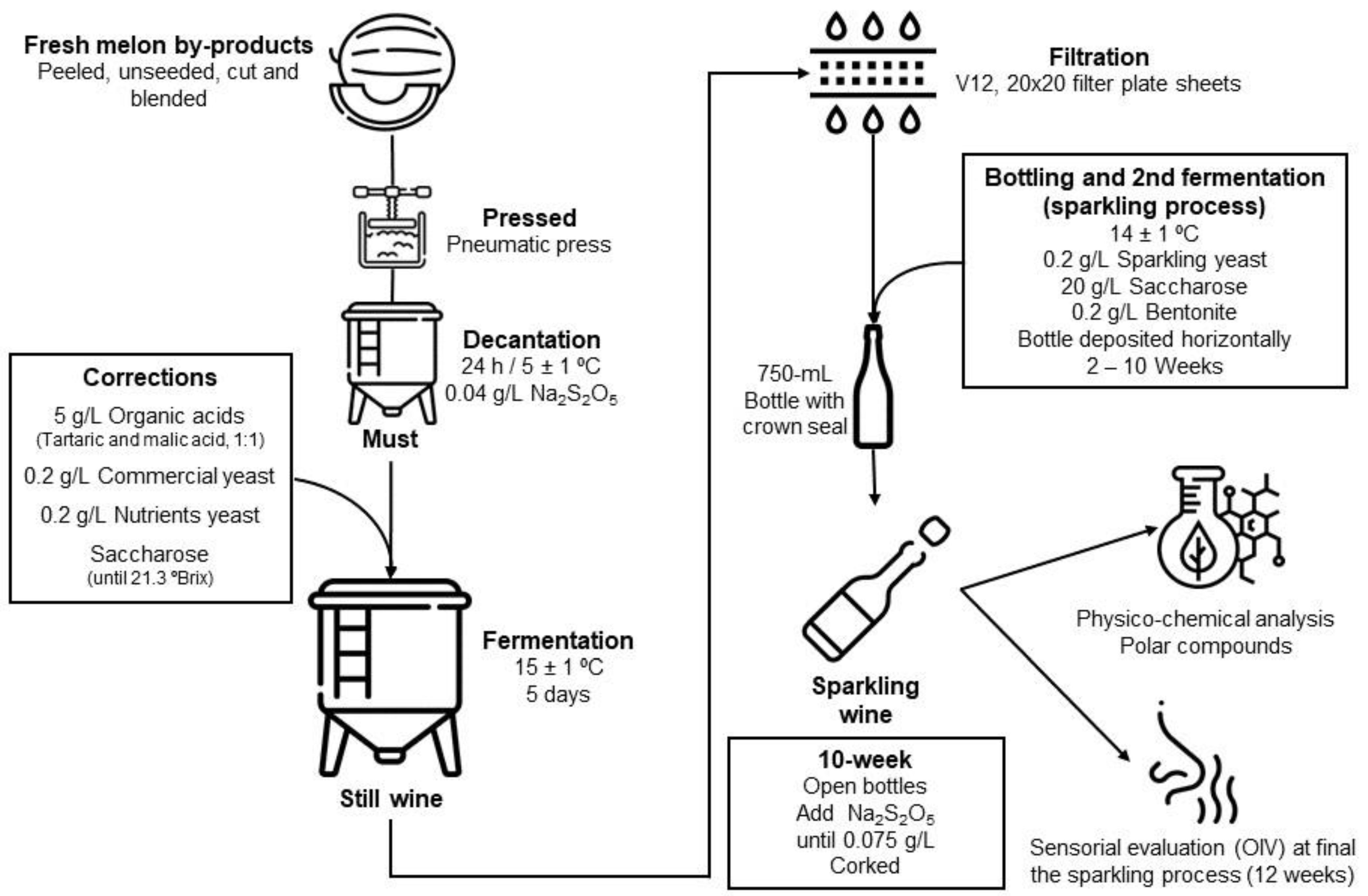
3.1. Physico-Chemical Characterization for First Alcoholic Fermentation (Still Wine) and During the Second Fermentation Process (Sparkling Wine)
Table 3 shows the principal physico-chemical parameters of the must, still wine, and sparkling melon wine. After melon must optimization (
Section 2.1), once commercial saccharose had been added, the TSS reached 21.3 °Brix and 90.55 g/L of residual sugar (as the sum of total individual sugar,
Table 4). The enrichment with tartaric acid and malic acid (5 g/L of 1:1 tartaric and malic acid) provided 6.44 g/L for TA, 0.10 g/L for volatile acidity, and a pH of 4.4. The initial sodium metabisulfite added in the must provided 11.5 mg/L and 25.6 mg/L, free and total SO
2, respectively, and it avoided prior contamination before alcoholic fermentation. The color of the must was defined as a pale greenness (109 °h) resembling the typical green color of the melon pulp.
