Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Pretreatment
2.2. Preparation of DES
2.3. Extraction of TPs
2.3.1. Hot Water Extraction
2.3.2. DES-Based Ultrasound-Assisted Extraction
2.4. Experimental Design
2.5. Antioxidant Activity of TPs
2.5.1. ABTS Radical Scavenging Activity
2.5.2. DPPH Radical Scavenging Activity
2.5.3. FRAP
2.5.4. ORAC
2.6. Molecular Weight and Its Distribution
2.7. Monosaccharide Composition Determination
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
2.9. α-Amylase and α-Glucosidase Inhibition Assays
2.10. Glucose Uptake
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of TPs Extraction Process
3.2. The Antioxidant Activity In Vitro
3.3. Comparison of HWP and CHP
3.3.1. Extraction Yields and Total Carbohydrate
3.3.2. Monosaccharide Compositions
3.3.3. Molecular Weight
3.3.4. FT-IR Spectra
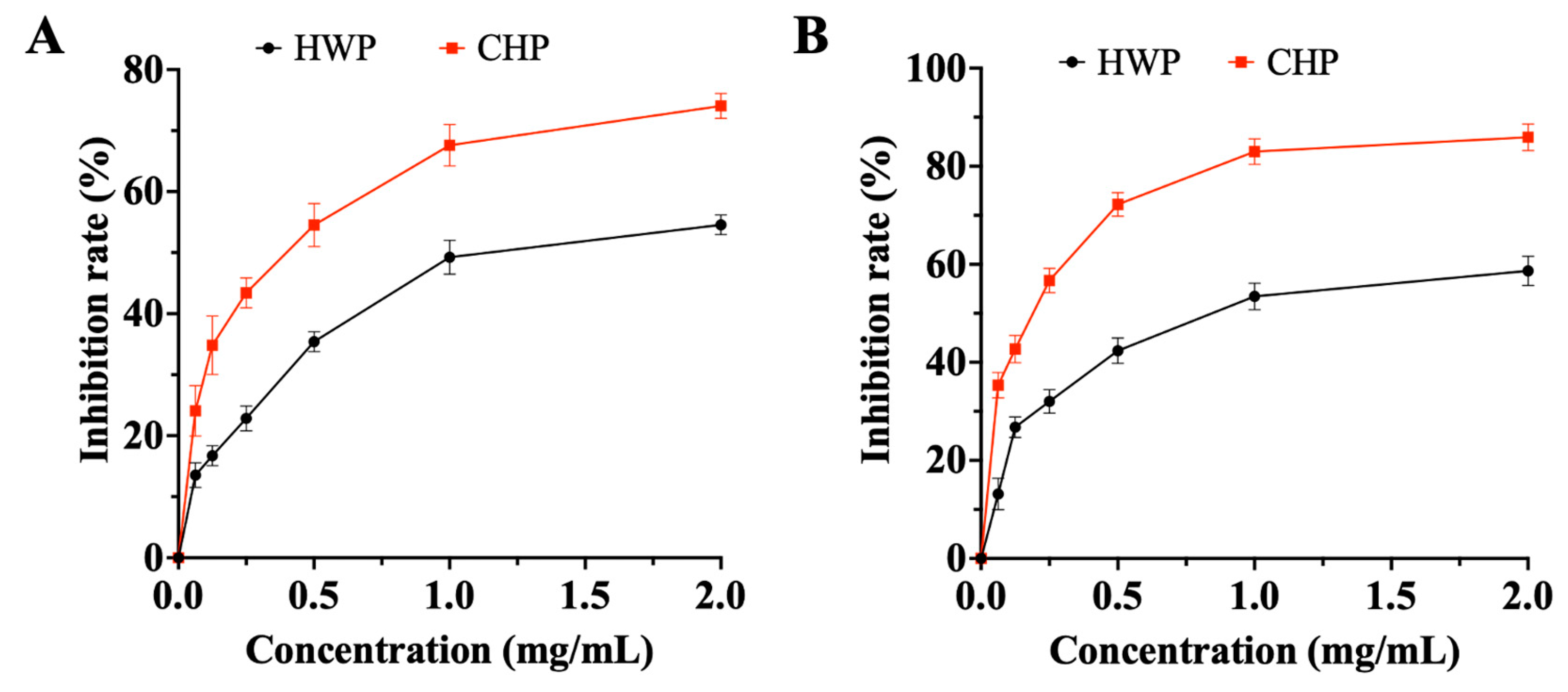
3.3.5. The α-Amylase and α-Glucosidase Inhibitory Effect
3.3.6. Hypoglycemic Effect of TPs in L6 Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R. The reference genome of tea plant and resequencing of 81 diverse accessions provide insights into its genome evolution and adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Kong, Y.-S.; Liu, P.-P.; Wang, J.-J.; Zhu, Y.; Wang, G.-M.; Sun, M.-F.; Chen, Y.; Guo, G.-Y.; Liu, Z.-H. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- Du, Y.; Chen, H.; Zhong, W.; Wu, L.; Ye, J.; Lin, C.; Zheng, X.; Lu, J.; Liang, Y. Effect of temperature on accumulation of chlorophylls and leaf ultrastructure of low temperature induced albino tea plant. Afr. J. Biotechnol. 2008, 7, 12. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Andrae-Marobela, K.; Okatch, H.; Xiao, J. Tea polysaccharides as food antioxidants: An old woman’s tale? Food Chem. 2013, 138, 1923–1927. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, F.; Wei, X. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, J.; Wang, G.; Mao, G. Extraction and free radical scavenging activity of polysaccharide from ‘Anji Baicha’ (Camellia sinensis (L.) O. Kuntze). Int. J. Biol. Macromol. 2016, 84, 161–165. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Sun, Y.; Yang, S.; Yang, X. Characterisation of polysaccharides from green tea of Huangshan Maofeng with antioxidant and hepatoprotective effects. Food Chem. 2013, 141, 3415–3423. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Wu, J.; Chen, R.; Tan, L.; Bai, H.; Tian, L.; Lu, J.; Gao, M.; Bai, C.; Sun, H.; Chi, Y. Ultrasonic disruption effects on the extraction efficiency, characterization, and bioactivities of polysaccharides from Panax notoginseng flower. Carbohydr. Polym. 