 15–17 September 2021. [Google Scholar]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of Cacao Pod Husks by Pyrolysis and Catalytic Reaction to Produce Useful Chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Porto de Souza Vandenberghe, L.; Kley Valladares-Diestra, K.; Amaro Bittencourt, G.; Fátima Murawski de Mello, A.; Sarmiento Vásquez, Z.; Zwiercheczewski de Oliveira, P.; Vinícius de Melo Pereira, G.; Ricardo Soccol, C. Added-Value Biomolecules’ Production from Cocoa Pod Husks: A Review. Bioresour. Technol. 2022, 344, 126252. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) Pod Husk: Renewable Source of Bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Ouattara, L.; Kouadio, E.; Soro, D.; Soro, Y.; Benjamin Yao, K.; Adouby, K.; Drogui, A.; Tyagi, D.; Aina, P. Cocoa Pod Husk: A Review. Bioresources 2021, 16, 1988–2000. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Musita, N. Characteristics of Pectin Extracted from Cocoa Pod Husks. Pelita Perkeb. Coffee Cocoa Res. J. 2021, 37. [Google Scholar] [CrossRef]
- De Cindio, B.; Gabriele, D.; Lupi, F.R. Pectin: Properties Determination and Uses. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological Investigation of Pectin-Based Emulsion Gels for Pharmaceutical and Cosmetic Uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Mamani Crisṕin, P.L.; Ruiz Caro, R.; Veiga, M.D. Pectin: Pharmaceutical and Therapeutic Uses. An. R. Acad. Nac. Farm. 2012, 78, 82–97. [Google Scholar]
- Satapathy, S.; Rout, J.R.; Kerry, R.G.; Thatoi, H.; Sahoo, S.L. Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical Bioprocessing. Front. Nutr. 2020, 7, 117. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Z.; Novak, Z. Encapsulation of Pharmaceuticals into Pectin Aerogels for Controlled Drug Release. Adv. Technol. 2015, 4, 49–52. [Google Scholar] [CrossRef]
- Tyagi, V.; Sharma, P.K.; Malviya, R. Pectins And Their Role in Food and Pharmaceutical Industry. J. Chronother. Drug Deliv. 2015, 6, 65–77. [Google Scholar]
- Srivastava, P.; Malviya, R. Sources of Pectin, Extraction and Its Applications in Pharmaceutical Industry—An Overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Sutapa, B.M.; Dhruti, A.P.G.; Gopa, R.B. Therapeutic and Pharmaceutical Benefits of Native and Modified Plant Pectin. J. Med. Plants Res. 2018, 12. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Kley Valladares-Diestra, K.; Porto de Souza Vandenberghe, L.; Ricardo Soccol, C. A Biorefinery Approach for Pectin Extraction and Second-Generation Bioethanol Production from Cocoa Pod Husk. Bioresour. Technol. 2022, 346, 126635. [Google Scholar] [CrossRef]
- Zoghi, A.; Vedadi, S.; Esfahani, Z.H.; Gavlighi, H.A.; Khosravi-Darani, K. A Review on Pectin Extraction Methods Using Lignocellulosic Wastes. Biomass. Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Desniorita, N.N.; Novelina, K.S. Sustainable Design of Biorefinery Processes on Cocoa Pod: Optimization of Pectin Extraction Process with Variations of pH, Temperature, and Time. Int. J. Adv. Sci. Eng. Inf. Technol. 2019, 9, 2104–2113. [Google Scholar] [CrossRef]
- Sabater, C.; Villamiel, M.; Montilla, A. Integral Use of Pectin-Rich by-Products in a Biorefinery Context: A Holistic Approach. Food Hydrocoll. 2022, 128, 107564. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Food Waste Management, Valorization, and Sustainability in the Food Industry. In Food Waste Recovery: Processing Technologies and Industrial Techniques; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780128004197. [Google Scholar]