After the first alcoholic fermentation, the still wine was obtained and TSS dropped to <9 °Brix and remained stable during the sparkling process without significant differences. The alcoholic grade reached 12.3° and was stable in the sparkling melon process (12.3° to 12.5°). Likewise, residual sugar decreased with fermentation, from 90.55 g/L in the must to 5.72 g/L in the still melon wine, and after the incorporation of 20 g/L of commercial saccharose before the second fermentation, the residual sugar decreased to 5.38–10.23 g/L in the sparkling wine; a decrease in the sugar concentration with the storage time was detected.
Regarding the sugar-free extract, the high value obtained in the must (142 g/L) was due to saccharose added and the presence of suspension pulp, dropping with the fermentation process and the filtration process in the still melon wine (25 g/L) and remaining quite stable during the sparkling wine process (20.6 to 25.1 g/L). Sugar-free extract is an important qualitative parameter for evaluating fullness and harmony in wine, this parameter should usually be below 25 g/L [
23], as obtained in the sparkling wine. The pressure reached 2 atm in the second week after second fermentation and remained stable throughout the sparkling process.
TA was maintained in the range of 5.62 to 6.76 g TA/L, without significant differences during the time studied for the sparkling process. As expected, the volatile acidity increased with the first alcoholic fermentation, 0.27 g/L was obtained in the still melon wine, and it ranged from 0.23 to 0.35 g/L during the sparkling process. The pH slightly decreased to 3.9 in the still wine with similar and stable values for the sparkling wines (4.0 to 4.1).
Regarding the color parameters, the first fermentation, induced an increase in the °h (178°) and a slight reduction in L*, with a green color being obtained that was maintained during the studied sparkling process. Chrome decreased from 20.7 (melon must) toward 0.8 to 1.2, which means a decline in the green color intensity, due to the filtration process and its clarification [
32].
The TPC and antioxidant capacities (FRAP and TEAC) in enriched melon must were 173.6 mg GAE/L, 0.94 mmol Fe
+2/L, and 0.47 mmol TE/L, respectively. Fermentation led to a decrease in those values, with 153 mg GAE/L being obtained in the still wine, and the antioxidant capacity was 0.73 mg Fe
+2/L and 0.43 mmol TE/L. These values were similar for the sparkling melon wine during the eight weeks of storage, excepting the end of the storage time when an increase in TPC (182 mg GAE/L) and antioxidant activity (1.15 mg Fe
+2/L and 0.78 mmol TE/L) was found, probably due to the interference of metabisulfite as an antioxidant agent [
33], which was added in week 10.
