Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
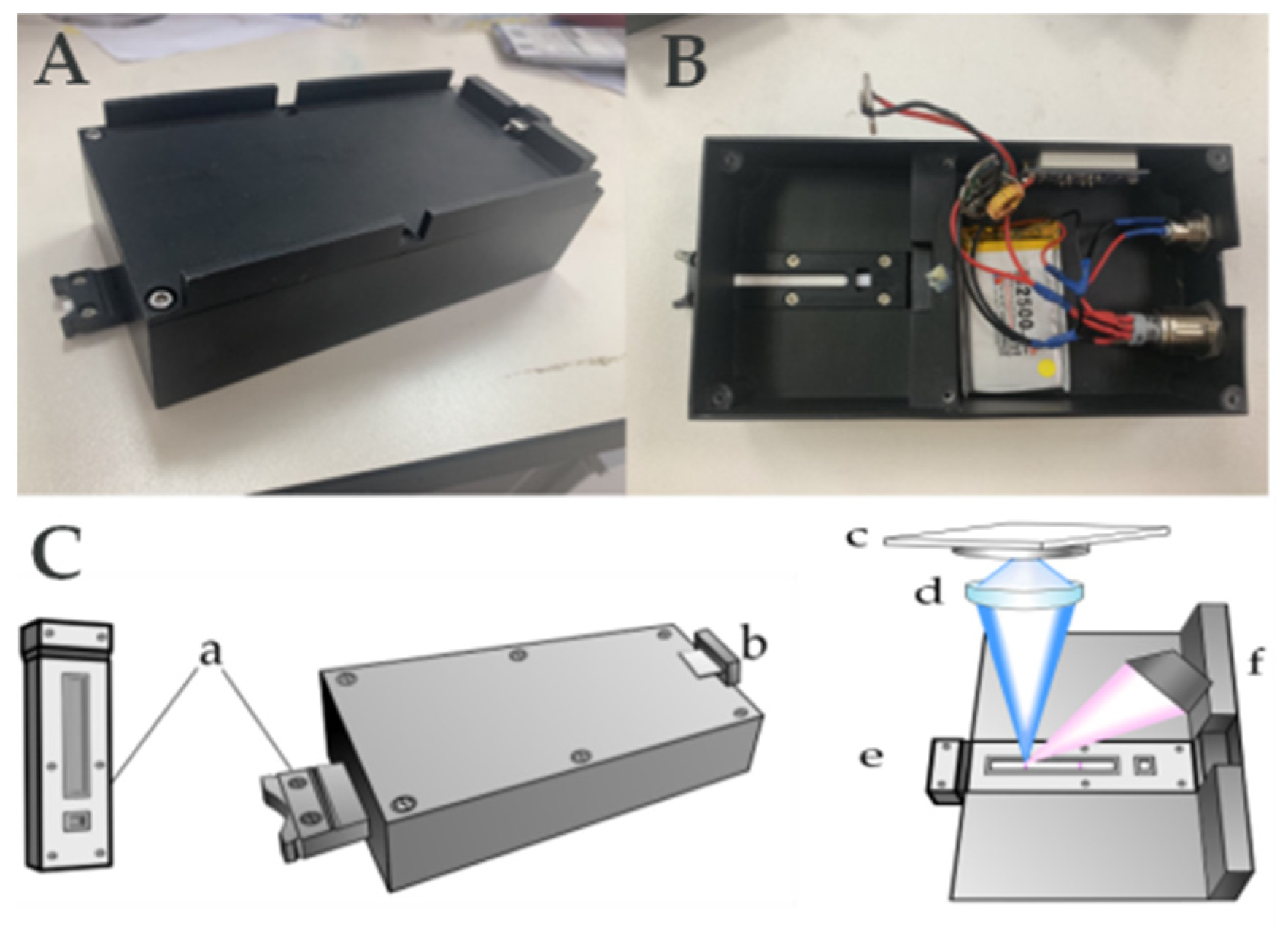
2.2. Apparatus
2.3. Development of the POCT Portable Reader
2.4. Preparation of the Anti-OTA Monoclonal Antibody
2.5. Conjugation of OTA mAb and Eu-Nanospheres (EuNPs)
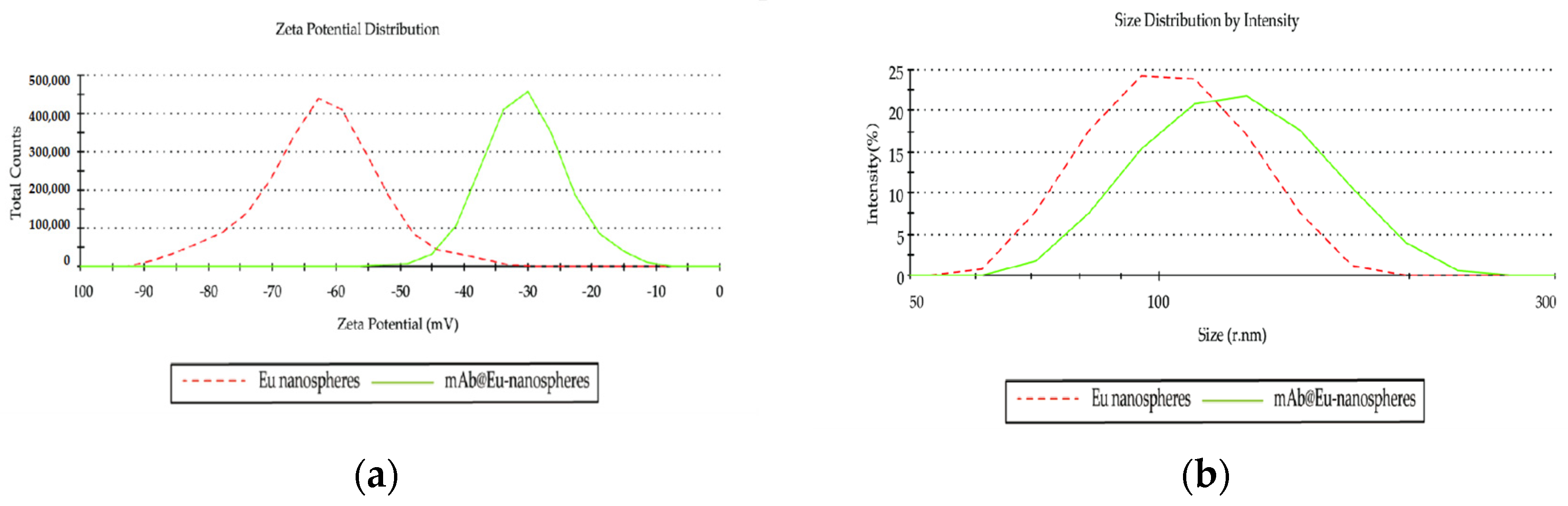
2.6. Characterization of the mAb@Eu-Nanospheres
2.7. iPOCT Assembly
2.8. Preparation of the Running Buffer
2.9. Sample Treatment
2.10. Validation of the POCT
3. Results and Discussion
3.1. Optimization of mAb@Eu-Nanospheres
3.2. Optimization of iPOCT
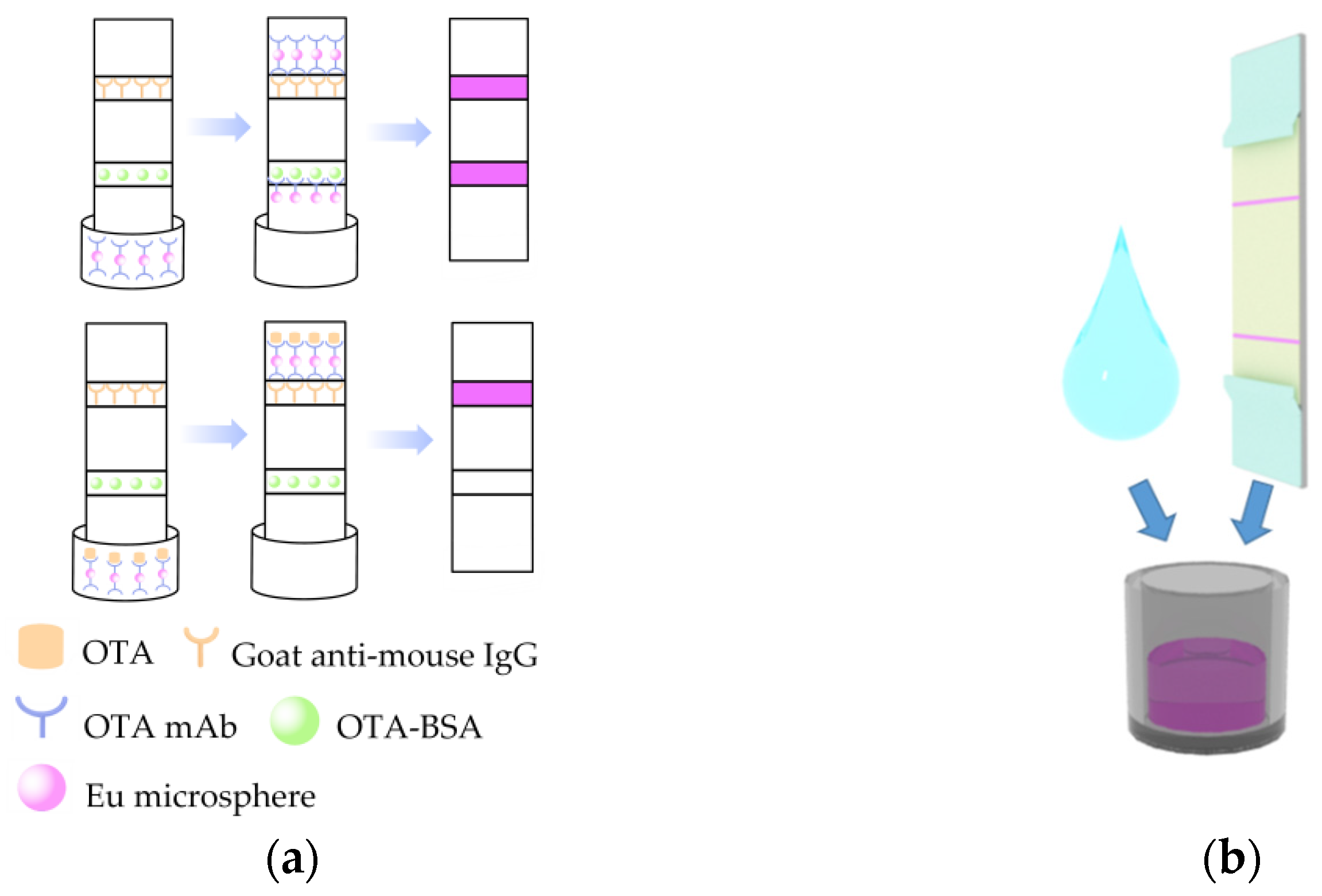
3.3. Procedure of the iPOCT
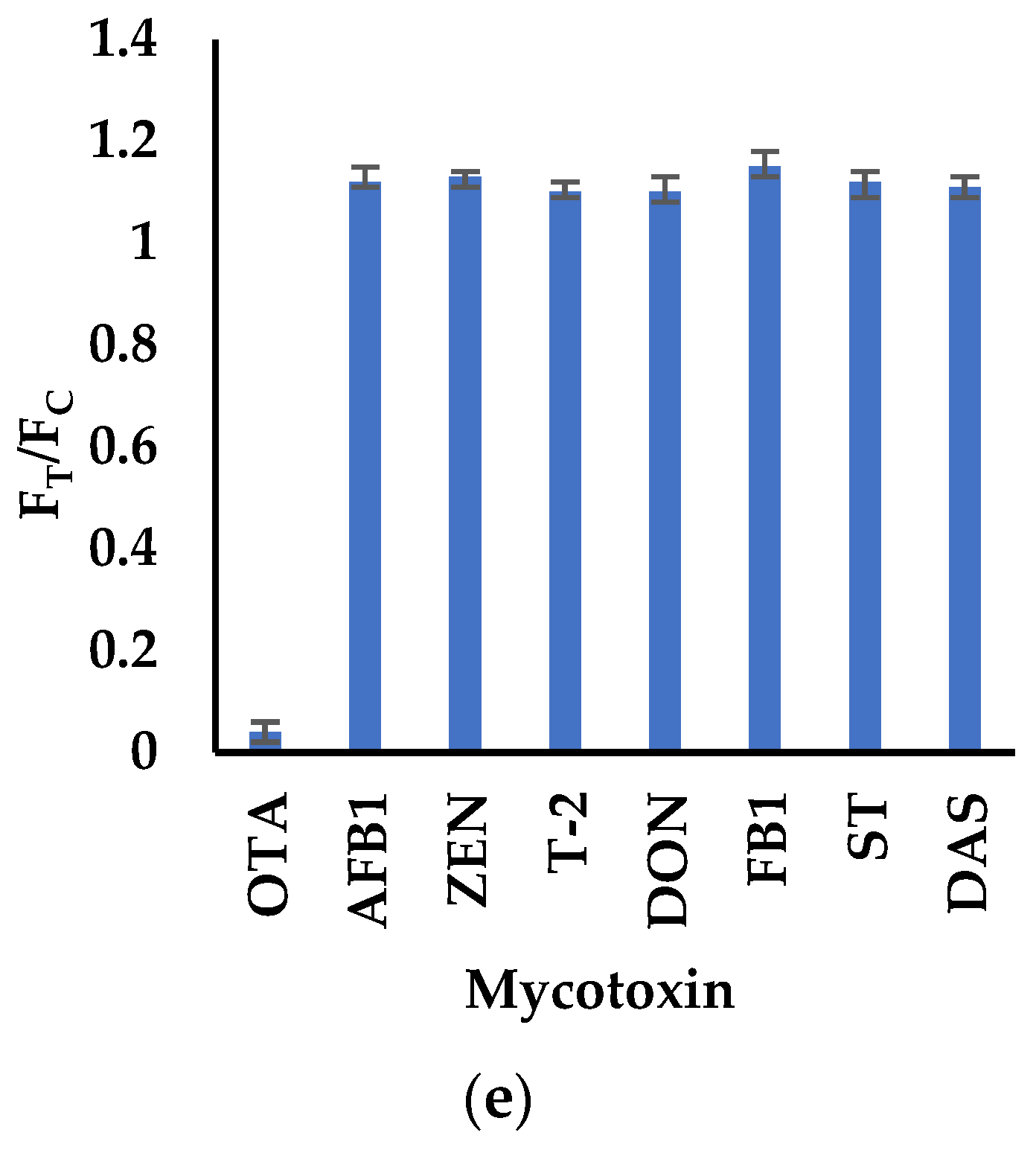
3.4. Validation of the POCT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Monitor Ion | Mode | Precursor Ion | Production | Dwell Time (ms) | Q1 (V) | CE | Q3 (V) |
|---|---|---|---|---|---|---|---|
| [M + H]+ | ESI+ | 404.1 | 239.0 | 100 | −12 | −23 | −18 |
| 404.1 | 358.0 | 100 | −12 | −16 | −26 |
| Fapas QC Naterial Data Sheet | Matrix | Weight/Volume of Contents | Analyte | Assigned Value Xa | Range for |Z| ≤ 2 | Units | No. of Data Points Producing Xa |
|---|---|---|---|---|---|---|---|
| T17131QC | Wheat | 55 g | Ochratoxin A | 1.66 | 0.85–2.43 | μg/kg | 44 |
References
