Protein-Based High Internal Phase Pickering Emulsions: A Review of Their Fabrication, Composition and Future Perspectives in the Food Industry
Abstract
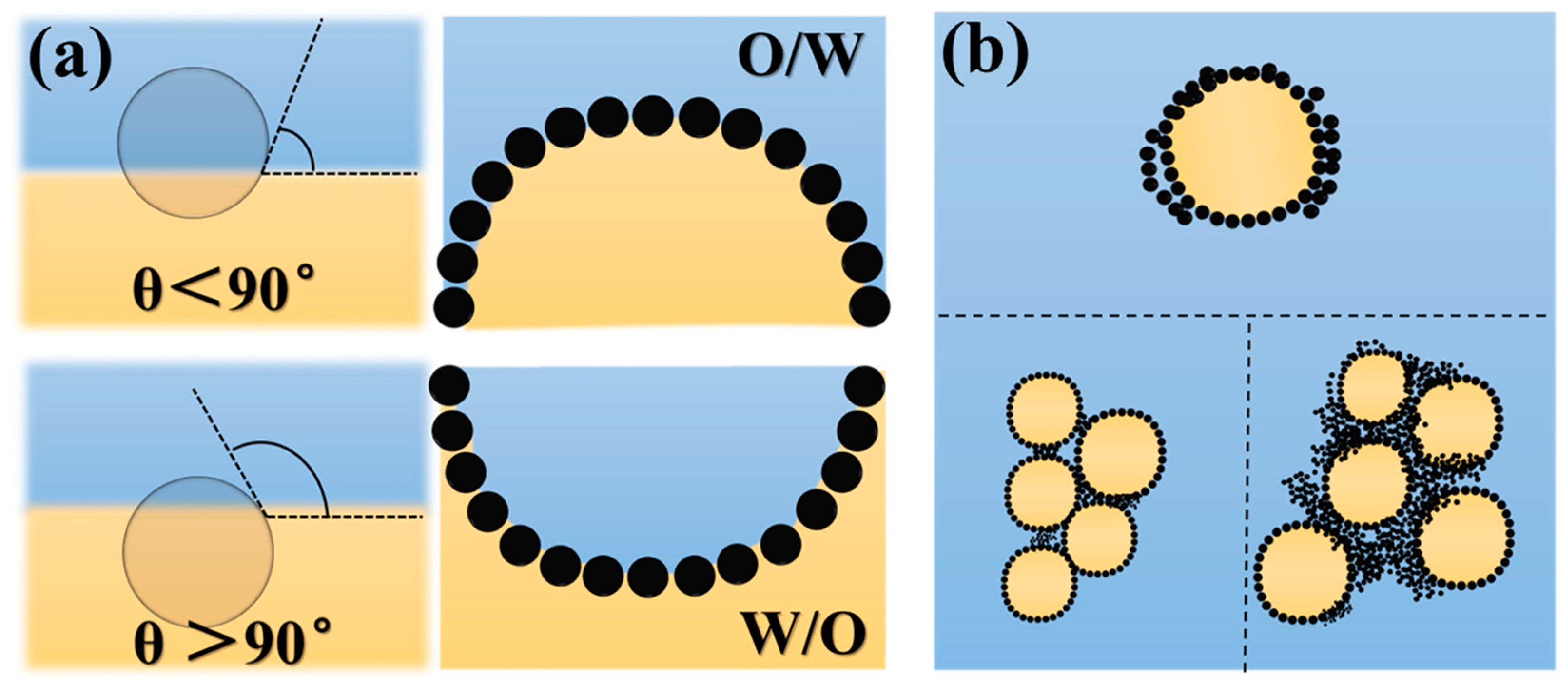
1. Introduction
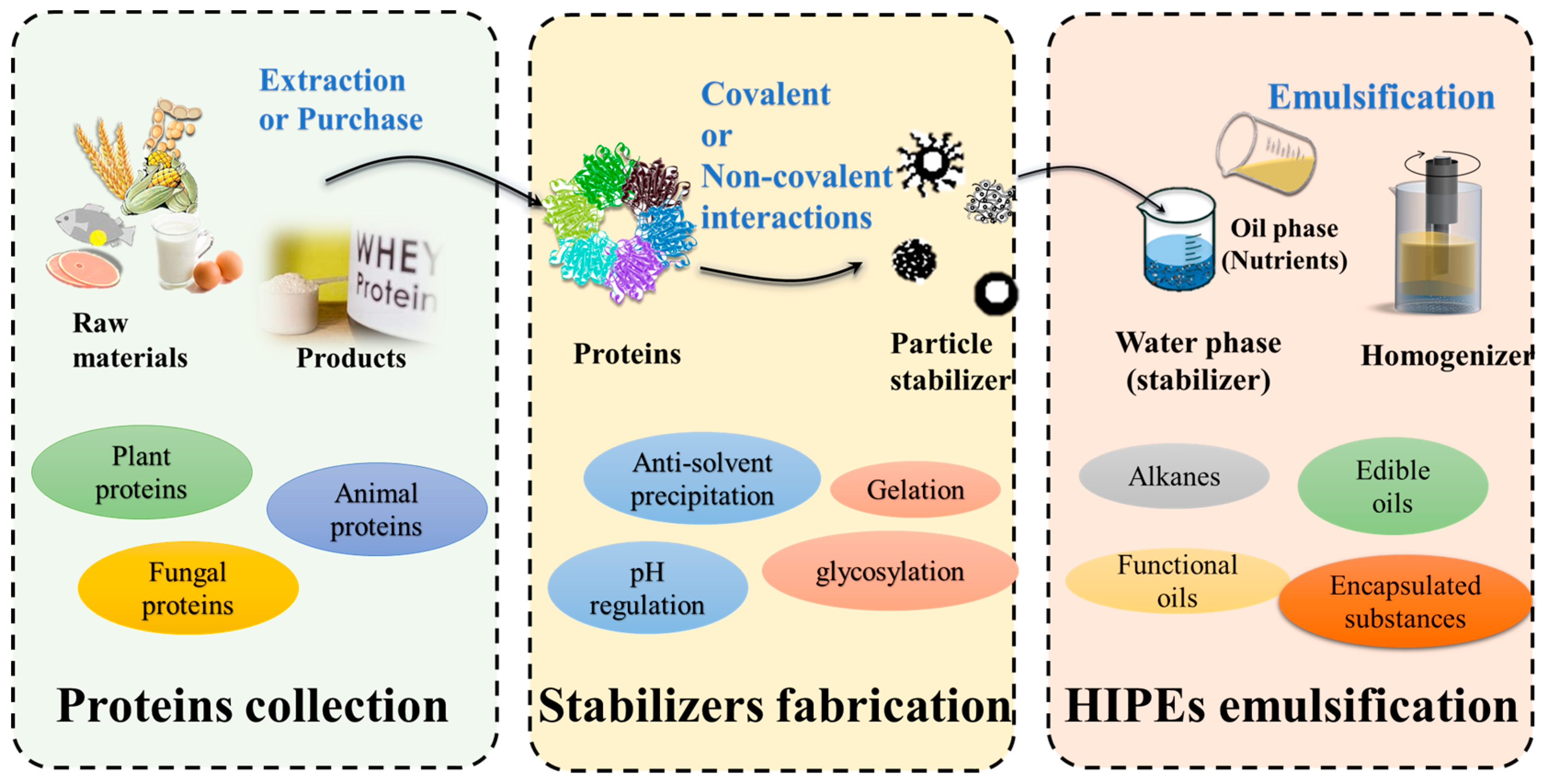
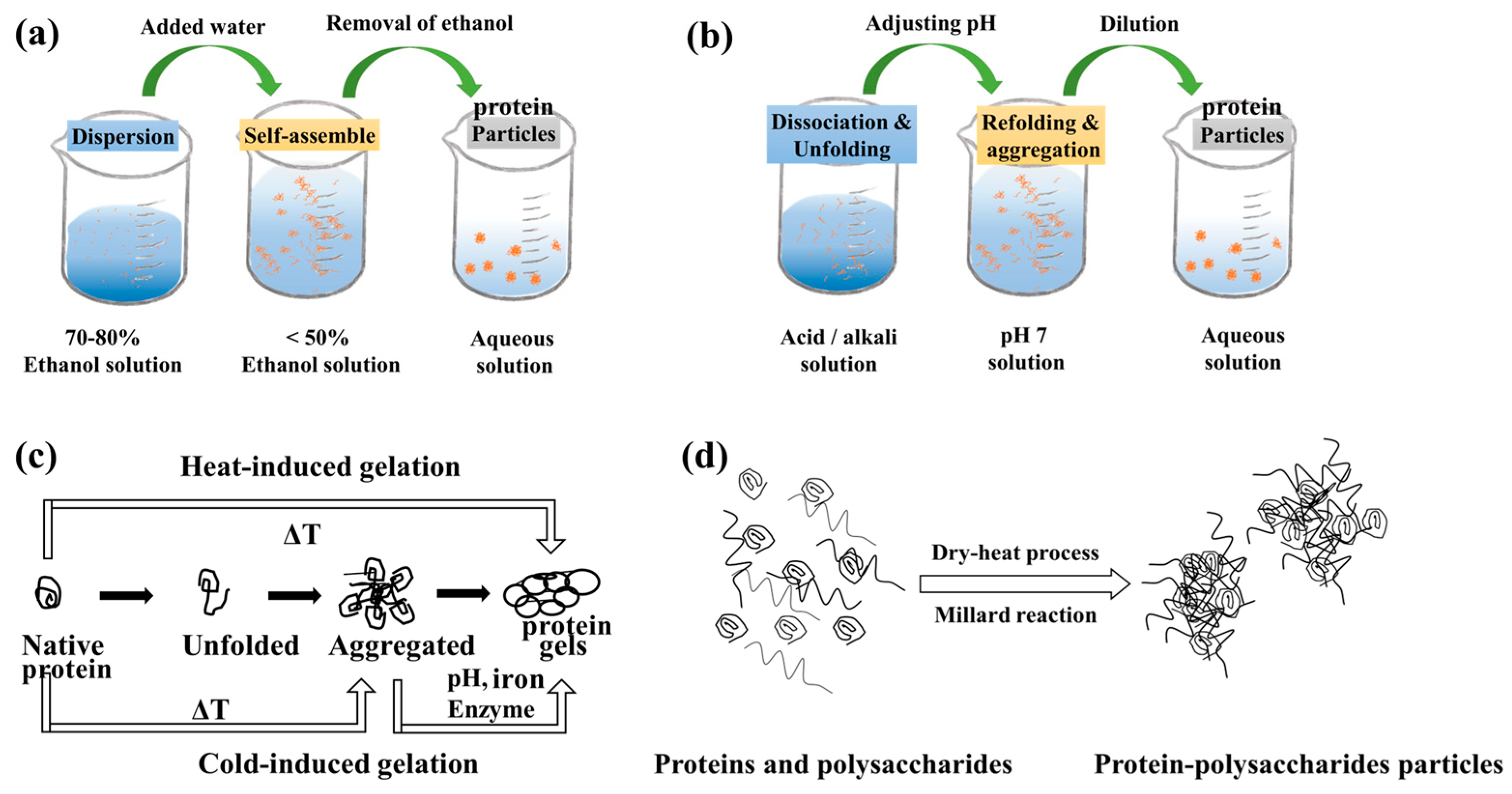
2. Preparation Route for Protein-Based HIPEs
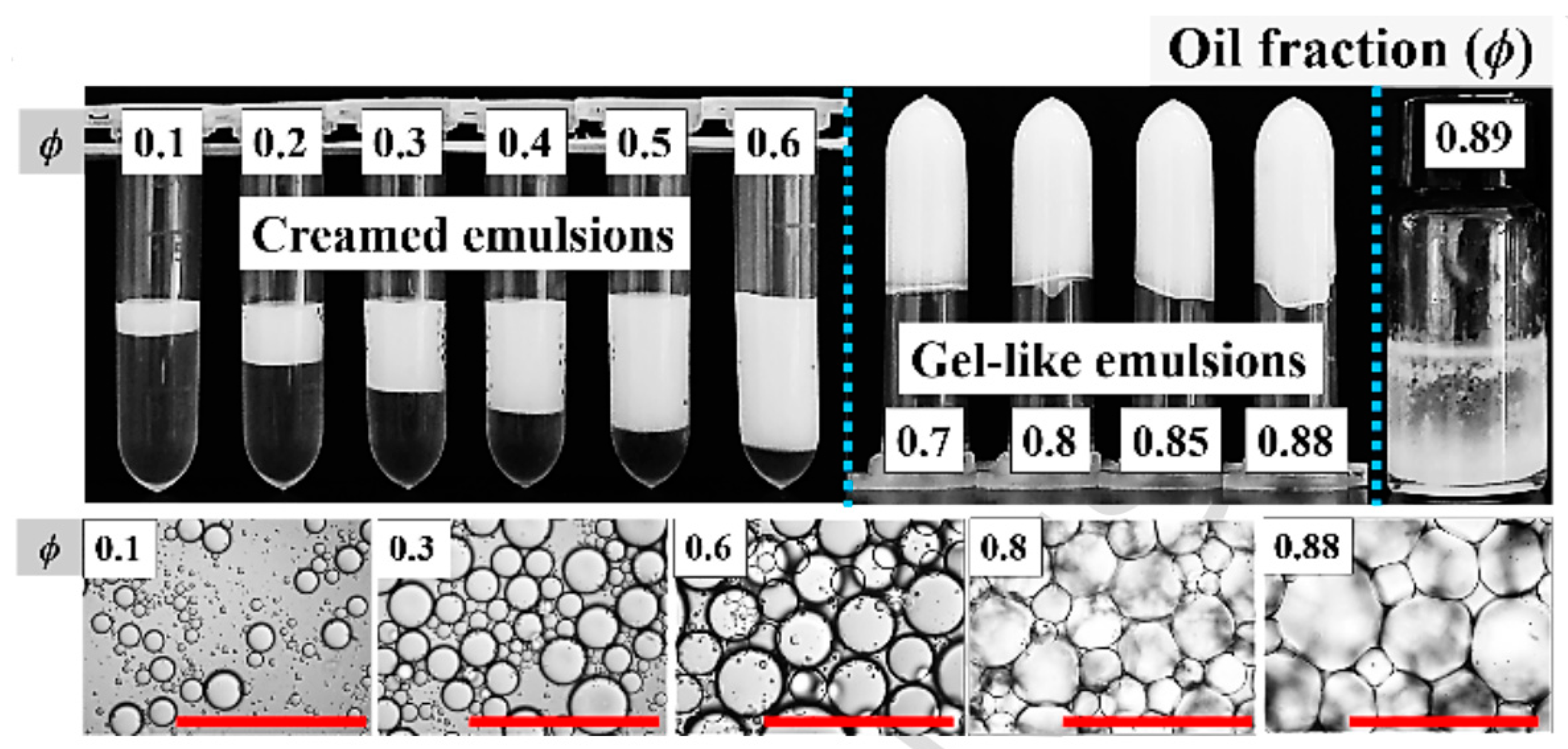
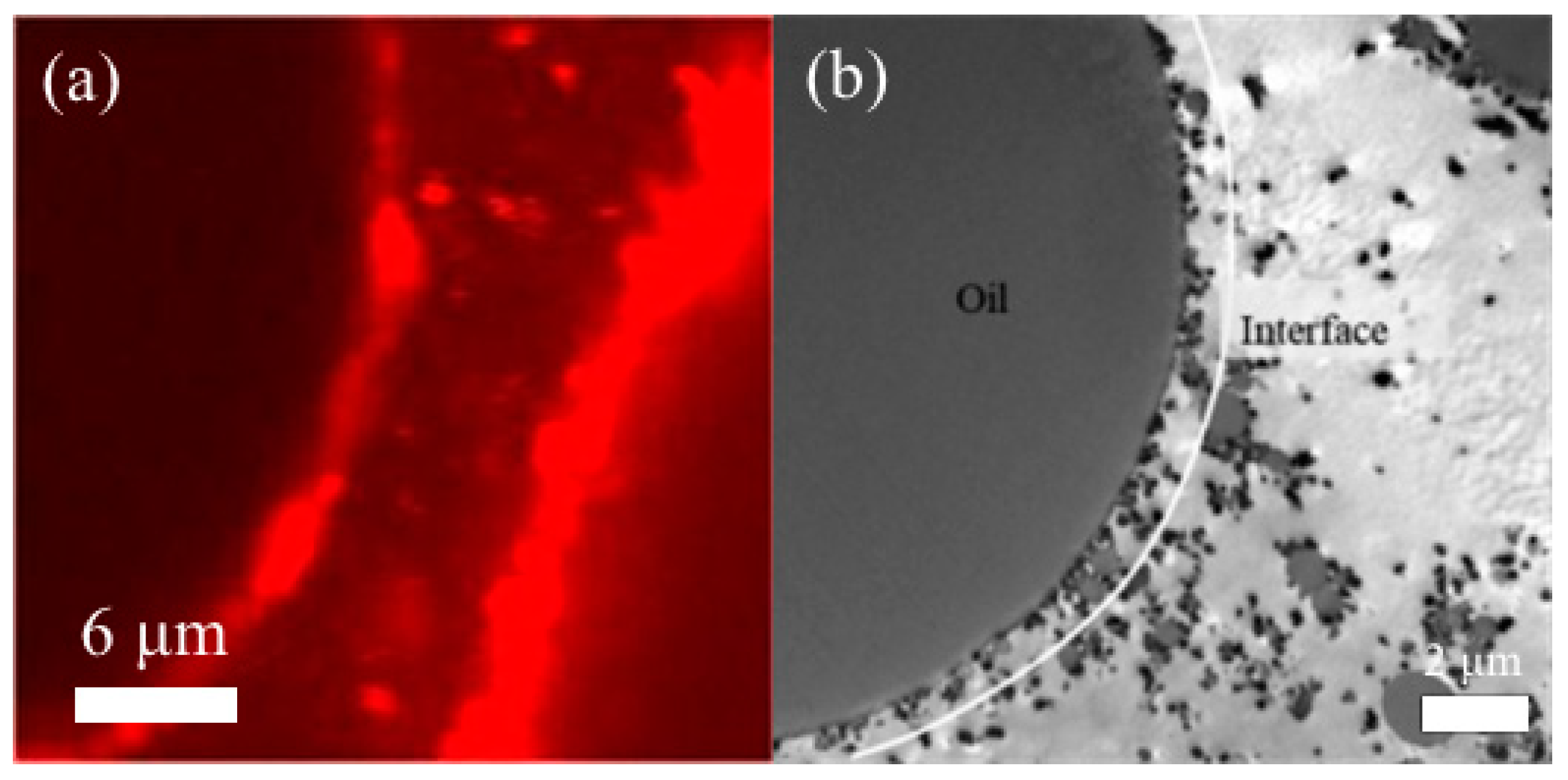
3. Microscopic Characteristics and Stability of HIPEs
4. Fabrication of Protein-Based HIPE Stabilizers
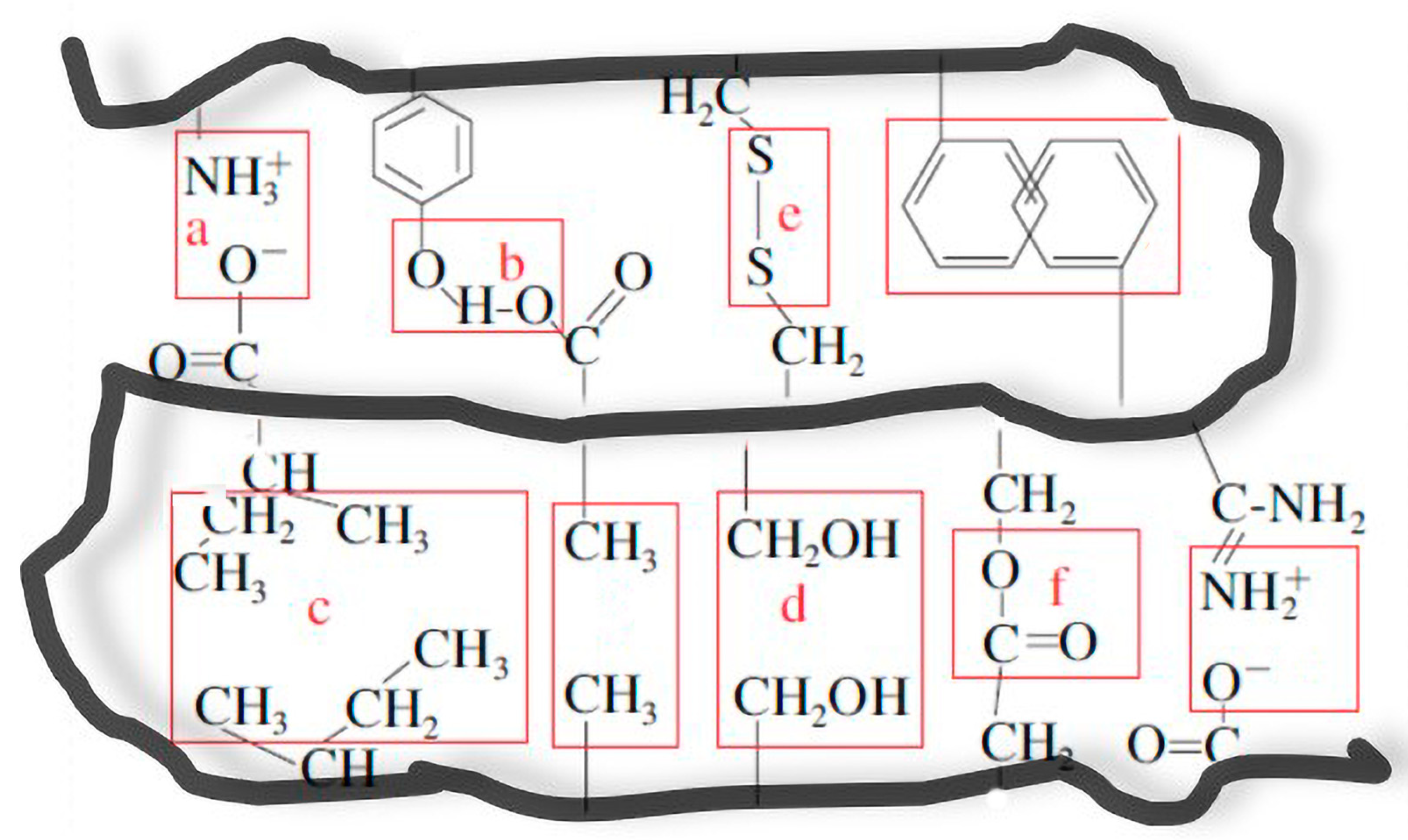
4.1. Non-Covalent Interactions
4.2. Covalent Interactions
5. Critical Parameters of Stabilizers
6. Exploration of Protein-Based Stabilizers
6.1. Plant Proteins
6.2. Animal Proteins
6.3. Fungal Proteins
7. Other Ingredients in Protein-Based HIPEs
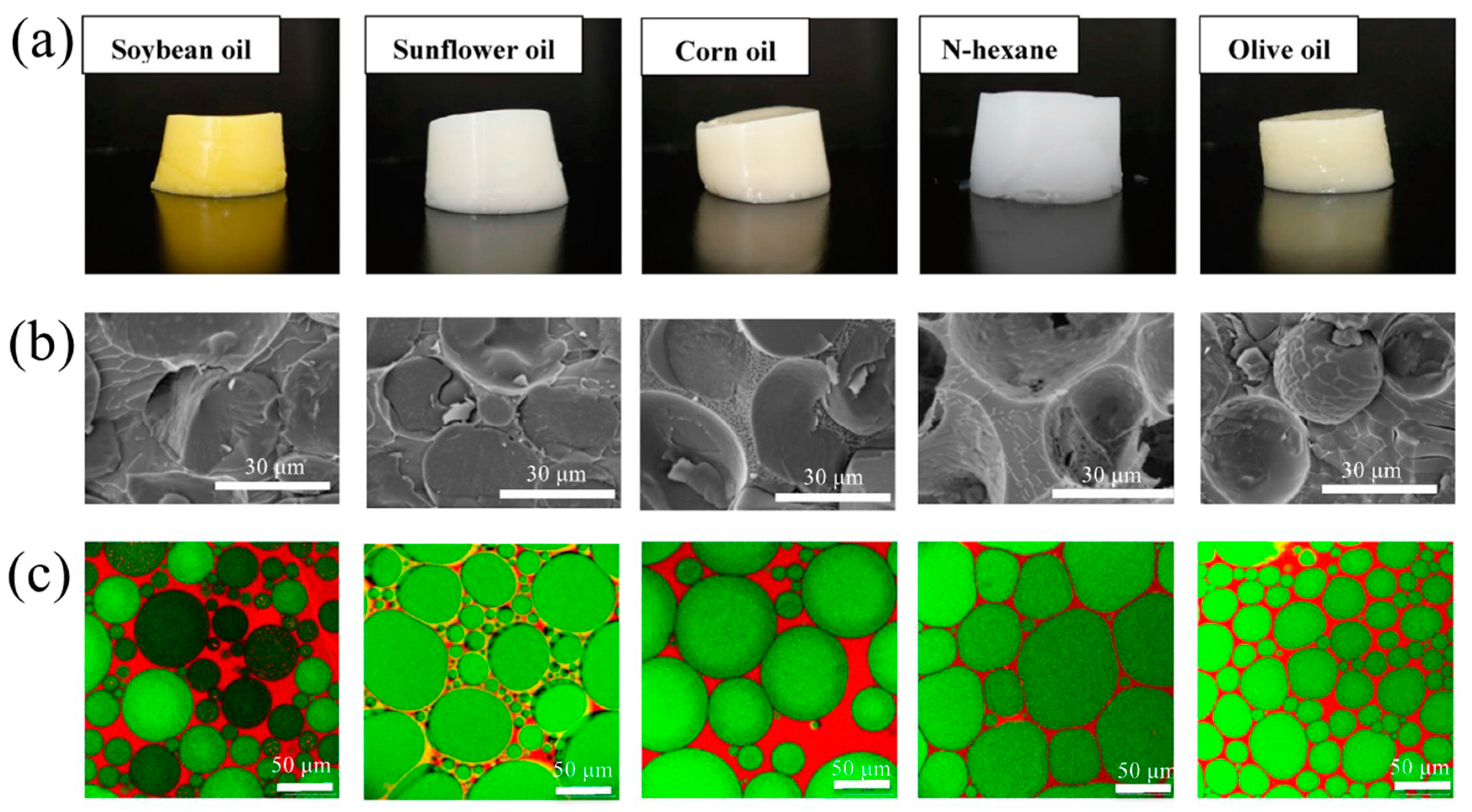
7.1. Oil Phase
7.1.1. Hydrocarbons
7.1.2. Edible Oil
7.2. Encapsulated Substances
8. Application in the Food Field
8.1. Fat Substitutes
8.2. Delivery of Nutrients
8.3. Detergent
8.4. Assistance with Other Delivery Systems
9. Future Perspectives
9.1. Development of Protein-Based Stabilizers
9.2. Improvement of Emulsification Technology for HIPEs
9.3. Promoting the Research of HIPE Application in Foods
9.4. Quality Control of Stabilizers and Protein-Based HIPEs
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, P.A. Food Emulsions: Principles, Practice, and Techniques. Int. J. Food Sci. Tech. 2001, 36, 223–224. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid. Interface Sci. 2020, 277, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Zhao, Q.; Zaaboul, F.; Liu, Y.; Li, J. Recent advances on protein-based Pickering high internal phase emulsions (Pickering HIPEs): Fabrication, characterization, and applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1934–1968. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, G.H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Linke, C.; Drusch, S. Pickering emulsions in foods—Opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 1971–1985. [Google Scholar] [CrossRef]
- Lissant, K.J. The geometry of high-internal-phase-ratio emulsions. J. Colloid Interface Sci. 1966, 22, 462–468. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering. 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Hao, Z.Z.; Peng, X.Q.; Tang, C.H. Edible pickering high internal phase emulsions stabilized by soy glycinin: Improvement of emulsification performance and pickering stabilization by glycation with soy polysaccharide. Food Hydrocoll. 2020, 103, 105672. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, M.; Zhao, X.; Li, Y.; Qi, B.; Jiang, L. Stability and digestion characteristics of pickering high internal phase emulsions formed by acid-induced soy lipophilic protein, β-conglycinin, and globulin. LWT-Food Sci. Technol. 2022, 153, 112554. [Google Scholar] [CrossRef]
- Lim, H.N.; Kassim, A.; Huang, N.M.; Radiman, S.; Yarmo, M.A.; Yeong, S.K.; Khiew, P.S.; Chiu, W.S. Three-Component Olive Oil-In-Water High Internal Phase Emulsions Stabilized by Palm Surfactant and Their Moisturizing Properties. J. Dis-pers. Sci. Technol. 2009, 31, 95–101. [Google Scholar] [CrossRef]
- Bai, Y.G.; Pei, X.P.; Zhao, B.; Xu, K.; Zhai, K.K.; Wang, C.; Zhang, F.; Tan, Y.; Zhang, B.C.; Wang, Y.C.; et al. Multiple pickering high internal phase emulsions stabilized by modified diatomite particles via one-step emulsification process. Chem. Eng. Sci. 2020, 212, 115341. [Google Scholar] [CrossRef]
- Yang, K.; Kang, Y.Y.; Ahn, H.J.; Kim, D.G.; Park, N.K.; Choi, S.Q.; Won, J.C.; Kim, Y.H. Porous boron nitride/polyimide composite films with high thermal diffusivity and low dielectric properties via high internal phase Pickering emulsion method. J. Ind. Eng. Chem. 2020, 82, 173–179. [Google Scholar] [CrossRef]
- Wang, C.; Pei, X.; Tan, J.; Zhang, T.; Zhai, K.; Zhang, F.; Bai, Y.; Deng, Y.; Zhang, B.; Wang, Y.; et al. Thermoresponsive starch-based particle-stabilized Pickering high internal phase emulsions as nutraceutical containers for controlled release. Int. J. Biol. Macromol. 2020, 146, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.; Du, M.; Lin, S.Y.; Xu, X.B.; Yu, P. In-situ dispersion of casein to form nanoparticles for Pickering high internal phase emulsions. LWT-Food Sci. Technol. 2021, 139, 110538. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.T.; Tang, C.H. Novel edible pickering high-internal-phase-emulsion gels efficiently stabilized by unique polysaccharide-protein hybrid nanoparticles from Okara. Food Hydrocoll. 2020, 98, 105285. [Google Scholar] [CrossRef]
- Zhu, C.P.; Zhang, H.H.; Huang, G.Q.; Xiao, J.X. Whey protein isolate-low methoxyl pectin coacervates as a high internal phase Pickering emulsion stabilizer. J. Dispers. Sci. Technol. 2021, 42, 1009–1020. [Google Scholar] [CrossRef]
- Liu, Y.K.; Yan, C.; Chen, J.; Wang, Y.; Liang, R.H.; Zou, L.Q.; McClements, D.J.; Liu, W. Enhancement of beta-carotene stability by encapsulation in high internal phase emulsions stabilized by modified starch and tannic acid. Food Hydrocoll. 2020, 109, 106083. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.H.; Wang, Y.; Chen, J.; Zhong, Y.J.; Liang, R.H.; Peng, S.F.; McClements, D.J.; Liu, W. Tunable high internal phase emulsions (HIPEs) formulated using lactoferrin-gum Arabic complexes. Food Hydrocoll. 2021, 113, 106445. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, L.; Tian, S.; Wen, H.; Gou, X.; Ngai, T. Gelatin Particle-Stabilized High-Internal Phase Emulsions for Use in Oral Delivery Systems: Protection Effect and in Vitro Digestion Study. J. Agric. Food Chem. 2017, 65, 900–907. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.; McClements, D.J.; Zhou, L.; Wu, J.; Zou, L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocoll. 2019, 88, 210–217. [Google Scholar] [CrossRef]