3.3. Volatile Compounds in Melon Wines and Sparkling Wine
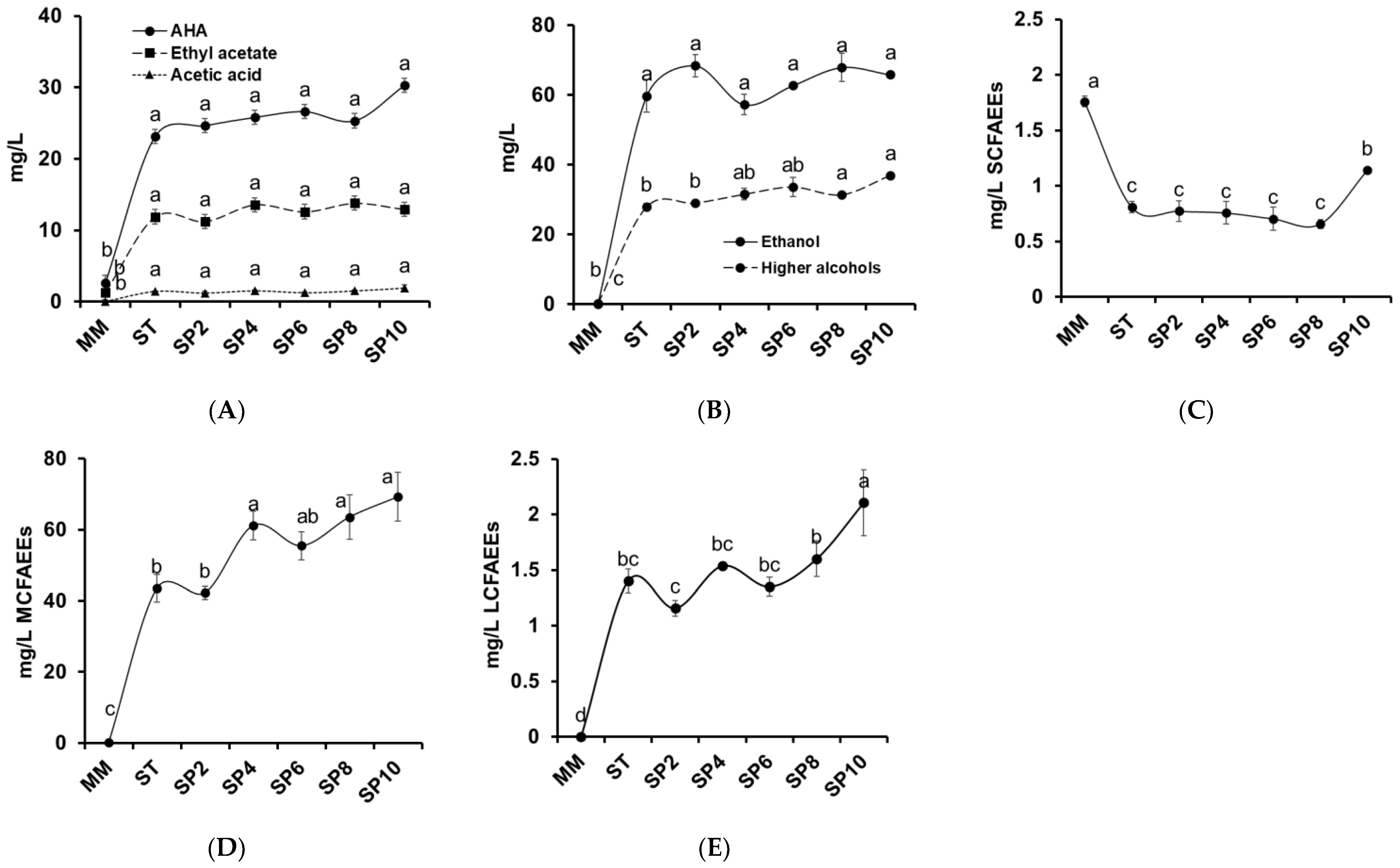
The volatile compounds identified in these melon wines were classified as: ethyl acetate, acetic acid, acetate higher alcohols (AHA), short-chain (C3-C5) fatty acid ethyl ester (SCFAEE), medium-chain (C6-C12) fatty acid ethyl ester (MCFAEE), long-chain (C13-C22) fatty acid ethyl ester (LCFAEE), other esters, ethanol, higher alcohols (HA), and other compounds. The aroma evolution during the first alcoholic fermentation and the sparkling process is shown in
Figure 2, except for other ester and other compound groups which can be consulted in
Table 6.