- Dong, G. A new story for wheat into China. Nat. Plants 2018, 4, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Balkan endemic nephropathy—Still continuing enigma, risk assessment and underestimated hazard of joint mycotoxin exposure of animals or humans. Chem. Biol. Interact. 2017, 261, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, T.; Qi, X.; Gao, J.; Huang, K.; He, X.; Luo, H.; Xu, W. Limited link between oxidative stress and ochratoxin A-induced renal injury in an acute toxicity rat model. Toxins 2016, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Co-occurrence of citrinin and ochratoxin A in rice in Asia and its implications for human health. J. Sci. Food Agric. 2017, 98, 2055–2059. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Ma, Z.; Zhang, Q.; Li, H. The occurrence and contamination level of ochratoxin A in plant and animal-derived food commodities. Molecules 2021, 26, 6928. [Google Scholar] [CrossRef]
- Jonatova, P.; Dzuman, Z.; Prusova, N.; Hajslova, J.; Stranska-Zachariasova, M. Occurrence of ochratoxin A and its stereoisomeric degradation product in various types of coffee available in the Czech market. World Mycotoxin J. 2020, 13, 97–107. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Development and validation of a method based on a QuEChERS procedure and heart-cutting GC-MS for determination of five mycotoxins in cereal products. J. Sep. Sci. 2010, 33, 600–609. [Google Scholar] [CrossRef]
- Hajslova, J.; Zachariasova, M.; Cajka, T. Analysis of multiple mycotoxins in food. Methods Mol. Biol. 2011, 747, 233–258. [Google Scholar] [CrossRef]