2022, 291, 119535. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Men, Z.; Song, L.; Lv, Y.; Wu, P.; Lou, B.; Zhang, Y.; Shi, N.; Chen, Q. Insight into effective denitrification and desulfurization of liquid fuel with deep eutectic solvents: An innovative evaluation criterion to filtrate extractants using the compatibility index. Green Chem. 2018, 20, 3112–3120. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.; Ferreira, I.C.; Ferreira, O. Enhanced extraction of phenolic compounds using choline chloride based deep eutectic solvents from Juglans regia L. Ind. Crops Prod. 2018, 115, 261–271. [Google Scholar] [CrossRef]
- Zhang, Y.; He, L.; Li, Q.; Cheng, J.; Wang, Y.; Zhao, J.; Yuan, S.; Chen, Y.; Shi, R. Optimization of ultrasonic-assisted deep eutectic solvent for the extraction of polysaccharides from Indocalamus tessellatus leaves and their biological studies. Sustain. Chem. Pharm. 2022, 30, 100855. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q. Study on extraction and antioxidant activity of polysaccharides from Radix Bupleuri by natural deep eutectic solvents combined with ultrasound-assisted enzymolysis. Sustain. Chem. Pharm. 2022, 30, 100877. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-C.; Chu, D.; Zhang, J.-X.; Zheng, Y.-F.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Zhang, H.; Li, J.; Tao, W.; Linhardt, R.J.; Chen, S.; Ye, X. Physicochemical properties and conformations of water-soluble peach gums via different preparation methods. Food Hydrocoll. 2019, 95, 571–579. [Google Scholar] [CrossRef]
- Bodini, R.B.; Sobral, P.d.A.; Fávaro-Trindade, C.S.; Carvalho, R.d. Properties of gelatin-based films with added ethanol–propolis extract. LWT-Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-activity relationship of procyanidins on advanced glycation end products formation and corresponding mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Islam, M.; Sarkar, S.M.; Murugan, A.; Makky, E.A.; Rashid, S.; Yusoff, M.M. Anti-amylolytic activity of fresh and cooked okra (Hibiscus esculentus L.) pod extract. Biocatal. Agric. Biotechnol. 2014, 3, 373–377. [Google Scholar] [CrossRef]