Acetate esters and acetic acid are presented in
Figure 2A. Initially, the content of ethyl acetate in the must was low (1.30 mg/L) compared to its increment in the first alcoholic fermentation (11.88 mg/L) and stabilization during the sparkling process (11.22 to 13.79 mg/L). These acetate esters, common in wine, are responsible for the negative effect on flavor and spoilage character when they exceed 150 to 200 mg/L [
40], far higher than our values. However, acetate esters under 80 mg/L concentration may improve the aroma of wine [
41]. Acetic acid was not detected in must but appeared with the fermentations, 1.23 to 1.95 mg/L.
AHA increased from 2.57 mg/L in the must to 23.14 in the still wine and to 27.81 mg/L in the sparkling wine (10 weeks). Isoamyl acetate was the principal AHA
Table 6, representing around 60% of all AHA compound. In wines, this compound is responsible for providing a fruity character in the wine aroma [
42]. Zhang et al. [
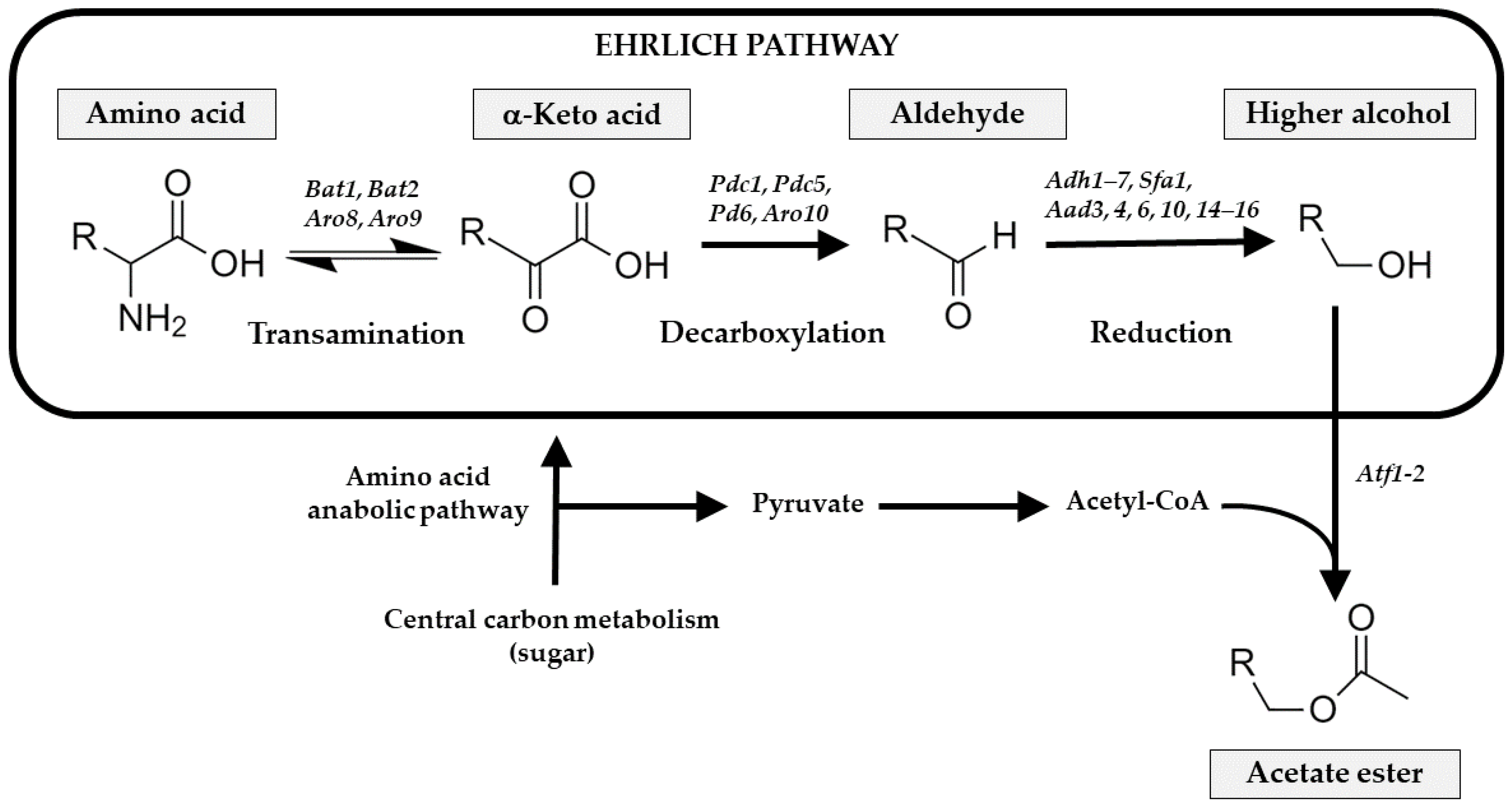
43] found that
S. cerevisiae catabolites the leucine into 3-methyl-butyraldehyde and isoamyl alcohol, via the Ehrlich pathway
Figure 3, and the final esterification step with active acetic acid or acetyl-CoA to isoamyl acetate; its yields are regulated by the initial concentration of leucine. Our results follow that trend, with an increased concentration of isoamyl acetate, showing a negative correlation with the leucine concentration.
The next principal AHA compounds were 2-phenylethyl acetate and isobutyl acetate, which increased during the first fermentation (3.84 mg/L) and the sparkling process (5.13 mg/L). As with the isoamyl acetate and leucine correlation, a similar scenario occurred with 2-phenylethyl acetate and phenylalanine, which
S. cerevisiae catabolized into 2-phenylethanol and 2-phenylethyl acetate [