- Yan, C.; McClements, D.J.; Zou, L.; Liu, W. A stable high internal phase emulsion fabricated with OSA-modified starch: An improvement in beta-carotene stability and bioaccessibility. Food Funct. 2019, 10, 5446–5460. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Qian, Y.; Wang, C.; Yang, D.; Qiu, X.; Binks, B.P. Tumor microenvironment-responsive, high internal phase Pickering emulsions stabilized by lignin/chitosan oligosaccharide particles for synergistic cancer therapy. J. Colloid Interface Sci. 2021, 591, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Z.; Yu, X.H.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Fabrication and Characterization of Novel Water-Insoluble Protein Porous Materials Derived from Pickering High Internal-Phase Emulsions Stabilized by Gliadin-Chitosan-Complex Particles. J. Agric. Food Chem. 2019, 67, 3423–3431. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Z.L.; Zhu, J.Y.; Yin, S.W.; Tang, C.H.; Wu, L.Y.; Yang, X.Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef]
- Zong, Y.; Kuang, Q.; Liu, G.; Wang, R.; Feng, W.; Zhang, H.; Chen, Z.; Wang, T. All-natural protein-polysaccharide conjugates with bead-on-a-string nanostructures as stabilizers of high internal phase emulsions for 3D printing. Food Chem. 2022, 388, 133012. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, X.; Li, T.; Zhou, J.; Yang, Y.; Bian, X.; Wang, L. High internal phase emulsions stabilized solely by sonicated quinoa protein isolate at various pH values and concentrations. Food Chem. 2022, 378, 132011. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, R.; Feng, W.; Chen, Z.; Wang, T. High internal phase Pickering emulsions stabilized by co-assembled rice proteins and carboxymethyl cellulose for food-grade 3D printing. Carbohydr. Polym. 2021, 273, 118586. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Ding, J.; Ni, X.; Li, C.; Wang, J.; Yang, C. High ethanol tolerance of oil-in-water Pickering emulsions stabilized by protein nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2022, 632, 127777. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, Y.; Jin, H.; Sui, X.; Jiang, L. Deciphering the Structural Network That Confers Stability to High Internal Phase Pickering Emulsions by Cross-Linked Soy Protein Microgels and Their In Vitro Digestion Profiles. J. Agric. Food Chem. 2020, 68, 9796–9803. [Google Scholar] [CrossRef]
- Gong, X.H.; Rohm, K.; Su, Z.H.; Zhao, B.R.; Renner, J.; Manas-Zloczower, I.; Feke, D.L. Porous hydrogels templated from soy-protein-stabilized high internal phase emulsions. J. Mater. Sci. 2020, 55, 17284–17301. [Google Scholar] [CrossRef]
- Jiao, B.; Shi, A.; Wang, Q.; Binks, B.P. High-Internal-Phase Pickering Emulsions Stabilized Solely by Peanut-Protein-Isolate Microgel Particles with Multiple Potential Applications. Angew. Chem. Int. Ed. 2018, 57, 9274–9278. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, L.; Yang, F.; Yao, J.; Ma, Y.; Liu, J. Construction of high internal phase Pickering emulsions stabilized by bamboo fungus protein gels with the effect of pH. Food Chem. 2022, 369, 130954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Gao, G.; Wang, H.; He, X.; Chen, T.; Ke, L.; Rao, P.; Wang, Q. Boiling Licorice Produces Self-Assembled Protein Nanoparticles: A Novel Source of Bioactive Nanomaterials. J. Agric. Food Chem. 2019, 67, 9354–9361. [Google Scholar] [CrossRef]
- Ma, L.; Zou, L.Q.; McClements, D.J.; Liu, W. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: Gliadin nanoparticles/gum Arabic. Food Hydrocoll. 2020, 100, 105381. [Google Scholar] [CrossRef]
- Yi, J.; Gao, L.; Zhong, G.; Fan, Y. Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for beta-carotene stabilization and delivery. Food Funct. 2020, 11, 768–778. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Tan, Z.; Zhang, G.; Xu, X.; Song, L. Improved thermal and oxidation stabilities of pickering high internal phase emulsions stabilized using glycated pea protein isolate with glycation extent. LWT-Food Sci. Technol. 2022, 162, 113465. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Tang, C. Novel pickering high internal phase emulsion gels stabilized solely by soy β-conglycinin. Food Hydrocoll. 2019, 88, 21–30. [Google Scholar] [CrossRef]
- Wei, Z.H.; Cheng, Y.J.; Zhu, J.Y.; Huang, Q.R. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Boostani, S.; Riazi, M.; Marefati, A.; Rayner, M.; Hosseini, S.M.H. Development and characterization of medium and high internal phase novel multiple Pickering emulsions stabilized by hordein nanoparticles. Food Chem. 2022, 372, 131354. [Google Scholar] [CrossRef] [PubMed]