- Garcia-Garcia, G.; Woolley, E.; Rahimifard, S.; Colwill, J.; White, R.; Needham, L. A Methodology for Sustainable Management of Food Waste. Waste Biomass Valorization 2017, 8, 2209–2227. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ooi, J.K.; Woon, K.S. An Integrated Life Cycle Multi-Objective Optimization Model for Health-Environment-Economic Nexus in Food Waste Management Sector. Sci. Total Environ. 2022, 816, 151541. [Google Scholar] [CrossRef] [PubMed]
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation and Industrial Uses: A Review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Azmin, S.N.H.M.; Hayat, N.A.B.M.; Nor, M.S.M. Development and Characterization of Food Packaging Bioplastic Film from Cocoa Pod Husk Cellulose Incorporated with Sugarcane Bagasse Fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Akinjokun, A.I.; Petrik, L.F.; Ogunfowokan, A.O.; Ajao, J.; Ojumu, T.V. Isolation and Characterization of Nanocrystalline Cellulose from Cocoa Pod Husk (CPH) Biomass Wastes. Heliyon 2021, 7, e06680. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Tang, M.; Pertino, M.W.; Simirgiotis, M.J. UHPLC-MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants 2022, 11, 595. [Google Scholar] [CrossRef]
- Adi-Dako, O.; Ofori-Kwakye, K.; Frimpong Manso, S.; el Boakye-Gyasi, M.; Sasu, C.; Pobee, M. Physicochemical and Antimicrobial Properties of Cocoa Pod Husk Pectin Intended as a Versatile Pharmaceutical Excipient and Nutraceutical. J. Pharm. 2016, 2016, 7608693. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S. Effect of Extraction Conditions on the Yield and Chemical Properties of Pectin from Cocoa Husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Hamidon, N.H.; Zaidel, D.N.A. Effect of Extraction Conditions on Pectin Yield Extracted from Sweet Potato Peels Residues Using Hydrochloric Acid. Chem. Eng. Trans. 2017, 56, 979–984. [Google Scholar] [CrossRef]
- Sarah, M.; Hisham, H.; Rizki, M.; Erwinda, R. Effect of Power and Time in Pectin Production from Cocoa Pod Husk Using Microwave-Assisted Extraction Technique. Int. J. Renew. Energy Dev. 2020, 9, 125–130. [Google Scholar] [CrossRef]
- Pangestu, R.; Amanah, S.; Juanssilfero, A.B.; Yopi; Perwitasari, U. Response Surface Methodology for Microwave-Assisted Extraction of Pectin from Cocoa Pod Husk (Theobroma cacao) Mediated by Oxalic Acid. J. Food Meas. Charact. 2020, 14, 2126–2133. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Astudillo, I.C.P. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules 2021, 26, 1473. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; Petkowicz, C.L.D.O. Optimization of Nitric Acid-Mediated Extraction of Pectin from Cacao Pod Husks (Theobroma cacao L.) Using Response Surface Methodology. Carbohydr. Polym. 2011, 84, 1230–1236. [Google Scholar] [CrossRef]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction Optimization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) with Ascorbic Acid Using Response Surface Methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef]
- Marsiglia, D.E.; Ojeda, K.A.; Ramírez, M.C.; Sánchez, E. Pectin Extraction from Cocoa Pod Husk (Theobroma cacao L.) by Hydrolysis with Citric and Acetic Acid. Int. J. ChemTech Res. 2016, 9, 497–507. [Google Scholar]
- Myers, R.; Montgomery, D.; Anderson-Cook, C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- Khuri, A.I. A General Overview of Response Surface Methodology. Biom. Biostat. Int. J. 2017, 5, 87–93. [Google Scholar] [CrossRef]