30], and this metabolic trend is shown in the decrement of phenylalanine and the increment in its alcoholic and acetate metabolites. Another AHA that increased during the first alcoholic fermentation was 3,6-nonadien-1-yl acetate, probably derived from 3,6-nonadien-1-ol due to the activity of alcohol acetyltransferases, which catalyzed alcohols and acetyl-coenzyme A for the formation of acetate ester [
44].
Ethanol and higher alcohols are presented in
Figure 2B. The melon wine fermentations converted sugar into alcohol; an ethanol concentration of 59.73 mg/L was obtained in the still wine, and it reached 65.83 mg/L in the sparkling wine (10 weeks).
Higher alcohols also increased with a slight rise during the sparkling process, principally isoamyl alcohol, derived from the deamination, decarboxylation, and reduction of leucine as explained above, via the Ehrlich pathway. Isoamyl alcohol was not detected in the must
Table 6 and developed with the first fermentation and remained stable throughout the sparkling processes (18.70 to 24.17 mg/L). 2-Phenylethanol was the third most abundant higher alcohol, derived from phenylalanine, and ranged from 4.71 mg/L in the still wine to 8.16 mg/L in the sparkling processes (10 weeks). Isobutyl alcohol and 2,3-butanediol were identified with alcoholic fermentation. As reported by Wess et al. [
45],
S. cerevisiae metabolizes valine amino acid by the Ehrlich pathway to produce isobutyl alcohol in the cytosol. Another alcohol that competes with the ethanol pathway is 2,3-butanediol which, from 2-acetolactate after a spontaneous decarboxylation and followed by oxidation by butanediol dehydrogenases, is reduced to 2,3-butanediol [
45]; this was detected in very low concentrations in both fermentation processes. Another higher alcohol identified was (E,Z)-3,6-nonadien-1-ol, which was found in the must, as reported in other melon cultivars [
46] and doubled its concentration with fermentations.