- Saffarionpour, S. Preparation of Food Flavor Nanoemulsions by High- and Low-Energy Emulsification Approaches. Food Eng. Rev. 2019, 11, 259–289. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Wang, Y.; Boesch, C.; Zhao, Y.; Sarkar, A. Pickering emulsions stabilized by colloidal gel particles complexed or conjugated with biopolymers to enhance bioaccessibility and cellular uptake of curcumin. Curr. Res. Food Sci. 2020, 3, 178–188. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of recent advances in the preparation, properties, and applications of high internal phase emulsions. Trends Food Sci. Tech. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Zou, Y.A.; Yang, X.Q.; Scholten, E. Tuning particle properties to control rheological behavior of high internal phase emulsion gels stabilized by zein/tannic acid complex particles. Food Hydrocoll. 2019, 89, 163–170. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P. High internal phase Pickering emulsions. Curr. Opin. Colloid Interface Sci. 2022, 57. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Chen, H.; Deng, Y.; Wei, Z.; Zhang, Y.; Tang, X.; Li, P.; Zhao, Z.; Zhou, P.; et al. Rice bran-modified wheat gluten nanoparticles effectively stabilized pickering emulsion: An interfacial antioxidant inhibiting lipid oxidation. Food Chem. 2022, 387, 132874. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, K.; Luo, Y.; Li, Y.; Cheng, N.; Mei, X. Preparation and characterization of high internal phase Pickering emulsions stabilized by hordein-chitosan composite nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2023, 659. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, C.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z. Preparation of high internal phase Pickering emulsion gels stabilized by glycyrrhizic acid-zein composite nanoparticles: Gelation mechanism and 3D printing performance. Food Hydrocoll. 2023, 135. [Google Scholar] [CrossRef]
- Ding, J.; Li, Y.; Wang, Q.; Chen, L.; Mao, Y.; Mei, J.; Yang, C.; Sun, Y. Pickering high internal phase emulsions with excellent UV protection property stabilized by Spirulina protein isolate nanoparticles. Food Hydrocoll. 2023, 137. [Google Scholar] [CrossRef]
- Li, R.R.; He, Q.; Guo, M.; Yuan, J.H.; Wu, Y.Y.; Wang, S.N.; Rong, L.Y.; Li, J.R. Universal and simple method for facile fabrication of sustainable high internal phase emulsions solely using meat protein particles with various pH values. Food Hydrocoll. 2020, 100, 105444. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, Y.; Yi, X.; Li, Z.; Luo, Y. Effect of four plant oils on the stability of high internal phase Pickering emulsions stabilized by ovalbumin-tannic acid complex. Int. J. Biol. Macromol. 2022, 222 Pt B, 1633–1641. [Google Scholar] [CrossRef]
- Tan, H.; Han, L.; Yang, C. Effect of oil type and β-carotene incorporation on the properties of gelatin nanoparticle-stabilized pickering emulsions. LWT-Food Sci. Technol. 2021, 141, 110903. [Google Scholar] [CrossRef]
- Xu, Y.T.; Wang, Y.H.; Chen, F.P.; Tang, C.H. Whether ovalbumin performs as a particulate or polymeric emulsifier is largely determined by pH. Food Hydrocoll. 2020, 103, 105694. [Google Scholar] [CrossRef]
- Feng, T.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Plant protein-based antioxidant Pickering emulsions and high internal phase Pickering emulsions against broad pH range and high ionic strength: Effects of interfacial rheology and microstructure. LWT-Food Sci. Technol. 2021, 150, 111953. [Google Scholar] [CrossRef]
- Ju, M.N.; Zhu, G.; Huang, G.; Shen, X.C.; Zhang, Y.; Jiang, L.Z.; Sui, X.N. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Li, J.; Li, B. Fabrication of processable and edible high internal phase Pickering emulsions stabilized with gliadin/sodium carboxymethyl cellulose colloid particles. Food Hydrocoll. 2022, 128, 107571. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Li, D.; Huang, Q. Effects of pectin polydispersity on zein/pectin composite nanoparticles (ZAPs) as high internal-phase Pickering emulsion stabilizers. Carbohydr. Polym. 2019, 219, 77–86. [Google Scholar] [CrossRef]
- McManus, J.J.; Charbonneau, P.; Zaccarelli, E.; Asherie, N. The physics of protein self-assembly. Curr. Opin. Colloid Interface Sci. 2016, 22, 73–79. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; Guo, J.; Wu, L.Y.; Tang, C.H.; Yang, X.Q. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Boostani, S.; Hosseini, S.M.H.; Golmakani, M.T.; Marefati, A.; Hadi, N.B.A.; Rayner, M. The influence of emulsion parameters on physical stability and rheological properties of Pickering emulsions stabilized by hordein nanoparticles. Food Hydrocoll. 2020, 101, 105520. [Google Scholar] [CrossRef]
- Jing, X.; Chen, B.; Liu, T.; Cai, Y.; Zhao, Q.; Deng, X.; Zhao, M. Formation and stability of Pickering emulsion gels by insoluble soy peptide aggregates through hydrophobic modification. Food Chem. 2022, 387, 132897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Q.L.; Xiong, Y.L.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein isolate. Food Chem. 2020, 325, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, X.; Wang, X.; Zhang, X.; Gu, Y.; Xia, S.; Huang, Q. High internal phase pickering emulsions stabilized by pea protein isolate-high methoxyl pectin-EGCG complex: Interfacial properties and microstructure. Food Chem. 2021, 350, 129251. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Malchione, N.; Selig, M.J.; Abbaspourrad, A. Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels. Food Funct. 2018, 9, 982–990. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.C.; Coimbra, J.S.; de Oliveira, E.B.; Zuniga, A.D.; Rojas, E.E. Food Protein-polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M. Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: A review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Xu, Y.T.; Tang, C.H.; Binks, B.P. High internal phase emulsions stabilized solely by a globular protein glycated to form soft particles. Food Hydrocoll. 2020, 98, 105254. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107 Pt A, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Tu, Z.; Jia, H.; Gou, X.; Ngai, T. Hierarchical Porous Protein Scaffold Templated from High Internal Phase Emulsion Costabilized by Gelatin and Gelatin Nanoparticles. Langmuir 2018, 34, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.S.; Gao, Q.Y.; Luo, Z.G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus Plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Weiss, J.; Ahmad, T.; Zhang, C.; Zhang, H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Technol. 