- Pais-Chanfrau, J.M.; Núñez-Pérez, J.; del Carmen Espin-Valladares, R.; Vinicio Lara-Fiallos, M.; Enrique Trujillo-Toledo, L. Uses of the Response Surface Methodology for the Optimization of Agro-Industrial Processes. In Response Surface Methodology in Engineering Science; Palanikumar, K., Ed.; Intech: London, UK, 2021; pp. 1–21. ISBN 978-1-83968-918-5. [Google Scholar]
- Mada, T.; Duraisamy, R.; Guesh, F. Optimization and Characterization of Pectin Extracted from Banana and Papaya Mixed Peels Using Response Surface Methodology. Food Sci. Nutr. 2022, 10, 1222–1238. [Google Scholar] [CrossRef]
- Duwee, Y.S.; Kiew, P.L.; Yeoh, W.M. Multi-Objective Optimization of Pectin Extraction from Orange Peel via Response Surface Methodology: Yield and Degree of Esterification. J. Food Meas. Charact. 2022, 16, 1710–1724. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Thottiam Vasudevan, R.; Sriramulu, G. Optimized Extraction and Characterization of Pectin from Jackfruit (Artocarpus integer) Wastes Using Response Surface Methodology. Int. J. Biol. Macromol. 2018, 106, 698–703. [Google Scholar] [CrossRef]
- Pradhan, D.; Abdullah, S.; Pradhan, R.C. Optimization of Pectinase Assisted Extraction of Chironji (Buchanania lanzan) Fruit Juice Using Response Surface Methodology and Artificial Neural Network. Int. J. Fruit Sci. 2020, 20, S318–S336. [Google Scholar] [CrossRef]
- Seggiani, M.; Puccini, M.; Pierini, M.; Giovando, S.; Forneris, C. Effect of Different Extraction and Precipitation Methods on Yield and Quality of Pectin. Int. J. Food Sci. Technol. 2009, 44, 574–580. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products. In Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill: New Delhi, India, 1986. [Google Scholar]
- Pappas, C.S.; Malovikova, A.; Hromadkova, Z.; Tarantilis, P.A.; Ebringerova, A.; Polissiou, M.G. Determination of the Degree of Esterification of Pectinates with Decyl and Benzyl Ester Groups by Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) and Curve-Fitting Deconvolution Method. Carbohydr. Polym. 2004, 56, 465–469. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Amponsah, S.K.; Yeboah, S.; Kukuia, K.K.E.; N’Guessan, B.B.; Adi-Dako, O. A Pharmacokinetic Evaluation of a Pectin-Based Oral Multiparticulate Matrix Carrier of Carbamazepine. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5527452. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; Lúcia de Oliveira Petkowicz, C. Extraction and Characterization of Pectin from Cacao Pod Husks (Theobroma cacao L.) with Citric Acid. LWT—Food Sci. Technol. 2012, 49, 108–116. [Google Scholar] [CrossRef]

| Coded Factors | ||||||
|---|---|---|---|---|---|---|
| −1.414 | −1.000 | 0.000 | +1.000 | +1.414 | ||
| A: Time (min) | 60 | 64 | 75 | 86 | 90 | |
| B: Temperature (°C) | 70 | 73 | 80 | 87 | 90 | |
| C: Organic acid | C [1] citric acid | C [2] malic acid | C [3] fumaric acid | |||
| Std. | Block | Run | A: Time (min) | B: Temperature (°C) | C: Organic-Acid (−) | Yield (g/kg) |
|---|---|---|---|---|---|---|
| 32 | 1 | 1 | 86 | 87 | Fumaric acid | 4.0 |
| 31 | 1 | 2 | 64 | 87 | Fumaric acid | 3.6 |
| 17 | 1 | 3 | 64 | 87 | Malic acid | 2.4 |
| 33 | 1 | 4 | 75 | 80 | Fumaric acid | 1.0 |
| 18 | 1 | 5 | 86 | 87 | Malic acid | 5.4 |
| 4 | 1 | 6 | 86 | 87 | Citric acid | 4.6 |
| 21 | 1 | 7 | 75 | 80 | Malic acid | 4.4 |
| 29 | 1 | 8 | 64 | 73 | Fumaric acid | 1.6 |
| 5 | 1 | 9 | 75 | 80 | Citric acid | 5.0 |
| 1 | 1 | 10 | 64 | 73 | Citric acid | 4.2 |
| 15 | 1 | 11 | 64 | 73 | Malic acid | 2.2 |
| 6 | 1 | 12 | 75 | 80 | Citric acid | 5.0 |
| 19 | 1 | 13 | 75 | 80 | Malic acid | 4.8 |
| 16 | 1 | 14 | 86 | 73 | Malic acid | 4.6 |