- Yu, S.; Liu, L.; Bu, T.; Zheng, J.; Wang, W.; Wu, J.; Liu, D. Purification and characterization of hypoglycemic peptides from traditional Chinese soy-fermented douchi. Food Funct. 2022, 13, 3343–3352. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, G.L.; Huang, H.L. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem. 2022, 388, 133000. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhou, H.; Zhang, J.Y.; Li, F.L.; Wei, K.; Wei, X.L.; Wang, Y.F. Valorization of polysaccharides obtained from dark tea: Preparation, physicochemical, antioxidant, and hypoglycemic properties. Foods 2021, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.A.; Hao, L.; Zhang, L.A.; Zheng, Z.B.; Yang, Y.C. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium Meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar]
- Yan, J.-K.; Ding, Z.-C.; Gao, X.; Wang, Y.-Y.; Yang, Y.; Wu, D.; Zhang, H.-N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohydr. Polym. 2018, 193, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Wang, C.; Ma, H.; Ma, Y.; Yan, J.-K. Physicochemical and functional characteristics of polysaccharides from okra extracted by using ultrasound at different frequencies. Food Chem. 2021, 361, 130138. [Google Scholar] [CrossRef]
- Xu, K.; Jinfeng, d.; Wu, C.; Fan, G.; Li, X.; Sun, W.; Suo, A.; Li, Z.; Zhang, L. Effects of ultrasound-assisted Fenton treatment on structure and hypolipidemic activity of apricot polysaccharides. Food Biosci. 2022, 50, 102073. [Google Scholar] [CrossRef]
- Pan, X.; Xu, L.; Meng, J.; Chang, M.; Cheng, Y.; Geng, X.; Guo, D.; Liu, R. Ultrasound-assisted deep eutectic solvents extraction of polysaccharides from Morchella importuna: Optimization, physicochemical properties, and bioactivities. Front. Nutr. 2022, 9, 912014. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Chen, J.; Chen, H.; Wu, Q.; Zhang, K.; Li, D.; Li, Y.; Chen, Y. Effects of high-pressure homogenization extraction on the physicochemical properties and antioxidant activity of large-leaf yellow tea polysaccharide conjugates. Process Biochem. 2022, 122, 87–94. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; Julian McClements, D.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Omondi Onyango, S. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochemistry 2021, 70, 105355. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Feng, K.-L.; Huang, L.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Deep Eutectic Solvent-Assisted Extraction, Partially Structural Characterization, and Bioactivities of Acidic Polysaccharides from Lotus Leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, W.; Fu, M.-X.; Wang, A.-Q.; Wu, D.-T.; Guo, H.; Hu, Y.-C.; Gan, R.-Y.; Zou, L.; Liu, Y. Pressurized hot water extraction, structural properties, biological effects, and in vitro microbial fermentation characteristics of sweet tea polysaccharide. Int. J. Biol. Macromol. 