An aromatic higher alcohol, 2,4-di-tert-butylphenol increased from 1.75 to 2.35 mg/L after the first alcoholic fermentation, probably due to the oxidation of 1,3-di-tertbutyl-benzene, which was also identified
Table 6.
Fatty acid ethyl esters
Figure 2C–E were produced by
S. cerevisiae during the first alcoholic fermentation and sparkling process and determined the complex flavor and fruity aroma, obtained from the condensation of acyl-CoA with ethanol, catalyzed by ethanol-O-acyl transferase [
47].
The most abundant group of ethyl esters was MCFAEEs, followed by LCFAEEs. Both groups increased during the first fermentation and sparkling process, ranging from 43.25 to 69.23 mg/L and 1.16 to 2.11 mg/L, respectively. In contrast, SCFAEEs decreased with the first fermentation (0.81 mg/L) and showed a slight increase at the end of the studied sparkling process (1.13 mg/L). Five SCFAEEs were identified in the must
Table 6, principally ethyl 2-methyl butyrate and ethyl butanoate, followed by ethyl isobutyrate, ethyl propanoate, and ethyl pentanoate. These last three compounds decreased to a non-detected limit after the first alcoholic fermentation. However, ethyl 2-methyl butyrate decreased from 0.77 mg/L in the must to 0.17–0.32 mg/L after the first fermentation and sparkling process. Ethyl butanoate ranged from 0.46 to 0.68 mg/L during the experiment, increasing to 0.82 mg/L after 10 weeks. Generally, ethyl 2-methyl butyrate and ethyl butanoate are commonly identified in a large range of white and rosé wines fermented with
S. cerevisiae [
48].
Regarding MCFAEEs, five ethyl esters were identified throughout the experiment, with a gradual increase during the first alcoholic fermentation and sparkling process, with the maximum concentration being obtained at the end of the experiment (69.23 mg/L)
Figure 2D. Ethyl hexanoate was the only compound identified in the must. Instead, the first alcoholic fermentation provided the formation of new aromas such as ethyl octanoate, ethyl decanoate, ethyl hexanoate, ethyl 9-decenoate and ethyl laurate, with octanoate and decanoate being the compounds found in the highest concentrations. All these MCFAEE volatile compounds have been identified in other alcoholic fermentative beverages such as wines from grapes [
48,
49] and kiwi wines [
6].
Concerning LCFAEEs, ethyl tetradecanoate and ethyl hexadecanoate were not identified in the must but developed with alcoholic fermentation. The concentration of these compounds was low, with a stable trend throughout the sparkling process (0.57 to 0.75 mg/L, and 0.59–1.39 mg/L, respectively)
Table 6. Both compounds have been reported in other sparkling grape wines [
50] and fruit-based wines such as pomegranate and kiwi [
6,
51].
3.4. Odor Activity Value (OAV) and Relative Odor Contribution (ROC)
Table 7 shows the 15 individual volatile compounds which had at least OAV > 1 and contributed to the aroma of the melon-based wine.