| 3 | 1 | 15 | 64 | 87 | Citric acid | 1.8 |
| 35 | 1 | 16 | 75 | 80 | Fumaric acid | 1.0 |
| 30 | 1 | 17 | 86 | 73 | Fumaric acid | 1.4 |
| 2 | 1 | 18 | 86 | 73 | Citric acid | 4.2 |
| 20 | 1 | 19 | 75 | 80 | Malic acid | 4.4 |
| 34 | 1 | 20 | 75 | 80 | Fumaric acid | 4.6 |
| 7 | 1 | 21 | 75 | 80 | Citric acid | 5.8 |
| 23 | 2 | 22 | 90 | 80 | Malic acid | 5.6 |
| 40 | 2 | 23 | 75 | 80 | Fumaric acid | 1.0 |
| 28 | 2 | 24 | 75 | 80 | Malic acid | 4.4 |
| 9 | 2 | 25 | 90 | 80 | Citric acid | 9.4 |
| 10 | 2 | 26 | 75 | 70 | Citric acid | 6.8 |
| 22 | 2 | 27 | 60 | 80 | Malic acid | 1.6 |
| 12 | 2 | 28 | 75 | 80 | Citric acid | 5.0 |
| 38 | 2 | 29 | 75 | 70 | Fumaric acid | 2.4 |
| 24 | 2 | 30 | 75 | 70 | Malic acid | 3.6 |
| 14 | 2 | 31 | 75 | 80 | Citric acid | 4.8 |
| 41 | 2 | 32 | 75 | 80 | Fumaric acid | 1.2 |
| 26 | 2 | 33 | 75 | 80 | Malic acid | 4.6 |
| 11 | 2 | 34 | 75 | 90 | Citric acid | 5.2 |
| 37 | 2 | 35 | 90 | 80 | Fumaric acid | 4.0 |
| 8 | 2 | 36 | 60 | 80 | Citric acid | 5.4 |
| 39 | 2 | 37 | 75 | 90 | Fumaric acid | 3.0 |
| 13 | 2 | 38 | 75 | 80 | Citric acid | 5.0 |
| 25 | 2 | 39 | 75 | 90 | Malic acid | 5.0 |
| 27 | 2 | 40 | 75 | 80 | Malic acid | 4.4 |
| 42 | 2 | 41 | 75 | 80 | Fumaric acid | 1.0 |
| 36 | 2 | 42 | 60 | 80 | Fumaric acid | 0.8 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Block | 1.60 | 1 | 1.60 | |||
| Model | 88.00 | 3 | 29.33 | 20.70 | <0.0001 | significant |
| A-Time | 24.48 | 1 | 24.48 | 17.28 | 0.0002 | |
| C-OA 1 | 63.52 | 2 | 31.76 | 22.41 | <0.0001 | |
| Residual | 52.43 | 37 | 1.42 | |||
| Lack of Fit | 43.18 | 25 | 1.73 | 2.24 | 0.0728 | not significant |
| Pure Error | 9.25 | 12 | 0.7711 | |||
| Cor. Total | 142.03 | 41 |
| Factor | Goal | Lower Limit | Upper Limit | Importance |
|---|---|---|---|---|
| A: Time | In range | 64.39 | 85.61 | 3 |
| B: Temperature | Equal to 80.0 | 72.93 | 90.00 | 3 |
| C: Organic acid | In range | Citric acid | Fumaric acid | 3 |
| Yield | Maximise | 0.80 | 9.40 | 5 |
| Experiment | Time | Temperature | Organic Acid | |
|---|---|---|---|---|
| 1 | 85.607 | 80.00 | Citric acid | 6.167 |
| 2 | 85.607 | 80.00 | Malic acid | 5.110 |
| 3 | 85.607 | 80.00 | Fumaric acid | 3.196 |
| Organic Acid | Predicted Mean | Std. Dev. | n | SE Pred. | 95% PI Low | Data Mean | 95% PI High |
|---|---|---|---|---|---|---|---|
| Citric acid | 6.17 | 1.19 | 6 | 0.63 | 4.89 | 5.73 | 7.44 |
| Malic acid | 5.11 | 1.19 | 6 | 0.63 | 3.83 | 4.7 | 6.39 |
| Fumaric acid | 3.2 | 1.19 | 6 | 0.63 | 1.92 | 3.53 | 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrín-Chacón, J.P.; Núñez-Pérez, J.; Espín-Valladares, R.d.C.; Manosalvas-Quiroz, L.A.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods 2023, 12, 590. https://doi.org/10.3390/foods12030590
Jarrín-Chacón JP, Núñez-Pérez J, Espín-Valladares RdC, Manosalvas-Quiroz LA, Rodríguez-Cabrera HM, Pais-Chanfrau JM. Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods. 2023; 12(3):590. https://doi.org/10.3390/foods12030590
Chicago/Turabian StyleJarrín-Chacón, Jenny Paola, Jimmy Núñez-Pérez, Rosario del Carmen Espín-Valladares, Luis Armando Manosalvas-Quiroz, Hortensia María Rodríguez-Cabrera, and José Manuel Pais-Chanfrau. 2023. "Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study" Foods 12, no. 3: 590. https://doi.org/10.3390/foods12030590
APA StyleJarrín-Chacón, J. P., Núñez-Pérez, J., Espín-Valladares, R. d. C., Manosalvas-Quiroz, L. A., Rodríguez-Cabrera, H. M., & Pais-Chanfrau, J. M. (2023). Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods, 12(3), 590. https://doi.org/10.3390/foods12030590