2020, 106, 91–103. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Zaky, A.A.; Tie, S.; Cui, G.; Liu, R.; Abd El-Aty, A.M.; Tan, M. High internal phase Pickering emulsion by Spanish mackerel proteins-procyanidins: Application for stabilizing astaxanthin and surimi. Food Hydrocoll. 2022, 133. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Huang, X.N.; Wu, Z.L.; Yin, S.W.; Zhu, J.H.; Tang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yang, S.; Wei, Y.; Sun, C.; McClements, D.J.; Mao, L.; Gao, Y. Development of stable high internal phase emulsions by pickering stabilization: Utilization of zein-propylene glycol alginate-rhamnolipid complex particles as colloidal emulsifiers. Food Chem. 2019, 275, 246–254. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, T.; Smits, J.; Huang, X.N.; Maas, M.; Yin, S.W.; Ngai, T. Edible high internal phase Pickering emulsion with double-emulsion morphology. Food Hydrocoll. 2021, 111, 106405. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yuan, D.B.; Yang, X.Q. Development of antioxidant gliadin particle stabilized Pickering high internal phase emulsions (HIPEs) as oral delivery systems and the in vitro digestion fate. Food Funct. 2018, 9, 959–970. [Google Scholar] [CrossRef]
- Peng, L.P.; Tang, C.H. Outstanding antioxidant pickering high internal phase emulsions by co-assembled polyphenol-soy beta-conglycinin nanoparticles. Food Res. Int. 2020, 136, 109509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, D.H.; Shen, R.; Yang, X.B. Bacterial cellulose nanofibers improved the emulsifying capacity of soy protein isolate as a stabilizer for pickering high internal-phase emulsions. Food Hydrocoll. 2021, 112, 106279. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Y.; Li, X.; Tang, C. Improving the emulsification of soy β-conglycinin by alcohol-induced aggregation. Food Hydrocoll. 2020, 98, 105307. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Xu, Y.T.; Tang, C.H.; Binks, B.P. Ultraefficient stabilization of high internal phase emulsions by globular proteins in the presence of polyols: Importance of a core-shell nanostructure. Food Hydrocoll. 2020, 107, 105968. [Google Scholar] [CrossRef]
- Chen, X.H.; Tang, C.H. Transparent high internal phase emulsion gels stabilized solely by proteins. Colloids Surf. Physicochem. Eng. Asp. 2021, 608, 125596. [Google Scholar] [CrossRef]
- Dai, H.; Chen, X.; Peng, L.; Ma, L.; Sun, Y.; Li, L.; Wang, Q.; Zhang, Y. The mechanism of improved myosin gel properties by low dose rosmarinic acid addition during gel formation. Food Hydrocoll. 2020, 106. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, X.Z.; Zhang, Z.Y.; Ding, B.M.; Li, Z.S.; Luo, Y.C. High internal phase Pickering emulsions stabilized by tannic acid-ovalbumin complexes: Interfacial property and stability. Food Hydrocoll. 2022, 125, 107332. [Google Scholar] [CrossRef]
- Xu, Y.T.; Tang, C.H.; Liu, T.X.; Liu, R. Ovalbumin as an Outstanding Pickering Nanostabilizer for High Internal Phase Emulsions. J. Agric. Food Chem. 2018, 66, 8795–8804. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Li, H.; Zhang, H.; Chi, Y.; Xia, N.; Li, Z.; Jiang, L.; Zhang, X.; Rayan, A.M. Fabrication and digestive characteristics of high internal phase Pickering emulsions stabilized by ovalbumin-pectin complexes for improving the stability and bioaccessibility of curcumin. Food Chem. 2022, 389, 133055. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Zhang, L.M. Casein nanogels as effective stabilizers for Pickering high internal phase emulsions, Colloids Surf. Physicochem. Eng. Asp. 2019, 579, 123662. [Google Scholar] [CrossRef]
- Wu, C.; Na, X.K.; Ma, W.C.; Ren, C.; Zhong, Q.X.; Wang, T.; Du, M. Strong, elastic, and tough high internal phase emulsions stabilized solely by cod myofibers for multidisciplinary applications. Chem. Eng. J. 2021, 412, 128724. [Google Scholar] [CrossRef]
- Zhang, L.; Zaky, A.A.; Zhou, C.; Chen, Y.; Su, W.; Wang, H.; Abd El-Aty, A.M.; Tan, M. High internal phase Pickering emulsion stabilized by sea bass protein microgel particles: Food 3D printing application. Food Hydrocoll. 2022, 131, 107744. [Google Scholar] [CrossRef]
- Tan, H.; Sun, G.; Lin, W.; Mu, C.; Ngai, T. Gelatin particle-stabilized high internal phase emulsions as nutraceutical containers. ACS Appl. Mater. Interfaces 2014, 6, 13977–13984. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release. 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, M.; Zeng, M. Novel Pickering emulsion stabilized by natural fiber polysaccharide-protein extracted from Haematococcus pluvialis residues. Food Hydrocoll. 2023, 134. [Google Scholar] [CrossRef]
- Dai, H.; Li, Y.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Liu, T.; Feng, X.; Tang, M.; Hu, W.; et al. Fabrication of cross-linked β-lactoglobulin nanoparticles as effective stabilizers for Pickering high internal phase emulsions. Food Hydrocoll. 2020, 109. [Google Scholar] [CrossRef]
- Xu, B.; Liu, C.; Sun, H.; Wang, X.; Huang, F. Oil-in-water Pickering emulsions using a protein nano-ring as high-grade emulsifiers. Colloids Surf. B. Biointerfaces 2020, 187, 110646. [Google Scholar] [CrossRef]