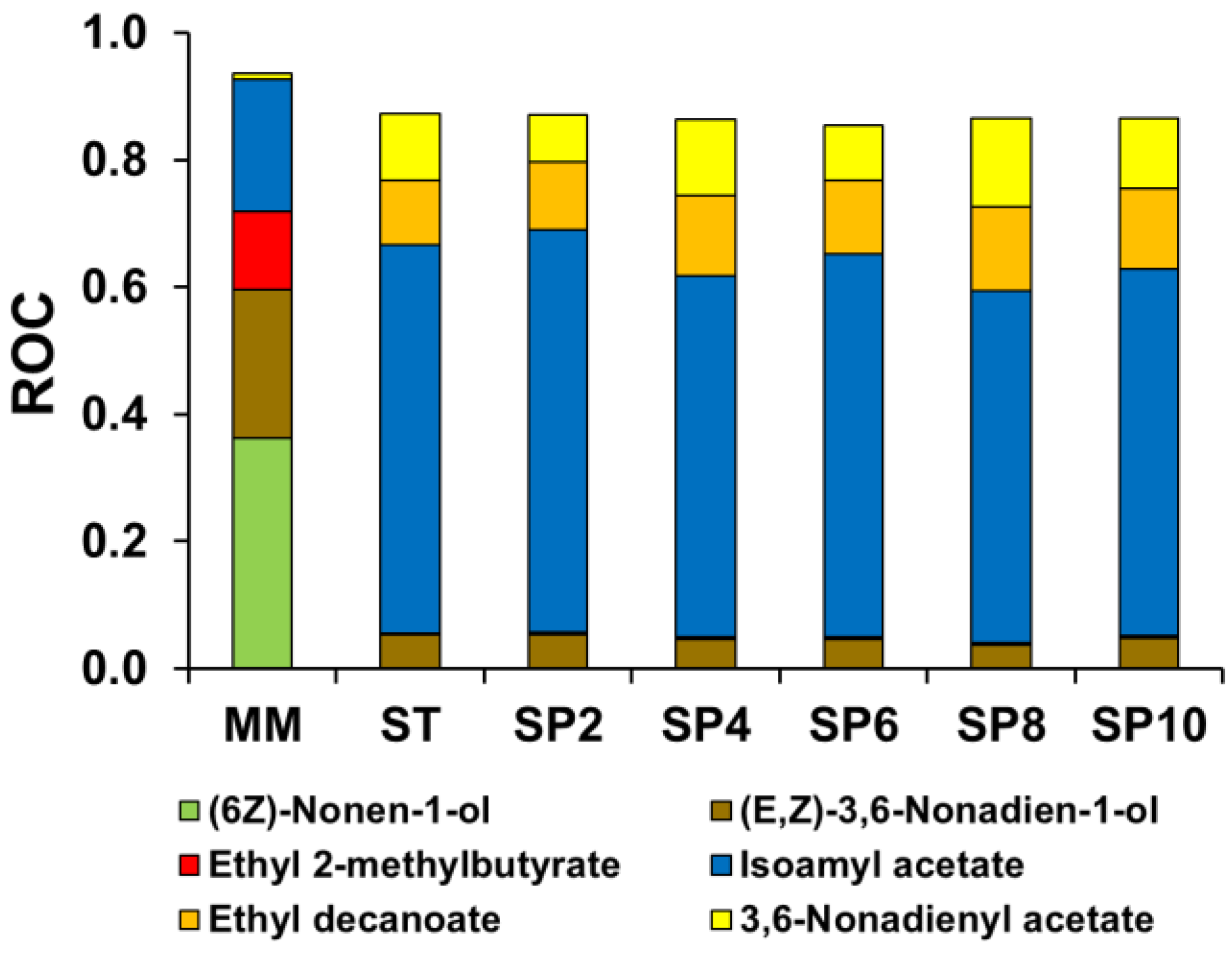
Figure 4 illustrates the principal aromas with a ROC value above 0.1. In the AHA groups, only four compounds exceeded the odor threshold: isobutyl acetate, isoamyl acetate, 3,6-nonadienyl acetate, and 2-phenylethyl acetate. As mentioned before, these compounds increased with the first alcoholic fermentation and sparkling process. Isoamyl acetate, followed by 3,6-nonadienyl acetate, were the AHA compounds with the highest OAV values in the must, with an increase in the alcoholic fermentations providing sweet banana and fruity odors in the still and sparkling wines
Table 7. Isoamyl acetate, with an initial (must) OAV value of 17.7, increased to 481 in the still wine and 647 at the end of the sparkling process and its ROC increased from 0.2 (must) to 0.55–0.61, during the first alcoholic fermentation and the sparkling process, meaning that the aroma was a major contribution to the melon-based wine.