2022, 222, 3215–3228. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, Y.; Zhou, C.; Xu, T.; Chen, X.; Wu, Q.; Zhang, K.; Li, Y.; Li, D.; Chen, Y. Effects of ultra-high pressure treatment on structure and bioactivity of polysaccharides from large leaf yellow tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Du, W.; Qian, L.; Xu, Y.; Huang, Y.; Xiong, Q.; Li, H.; Yuan, J. Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge. Int. J. Biol. Macromol. 2020, 150, 1011–1019. [Google Scholar] [CrossRef]
- Jahandideh, F.; Bourque, S.L.; Wu, J.P. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. X 2022, 13, 100222. [Google Scholar] [CrossRef]
- Ross, S.A.; Gulve, E.A.; Wang, M. Chemistry and biochemistry of type 2 diabetes. Chem. Rev. 2004, 104, 1255–1282. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.X.; Wu, D.T.; Zhao, Y.X.; Li, H.; Li, H.B.; Gan, R.Y. Structural characteristics of crude polysaccharides from 12 selected Chinese teas, and their antioxidant and anti-diabetic activities. Antioxidants 2021, 10, 1562. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Bian, X.; Wang, Y. Progress in research of alpha-glucosidase inhibitors and the structure-activity relationship. Chin. J. New Drugs 2014, 23, 189–195. [Google Scholar]
- Xu, L.; Chen, Y.; Chen, Z.; Gao, X.; Wang, C.; Panichayupakaranant, P.; Chen, H. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Miao, J.; Jing, S.; Li, X.; Huang, L.; Gao, W. The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol. 2017, 102, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhu, J.; Qian, Y.; Yue, W.; Xu, Y.; Zhang, D.; Yang, Y.; Gao, X.; He, H.; Wang, D. Effect of purity of tea polysaccharides on its antioxidant and hypoglycemic activities. J. Food Biochem. 2020, 44, e13277. [Google Scholar] [CrossRef]
- Jaiswal, N.; Gavin, M.G.; Quinn, W.J.; Luongo, T.S.; Gelfer, R.G.; Baur, J.A.; Titchenell, P.M. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol. Metab. 2019, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Identification of C21 Steroidal Glycosides from Gymnema sylvestre (Retz.) and Evaluation of Their Glucose Uptake Activities. Molecules 2021, 26, 6549. [Google Scholar]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and Anti-Diabetic Activities of Polysaccharides from Guava Leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
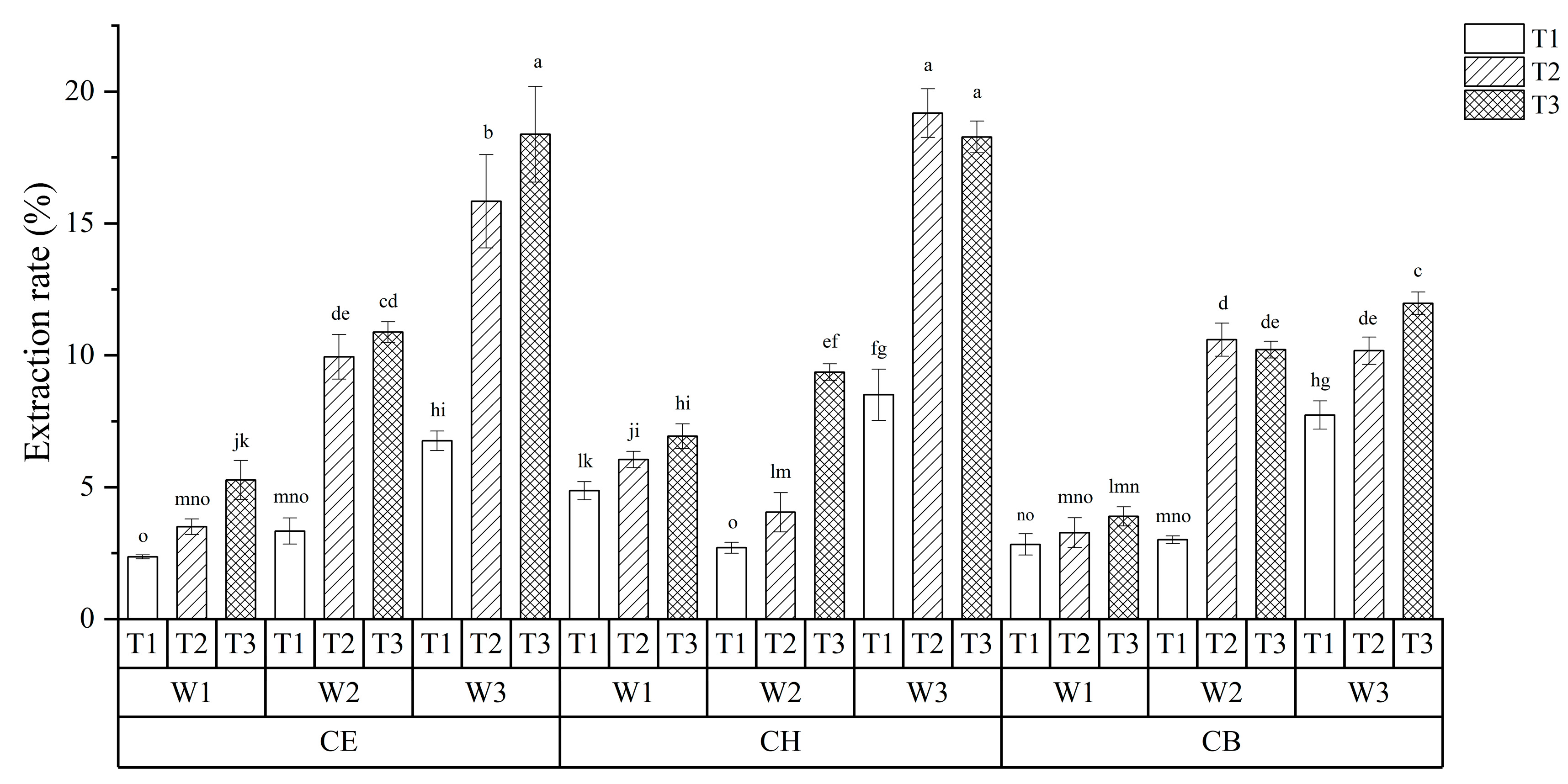



| Levels | Solvent | Extraction Time (min) | Water Content (w/w, %) |