- Su, D.; Mo, H.; Huang, J.; Li, Q.; Zhong, H.; Jin, B. Soy protein/beta-glucan/tannic acid complex coacervates with different micro-structures play key roles in the rheological properties, tribological properties, and the storage stability of Pickering high internal phase emulsions. Food Chem. 2022, 401, 134168. [Google Scholar] [CrossRef]
- Tang, C.H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 15. [Google Scholar] [CrossRef]
- Chen, S.; Du, Y.; Zhang, H.; Wang, Q.; Gong, Y.; Chang, R.; Zhang, J.; Zhang, J.; Yuan, Y.; Liu, B.; et al. The lipid digestion behavior of oil-in-water Pickering emulsions stabilized by whey protein microgels of various rigidities. Food Hydrocoll. 2022, 130, 107735. [Google Scholar] [CrossRef]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and rheology of particle stabilized emulsions: Effects of particle shape and inter-particle interactions. J. Colloid Interface Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Chen, Y.; Ding, B.; Ma, K.; Li, Z.; Luo, Y. High internal phase Pickering emulsions prepared by globular protein-tannic acid complexes: A hydrogen bond-based interfacial crosslinking strategy. J. Mol. Liq. 2023, 370. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; An, S.; Jiang, Q.; Gao, D.; Li, X. Construction of porous materials from Pickering high internal-phase emulsions stabilized by zein-Hohenbuehelia serotina polysaccharides nanoparticles and their adsortion performances. Food Hydrocoll. 2023, 134. [Google Scholar] [CrossRef]
- Li, X.-M.; Xie, Q.-T.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.-Q.; Jin, Z.-Y. Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel Pickering stabilizers: Physical stability and rheological properties. Food Hydrocoll. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Sun, Y.; Chai, X.; Han, W.; Farah, Z.; Tian, T.; Xu, Y.-J.; Liu, Y. Pickering emulsions stabilized by hemp protein nanoparticles: Tuning the emulsion characteristics by adjusting anti-solvent precipitation. Food Hydrocoll. 2023, 138. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, Y.; Zhang, W.; Xiang, R. Factors that affect Pickering emulsions stabilized by mesoporous hollow silica microspheres. J. Colloid Interface Sci. 2022, 633, 1012–1021. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, L.; Fu, Y.; Dai, H.; Zhang, Y. Fabrication and characterization of myofibrillar microgel particles as novel Pickering stabilizers: Effect of particle size and wettability on emulsifying capacity. Lwt 2021, 151. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, A.; Hemar, Y.; Gilbert, E.P.; de Campo, L.; Whitten, A.E.; Singh, H. Interfacial Structures of Droplet-Stabilized Emulsions Formed with Whey Protein Microgel Particles as Revealed by Small- and Ultra-Small-Angle Neutron Scattering. Langmuir 2019, 35, 12017–12027. [Google Scholar] [CrossRef]
- Esen, A. Separation of alcohol-soluble proteins (zeins) from maize into three fractions by differential solubility. Plant Physiol. 1986, 80, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sun, Y.; Yang, Y.; Zhou, X.; Cen, K.; Yu, C.; Xu, T.; Tang, X. Zein nanoparticle stabilized Pickering emulsion enriched with cinnamon oil and its effects on pound cakes. LWT-Food Sci. Technol. 2020, 122, 1–9. [Google Scholar] [CrossRef]
- Tao, S.; Guan, X.; Li, Y.; Jiang, H.; Gong, S.; Ngai, T. All-natural oil-in-water high internal phase Pickering emulsions featuring interfacial bilayer stabilization. J. Colloid Interface Sci. 2022, 607 Pt 2, 1491–1499. [Google Scholar] [CrossRef]
- Snyder, H. The Proteins of the Wheat Kernel. In Science; Carnegie Institution of Washington: Washington, DC, USA, 1907; Volume 26, p. 865. [Google Scholar]
- Woychik, J.H.; Boundy, J.A.; Dimler, R.J. Starch gel electrophoresis of wheat gluten proteins with concentrated urea. Arch. Biochem. Biophys. 1961, 94, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Takenaka, Y.; Gidamis, A.B.; Mikami, B.; Utsumi, S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 2001, 305, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G. Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zheng, J.; Liu, F.; Qiu, C.Y.; Lin, W.F.; Tang, C.H. Freeze-thaw stability of Pickering emulsions stabilized by soy protein nanoparticles. Influence of ionic strength before or after emulsification. Food Hydrocoll. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, W.; Zhang, Y.; Tang, C. Freeze-thaw-stable high internal phase emulsions stabilized by soy protein isolate and chitosan complexes at pH 3.0 as promising mayonnaise replacers. Food Res. Int. 2022, 156, 111309. [Google Scholar] [CrossRef]
- Ning, F.J.; Ge, Z.Z.; Qiu, L.; Wang, X.Q.; Luo, L.P.; Xiong, H.; Huang, Q.R. Double-induced se-enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade pickering emulsions. Food Hydrocoll. 2020, 99, 105308. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gu, Q.; Hong, X.; Liu, Y.; Li, J. Novel protein-based nanoparticles from perilla oilseed residues as sole Pickering stabilizers for high internal phase emulsions. LWT-Food Sci. Technol. 2021, 145, 111340. [Google Scholar] [CrossRef]
- Smithers, G.W.; Ballard, F.J.; Copeland, A.D.; De Silva, K.J.; Dionysius, D.A.; Francis, G.L.; Goddard, C.; Grieve, P.A.; McIn-tosh, G.H.; Mitchell, I.R.; et al. New opportunities from the isolation and utilization of whey proteins. J. Dairy Sci. 1996, 79, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Gulseren, I.; Guzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason SonoChem. 2007, 14, 173–183. [Google Scholar] [CrossRef]
- Holm, N.K.; Jespersen, S.K.; Thomassen, L.V.; Wolff, T.Y.; Sehgal, P.; Thomsen, L.A.; Christiansen, G.; Andersen, C.B.; Knudsen, A.D.; Otzen, D.E. Aggregation and fibrillation of bovine serum albumin. Biochim. Biophys. Acta. 2007, 1774, 1128–1138. [Google Scholar] [CrossRef]