Regarding the SCFAEEs, two volatile compounds contributed to the aroma of the melon-based wines: ethyl butanoate and ethyl 2-methyl butyrate. The first aroma was detected in the must (1.1), the still wine (1.1) and at the end of the sparkling process (1.4). Ethyl 2-methyl butyrate decreased its OAV value from 10.5 in the must to 2.3 in the still wine and increased to 4.3, at the end of the sparkling period studied. Both aromas provide a sweet and fruity connotation; however, only ethyl 2-methylbutyrate reached a ROC > 0.1 value in the must, after which other aromas became more relevant
Figure 4.
For the MCFAEEs, the trend of these fatty acid ethyl esters was, after AHA, the group with the largest increment in OAV; it increased with the fermentation process, and its evolution followed a similar pattern to other grape and fruit wines [
5,
52]. Ethyl hexanoate was the only MCFAEEs, with OAV > 1, present in the must (1.9), which increased with the fermentations and remained stable, ranging from 20.7 to 24.1. However, the compounds with high OAV were ethyl decanoate (78.9 to 141.9), whose odor description corresponds to sweet, fruity, nuts, and dried fruit, and ethyl octanoate (34.1 to 74.3) which provides pineapple, pear, and a soapy odor. The highest levels were obtained at the end of the sparkling process. However, only ethyl decanoate managed to contribute a ROC > 0.1, between 0.10 to 0.13, in the still and sparkling wines. Ethyl 9-decenoate had a value between 11.2 to 18.9 after alcoholic fermentations, providing a floral odor with a ROC > 0.1
Figure 4. For LCFAEEs group, only ethyl tetradecanoate had an OAV > 1 after first fermentation (1.5) and ranged 1.10—1.50 during the sparkling process. Regarding ethyl hexadecanoate, it only reached an OAV > 1 with 1.39 at 10 weeks of the sparkling process. Both volatile aromas contributed a fruity and fatty odor but with low contribution to the aroma of the still and sparkling melon wine (ROC > 0.1).
(6Z)-Nonen-1-ol and (E,Z)-3,6-nonadien-1-ol are common aromas in some melon cultivars [
46], and they were the only HA that reached their odor threshold with a melon-like and sweet odor description in the initial must (32.2 and 20.2, respectively), and with the highest ROC values of 0.4 and 0.23, respectively. After the first alcoholic fermentation, the contribution of (6Z)-nonen-1-ol disappeared but that of (E,Z)-3,6-nonadien-1-ol increased, with an OAV value of 33.6–56.44 during the experiment, with an ROC value of around 0.05.
3.5. Sensory Evaluation of Final Sparkling Wine
The sensory evaluation of the sparkling melon, after 10 weeks in the bottle, is shown in
Table 8. In accordance with the categories described by OIV (2009), this sparkling melon-based wine, obtained a total mark of 92.11 points, after the sum of all markers (visual, nose, taste, and harmony), achieving the “Grand Gold” category (>92 points). Considering visual aspects (14.44), the judges rated the sparkling wine as limpid to excellent limpidity, which means a good visual impression without cloudiness. Regarding the sensation perceived in the nose, melon-based wine had a very total absence of defects in genuineness (5.78), very strong qualitative intensity (7.78), and a very-to-excellent impression of quality (15.11). These results refer to the intensity of OAV
Table 7, such as isoamyl acetate, ethyl decanoate, 3,6-nonadienyl acetate and (E,Z)-3,6-nonadien-1-ol, which are the most relevant volatile aromas providing the highest ROC values that contributed to the complexity and fruity-aroma character in this sparkling melon wine. Concerning taste values (39.11), sparkling melon wine was characterized as having a total absence of defects in genuineness, strong intensity (7.11), a very good persistence of residual olfactory-gustatory sensation of flavors (7.22), and a very good impression for the taste quality (19.33). These results suggest that this melon wine is absent in oxidation flavor, volatile acidity, and a negative flavor that affects the taste and mouthfeel. Finally, the judges marked a very good general impression (9.89), which along with the total marks received, defined our sparkling melon-based wine as a potential marketable and novel product.