|---|---|---|---|
| 1 | CE | 20 | 10 |
| 2 | CH | 40 | 20 |
| 3 | CB | 60 | 30 |
| Group | DF | Sum_sq | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| sol | 2 | 48.368496 | 24.18425 | 48.48 | <0.0001 |
| water | 2 | 1052.052652 | 526.0263 | 1054.45 | <0.0001 |
| time | 2 | 513.006052 | 256.503 | 514.17 | <0.0001 |
| sol-water | 4 | 164.575719 | 41.14393 | 82.48 | <0.0001 |
| sol-time | 4 | 24.676096 | 6.169024 | 12.37 | <0.0001 |
| water-time | 4 | 137.223385 | 34.30585 | 68.77 | <0.0001 |
| sol-water-time | 8 | 93.045289 | 11.63066 | 23.31 | <0.0001 |
| Sample | HWP | CHP |
|---|---|---|
| Extraction yield (%) | 5.50 ± 0.62 b | 19.18 ± 0.92 a |
| Total carbohydrate (wt%) | 65.16 ± 6.06 b | 72.67 ± 4.99 a |
| Monosaccharide constituents (molar ratios) (mol%) | ||
| Fuc | 0.33 ± 0.03 b | 0.45 ± 0.00 a |
| Rha | 2.78 ± 0.65 a | 2.88 ± 0.04 a |
| Ara | 19.48 ± 1.62 b | 28.29 ± 0.22 a |
| Gal | 26.39 ± 2.25 a | 27.27 ± 0.12 a |
| Glc | 8.52 ± 0.94 a | 7.30 ± 0.09 b |
| Man | 4.27 ± 0.18 a | 3.34 ± 0.15 b |
| Xyl | 2.49 ± 0.26 a | 2.20 ± 0.16 a |
| Fru | 9.74 ± 1.18 a | 10.94 ± 0.06 a |
| GalA | 12.21 ± 2.55 a | 10.01 ± 0.25 b |
| GlcA | 13.77 ± 4.05 a | 7.44 ± 0.18 b |
| Sample | Fraction | Mw (Da) | Mn (Da) | Mass Fraction (%) | Mw/Mn |
|---|---|---|---|---|---|
| HWP | 1 | 2.558 × 106 (±0.989%) | 9.840 × 104 (±1.380%) | 66.80 | 2.600 (±1.697%) |
| 2 | 3.020 × 105 (±2.967%) | 2.922 × 105 (±3.156%) | 33.20 | 1.034 (±4.332%) | |
| CHP | 1 | 9.016 × 105 (±3.174%) | 6.384 × 105 (±2.997%) | 6.70 | 1.412 (±4.365%) |
| 2 | 5.837 × 104 (±4.434%) | 5.199 × 104 (±5.037%) | 93.30 | 1.123 (±6.711%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.; Liu, Q.; Sun, D.; Wang, Y.; Wang, W.; Liu, D. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method. Foods 2023, 12, 588. https://doi.org/10.3390/foods12030588
Xia B, Liu Q, Sun D, Wang Y, Wang W, Liu D. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method. Foods. 2023; 12(3):588. https://doi.org/10.3390/foods12030588
Chicago/Turabian StyleXia, Bing, Qi Liu, Da Sun, Yang Wang, Wenjun Wang, and Donghong Liu. 2023. "Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method" Foods 12, no. 3: 588. https://doi.org/10.3390/foods12030588
APA StyleXia, B., Liu, Q., Sun, D., Wang, Y., Wang, W., & Liu, D. (2023). Ultrasound-Assisted Deep Eutectic Solvent Extraction of Polysaccharides from Anji White Tea: Characterization and Comparison with the Conventional Method. Foods, 12(3), 588. https://doi.org/10.3390/foods12030588