- Tan, C.; Lee, M.C.; Abbaspourrad, A. Facile Synthesis of Sustainable High Internal Phase Emulsions by a Universal and Controllable Route. ACS Sustain. Chem. Eng. 2018, 6, 16657–16664. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, J.; Huang, C.H.; Tang, C.H.; Ou, S.Y. Pickering high internal phase emulsions stabilized by protein-covered cellulose nanocrystals. Food Hydrocoll. 2018, 82, 96–105. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, T.; Zhang, H.; Tao, N.; Wang, X.; Zhong, J. Gelatin molecular structures affect behaviors of fish oil-loaded traditional and Pickering emulsions. Food Chem. 2020, 309, 125642. [Google Scholar] [CrossRef]
- Geng, S.; Li, Y.; Lv, J.; Ma, H.; Liang, G.; Liu, B. Fabrication of food-grade Pickering high internal phase emulsions (HIPEs) stabilized by a dihydromyricetin and lysozyme mixture. Food Chem. 2022, 373 Pt B, 131576. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Luo, Y. High internal phase Pickering emulsions stabilized by egg yolk low density lipoprotein for delivery of curcumin, Colloids Surf. B. Biointerfaces. 2022, 211, 112334. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Xiong, Y.; Ding, B.; Li, Z.; Luo, Y. Preparation of high internal phase Pickering emulsions stabilized by egg yolk high density lipoprotein: Stabilizing mechanism under different pH values and protein concentrations. LWT-Food Sci. Technol. 2022, 157, 113091. [Google Scholar] [CrossRef]
- Du, M.; Sun, Z.; Liu, Z.; Yang, Y.; Liu, Z.; Wang, Y.; Jiang, B.; Feng, Z.; Liu, C. High efficiency desalination of wasted salted duck egg white and processing into food-grade pickering emulsion stabilizer. LWT-Food Sci. Technol. 2022, 161, 113337. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Fang, Z. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: A review. Trends Food Sci. Technol. 2020, 105, 468–482. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Z.; Zhang, Y.; Feng, Z.; Gu, P.; Huang, Y.; Liu, J.; Wu, Y.; Wang, D. Lentinan PLGA-stabilized pickering emulsion for the enhanced vaccination. Int. J. Pharm. 2022, 611, 121348. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, S.S.; Dogu-Baykut, E.; Kittipongpittaya, K.; McClements, D.J.; Decker, E.A. Increased antioxidant efficacy of tocopherols by surfactant solubilization in oil-in-water emulsions. J. Agric. Food Chem. 2014, 62, 10561–10566. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yan, J.; Guo, S.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Enhancing lycopene stability and bioaccessibility in homogenized tomato pulp using emulsion design principles. Innov. Food Sci. Emerg. Technol. 2021, 67. [Google Scholar] [CrossRef]
- Guo, B.Z.; Hu, X.T.; Wu, J.Y.; Chen, R.Y.; Dai, T.T.; Liu, Y.F.; Luo, S.J.; Liu, C.M. Soluble starch/whey protein isolate complex-stabilized high internal phase emulsion: Interaction and stability. Food Hydrocoll. 2021, 111, 106377. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.; Wan, Z.L.; Liu, Y.Y.; Ruan, Q.J.; Yang, X.Q. Wheat gluten-stabilized high internal phase emulsions as mayonnaise replacers. Food Hydrocoll. 2018, 77, 168–175. [Google Scholar] [CrossRef]
- Feng, T.; Fan, C.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Food-grade Pickering emulsions and high internal phase Pickering emulsions encapsulating cinnamaldehyde based on pea protein-pectin-EGCG complexes for extrusion 3D printing. Food Hydrocoll. 2022, 124, 107265. [Google Scholar] [CrossRef]
- Kan, X.H.; Yan, Y.M.; Ran, L.W.; Lu, L.; Mi, J.; Zhang, Z.J.; Li, X.Y.; Zeng, X.X.; Cao, Y.L. Evaluation of bioaccessibility of zeaxanthin dipalmitate from the fruits of Lycium barbarum in oil-in-water emulsions. Food Hydrocoll. 2020, 105, 105781. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, W.; Wang, K.; Liu, Y.; Liu, A.; Zhang, S.; Zhao, Y. Impact of melting point of palm oil on mechanical and water barrier properties of gelatin-palm oil emulsion film. Food Hydrocoll. 2016, 60, 243–251. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Wu, Y.; Zheng, Z.; Xie, Y.; Li, Y.; Li, B.; Pei, Y.; Liu, S. Construction of stable O/W/O multiple emulsions using beeswax to control the melting point of the continuous oil phase. Food Hydrocoll. 2023, 136. [Google Scholar] [CrossRef]
- Wei, Z.H.; Huang, Q.R. Development of high internal phase Pickering emulsions stabilised by ovotransferrin-gum arabic particles as curcumin delivery vehicles. Int. J. Food Sci. Technol. 2020, 55, 1891–1899. [Google Scholar] [CrossRef]
- Chen, Q.; Tai, X.; Li, J.; Li, C.; Guo, L. High Internal Phase Emulsions Synergistically Stabilized by Sodium Carboxymethyl Cellulose and Palm Kernel Oil Ethoxylates as an Essential Oil Delivery System. J. Agric. Food Chem. 2021, 69, 4191–4203. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; McClements, D.J.; Zhang, G. Curcumin: Recent Advances in the Development of Strategies to Improve Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Ma, L.; Yang, L.; Cong, P.; Lan, H.; Xue, C.; Xu, J. Co-oxidation of Antarctic krill oil with whey protein and myofibrillar protein in oil-in-water emulsions. J. Food Sci. 2020, 85, 3797–3805. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.Y.; Sun, Y.E.; Zeng, Q.Z.; Yang, X.Q. Complex coacervation of soy protein with chitosan: Constructing antioxidant microcapsule for algal oil delivery. Lwt 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.; Xing, G.; Qing, Z.; Decker, E.A.; McClements, D.J. Physical and oxidative stability of flaxseed oil-in-water emulsions fabricated from sunflower lecithins: Impact of blending lecithins with different phospholipid profiles. J. Agric. Food Chem. 2017, 65, 4755–4765. [Google Scholar] [CrossRef]
- Yan, C.; McClements, D.J.; Zhu, Y.; Zou, L.; Zhou, W.; Liu, W. Fabrication of OSA Starch/Chitosan Polysaccharide-Based High Internal Phase Emulsion via Altering Interfacial Behaviors. J. Agric. Food Chem. 2019, 67, 10937–10946. [Google Scholar] [CrossRef]
- Liu, B.; Li, T.; Wang, W.; Sagis, L.M.C.; Yuan, Q.; Lei, X.; Cohen Stuart, M.A.; Li, D.; Bao, C.; Bai, J.; et al. Corncob cellulose nanosphere as an eco-friendly detergent. Nat. Sustain. 2020, 3, 448–458. [Google Scholar] [CrossRef]
- Glaize, A.; Gutierrez-Rodriguez, E.; Hanning, I.; Diaz-Sanchez, S.; Gunter, C.; van Vliet, A.H.M.; Watson, W.; Thakur, S. Transmission of antimicrobial resistant non-O157 Escherichia coli at the interface of animal-fresh produce in sustainable farming environments, Int. J. Food Microbiol. 2020, 319, 108472. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, H.; Wang, Y.Y. Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: Potential mechanisms, combination with other methods, equipment innovations and future directions. Ultrason. Sonochem. 2022, 85, 105993. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, W.; Li, C.; Chen, X.; Cui, H.; Lin, L. Pickering emulsion stabilized by gliadin/soybean polysaccharide composite colloidal nanoparticle: Physicochemical properties and its application on washing of fresh-cut cabbage. Food Res. Int. 2022, 161, 111886. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, H.; Song, W. An overview of preparation and evaluation sustained-release injectable microspheres. J. Microencapsul. 2013, 30, 369–382. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, S.; Yang, Z.; Zhou, W.; Du, Z.; Huang, J.; Yi, H.; Wang, C. Facile fabrication of poly(L-lactic acid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates. Colloids Surf. B. Biointerfaces 2016, 140, 382–391. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Ning, Y.; Wang, C.; Tong, Z. Facile preparation of Artemisia argyi oil-loaded antibacterial microcapsules by hydroxyapatite-stabilized Pickering emulsion templating. Colloids Surf. B. Biointerfaces 2013, 112, 96–102. [Google Scholar] [CrossRef]
- Kaewsaneha, C.; Tangboriboonrat, P.; Polpanich, D.; Eissa, M.; Elaissari, A. Preparation of Janus colloidal particles via Pickering emulsion: An overview. Colloids Surf. Physicochem. Eng. Asp. 2013, 439, 35–42. [Google Scholar] [CrossRef]
- Zahn, N.; Kickelbick, G. Synthesis and aggregation behavior of hybrid amphiphilic titania Janus nanoparticles via surface-functionalization in Pickering emulsions. Colloids Surf. Physicochem. Eng. Asp. 2014, 461, 142–150. [Google Scholar] [CrossRef]
- Huang, X.N.; Zhu, J.J.; Xi, Y.K.; Yin, S.W.; Ngai, T.; Yang, X.Q. Protein-Based Pickering High Internal Phase Emulsions as Nutraceutical Vehicles of and the Template for Advanced Materials: A Perspective Paper. J. Agric. Food Chem. 2019, 67, 9719–9726. [Google Scholar] [CrossRef]

| Protein | Stabilizers | Preparation | Size (nm) | Contact Angle (°) | References |
|---|---|---|---|---|---|
| Zein | Zein–tannic acid complex particles | Anti-solvent | 68–108 | — | [46] |
| Zein–pectin complex particles | Anti-solvent | 583.74 | 86.44 | [77] | |
| Zein–pga–rhamnolipid complex particles | Anti-solvent | 504 | 84.05 | [78] | |
| Zein protein particles–lecithin | Anti-solvent | 100 | — | [79] | |
| Gliadin | Gliadin–chitosan complex particles | Anti-solvent | 190–564 | — | [80] |
| Gliadin–chitosan complex particles | Anti-solvent | 588.8 | 84.00 | [26] | |
| Rice bran-modified wheat gluten nanoparticle | pH adjustment | 250 | 104.6 | [48] | |
| Soy protein | Soy β-conglycinin–polyphenol complex nanoparticles | Anti-solvent | 25.5–62.2 | — | [81] |
| Soy protein isolate–bacterial cellulose nanofibers complex particles | Anti-solvent | 947–1177 | — | [82] | |
| Soy protein microgels | Heat treatment | 169.7–311.9 | — | [31] | |
| Soy protein–polysaccharide complex particles | Ultrasonication | 160 | — | [17] | |
| Aggregation of soy β-conglycinin | Anti-solvent precipitation | 500 | — | [83] | |
| Globulin particles | pH adjustment | 500–1000 | — | [10] | |
| Pea protein | Pea protein–high methoxyl pectin complex particles | pH adjustment | 347 | — | [84] |
| Glycated pea protein isolate particles | Heat treatment | 115.7–157.5 | 54.85–79.62 | [39] | |
| Peanut protein | Peanut protein microgel particles | Enzyme cross-link | 200–300 | 102.3 | [33] |
| Rice proteins | Rice protein–cellulose complex particles | pH adjustment | 132–144 | 96.3–129.27 | [29] |
| Fungal protein | Bamboo fungus protein gel particles | Enzyme cross-link | 227 | 77.6 | [35] |
| Shiitake mushroom protein–polysaccharide conjugates | Direct extraction | 300 | 75.5–115 | [27] | |
| Whey protein | Whey protein–low methoxyl pectin complex particles | pH adjustment | 916–1032 | 60.45–70.15 | [18] |
| Whey protein microgels particles | Ca2+ induced | 146.9 | 67 | [30] | |
| Whey protein microgels | Heat treatment | 90–350 | — | [68] | |
| Whey protein nanofibrils | Hydrothermal method | 200 | — | [85] | |
| BSA | BSA–trehalose complex particles | pH adjustment | 5.3–8 | — | [86] |
| BSA–sucrose complex particles | pH adjustment | 4–13 | — | [87] | |
| Glycated BSA particles | Galactose glycated | 4.2–6.5 | — | [71] | |
| Ovalbumin | S-ovalbumin particles | pH adjustment | 5.8 | — | [88] |
| Ovalbumin–tannic acid complexes particles | pH adjustment | — | 79.9–88.5 | [89] | |
| Ovalbumin particles | pH adjustment | 5.15 | — | [90] | |
| Ovalbumin–pectin complex particles | pH adjustment | 300–500 | — | [91] | |
| Casein | Casein nanogels particles | Glutaraldehyde cross-link | 179 | — | [92] |
| Meat protein | Pork proteins particles | pH adjustment | 821–940 | — | [34] |
| Cod myofibers | pH adjustment | 25–120 | — | [93] | |
| Sea bass protein microgel particles | Enzyme cross-link | 300–500 | 78.3–95.5 | [94] | |
| Gelatin type B | Gelatin particles | Glutaraldehyde cross-link | 200 | — | [95] |
| Gelatin particles | Glutaraldehyde cross-link | 235.9 | — | [73] |
| Oil Type | Encapsulated Compound | Stabilizer | Phase Fraction (%) | Droplet Size (μm) | Properties/Applications | References |
|---|---|---|---|---|---|---|
| Hexane | — | Gelatin particles | 80 | 40 | Degradable porous protein scaffold | [73] |
| Dodecane | β-carotene | Soy β-conglycinin particles | 88 | 24–60 | Heat, storage and freeze–thaw stability | [40] |
| β-carotene | Ovalbumin particles | 91 | 20–50 | Heat, storage stability | [90] | |
| — | BSA–trehalose complex particles | 80 | 20 | Storage, heat stability | [86] | |
| — | Glycated BSA particles | 92 | 30–50 | Heat, storage and freeze–thaw stability | [71] | |
| — | Ovalbumin–tannic acid complexes particles | 80 | 13–15 | Storage and freeze–thaw stability | [89] | |
| Squalane | — | Zein protein particles–soybean lecithin | 80 | 25 | Digestion stability | [79] |
| Corn oil | β-carotene | Gliadin–gum Arabic complex particles | 85 | 6.0–9.4 | Stability; nutrient protection | [37] |
| β-carotene | S-ovalbumin particles | 85 | 35 | pH, ionic and temperature stability | [88] | |
| -carotene | Ca2+ induced WPI particles | 80 | 16.6 | Stability; enhance bioavailability | [38] | |
| Curcumin | Gliadin–chitosan complex particles | 83 | 25.5–93.5 | Nutrient protection; alternative for PHOs | [26] | |
| Curcumin | Zein–pectin complex particles | 80 | 105.12 | Stability; nutrient protection | [77] | |
| — | Soluble starch–whey protein isolate complex particles | 75 | 5.55–10.60 | pH, thermal and ionic stability | [138] | |
| Astaxanthin | Sea bass protein microgel particles | 88 | 24–65 | Enhance bioavailability; 3D printing | [94] | |
| MCT oil | Curcumin | Whey protein nanogel particles | 80 | 15 | Enhance bioavailability and cellular uptake | [44] |
| Lutein | Lysozyme–dihydromyricetin–mixture | 90 | 48.5 | Nutrient protection | [130] | |
| — | Zein–PGA–rhamnolipid complex particles | 75 | 40–13 | Environmental stability | [78] | |
| Indomethacin | Casein nanogels | 80 | 50–160 | Stability and tunable drug release | [92] | |
| Olive oil | Quercetin | Pecan protein–xanthan gum complex particles | 80 | 30–70 | Enhance stability and bioavailability | [122] |
| — | Fibrous protein particles | 87 | 1–10 | Heat, storage and freeze–thaw stability | [34] | |
| Soy oil | Curcumin | Low density lipoprotein particles | 80 | 10 | Enhance stability and bioavailability | [131] |
| — | Soy protein–polysaccharide complex particles | 80 | 100 | Storage, heat and freeze–thaw stability | [17] | |
| — | Globulin particles | 80 | 562 | Oxidation stability; nutrients delivery | [10] | |
| — | Aggregation soy β-conglycinin | 80 | 25 | Structural stability | [83] | |
| — | Rice protein–cellulose complex particles | 85 | 20–60 | 3D printing | [29] | |
| — | Whey protein isolate–low methoxyl pectin | 80 | 1400 | Heating and centrifugal stability | [18] | |
| Sunflower oil | β-carotene | Gelatin particles | 80 | 10 | Tunable structure and release behavior | [95] |
| — | Zein–tannic acid complex particles | 87 | 5.5–9.5 | Microstructure tunable | [46] | |
| — | Soy Protein Microgels | 82 | 8–12 | Delayed digestive | [31] | |
| — | Cellulose nanofibers–soy protein complex particles | 75 | 947–1409 | Storage stability | [139] | |
| Algal oil | Curcumin | Gliadin–chitosan complex particles | 75 | 40 | Protect and increase the bioavailability | [80] |
| Peanut oil | — | Peanut protein particles | 87 | 10–30 | Novel porous material template | [33] |
| — | Whey protein nanofibrils | 80 | 11 | Stability; tunable rheology | [85] | |
| — | Shiitake mushroom protein–polysaccharide conjugates | 78 | 72.3 | Heat, storage stability; 3D printing | [27] | |
| — | Quinoa protein particles | 80 | 14–24 | Physical stability | [28] | |
| Flaxseed oil | β-carotene | Soy β-conglycinin–polyphenol complex nanoparticles | 80 | 50 | Heat, storage stability; oxidation protection | [81] |
| Camellia oil | Cinnamaldehyde | Pea protein–pectin–EGCG complexes | 83 | — | Enhance stability; 3D printing | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, X.; Zhou, L.; Chen, W.; Marchioni, E. Protein-Based High Internal Phase Pickering Emulsions: A Review of Their Fabrication, Composition and Future Perspectives in the Food Industry. Foods 2023, 12, 482. https://doi.org/10.3390/foods12030482
Zhang M, Li X, Zhou L, Chen W, Marchioni E. Protein-Based High Internal Phase Pickering Emulsions: A Review of Their Fabrication, Composition and Future Perspectives in the Food Industry. Foods. 2023; 12(3):482. https://doi.org/10.3390/foods12030482
Chicago/Turabian StyleZhang, Minghao, Xiang Li, Li Zhou, Weilin Chen, and Eric Marchioni. 2023. "Protein-Based High Internal Phase Pickering Emulsions: A Review of Their Fabrication, Composition and Future Perspectives in the Food Industry" Foods 12, no. 3: 482. https://doi.org/10.3390/foods12030482
APA StyleZhang, M., Li, X., Zhou, L., Chen, W., & Marchioni, E. (2023). Protein-Based High Internal Phase Pickering Emulsions: A Review of Their Fabrication, Composition and Future Perspectives in the Food Industry. Foods, 12(3), 482. https://doi.org/10.3390/foods12030482