- Elaridi, J.; Yamani, O.; Al Matari, A.; Dakroub, S.; Attieh, Z. Determination of ochratoxin A (OTA), ochratoxin B (OTB), T-2, and HT-2 toxins in wheat grains, wheat flour, and bread in lebanon by LC-MS/MS. Toxins 2019, 11, 471. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; von Holst, C.; Lippolis, V.; De Girolamo, A.; Logrieco, A.F.; Mol, H.G.J.; Pascale, M. Evaluation of mycotoxin screening tests in a verification study involving first time users. Toxins 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Fadlalla, M.H.; Ling, S.; Wang, R.; Li, X.; Yuan, J.; Xiao, S.; Wang, K.; Tang, S.; Elsir, H.; Wang, S. Development of ELISA and lateral flow immunoassays for ochratoxins (OTA and OTB) detection based on monoclonal antibody. Front. Cell. Infect. Microbiol. 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, L.; Duan, N.; Wang, W.; Yu, Q.; Wang, Z. A test strip for ochratoxin A based on the use of aptamer-modified fluorescence upconversion nanoparticles. Microchim. Acta 2018, 185, 497. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Liu, H.; Xu, L.; Kuang, H. Rapid and sensitive detection of ochratoxin A in rice flour using a fluorescent microsphere immunochromatographic test strip assay. Food Agric. Immunol. 2020, 31, 563–574. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Wu, Y.H.; Xiong, Q.R.; Xu, H.Y.; Xiong, Y.H.; Liu, K.; Jin, Y.; Lai, W.H. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014, 54, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.E.; Gadallah, A.S.; Mohamed, M.B.; Azzouz, I.M. Photoluminescence and upconversion on Ag/CdTe quantum dots. Opt. Laser Technol. 2014, 63, 8–12. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Tian, Y.; Cui, M.; Huang, B.; Luo, T.; Zhang, S.; Wang, H. Rapid, simultaneous detection of mycotoxins with smartphone recognition-based immune microspheres. Anal. Bioanal. Chem. 2021, 413, 3683–3693. [Google Scholar] [CrossRef]
- Bueno, D.; Muñoz, R.; Marty, J.L. Fluorescence analyzer based on smartphone camera and wireless for detection of Ochratoxin A. Sens. Actuators B Chem. 2016, 232, 462–468. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Z.; Min, Q.; Nan, W.; Kun, Z.; Daolin, D. Dual-label time-resolved fluoroimmunoassay as an advantageous approach for investigation of diethyl phthalate & dibutyl phthalate in surface water. Sci. Total. Environ. 2019, 695, 133793. [Google Scholar] [CrossRef]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper–plastic hybrid microfluidic device for smartphone-based colorimetric analysis of urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Yi, C.; Jiang, L.; Wu, J.; Chen, X.; Shen, X.; Sun, Y.; Lei, H. A Smartphone-based Quantitative Detection Device Integrated with Latex Microsphere Immunochromatography for On-site Detection of Zearalenone in Cereals and Feed. Sens. Actuators B 2019, 290, 170–179. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, P.; Wang, D.; Jiang, J.; Chen, X.; Liu, Y.; Zhang, Z.; Tang, B.Z.; Li, P. AIEgens enabled ultrasensitive point-of-care test for multiple targets of food safety: Aflatoxin B1 and cyclopiazonic acid as an example. Biosens. Bioelectron. 2021, 182, 113188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wen, K.; Wang, Z.; Jiang, H.; Beier, R.C.; Shen, J. An ultra-sensitive monoclonal antibody-based fluorescent microsphere immunochromatographic test strip assay for detecting aflatoxin M 1 in milk. Food Control 2016, 60, 588–595. [Google Scholar] [CrossRef]
- Martina, Z.; Fabio, D.N.; Donato, C.; Elisa, M.; Laura, A.; Massimo, G.; Mara, M.; Claudio, B.; Aldo, R. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin A in wine and coffee. Anal. Chim. Acta 2021, 1163, 338515. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, C.; Huang, Y.; Yan, J.; Chen, A. A Lateral Flow Strip Based Aptasensor for Detection of Ochratoxin A in Corn Samples. Molecules 2018, 23, 291. [Google Scholar] [CrossRef]

| Sample | OTA Spiked Level (ng/mL) | OTA Found (ng/mL) | RSD | |
|---|---|---|---|---|
| UPLC-MS/MS | iPOCT | |||
| 0.1 | 0.096 | 0.112 | 9.1 | |
| 0.5 | 0.501 | 0.487 | 5.4 | |
| Wheat | 1 | 1.022 | 1.028 | 6.7 |
| 1.5 | 1.503 | 1.507 | 4.7 | |
| 2 | 2.030 | 2.057 | 3.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Hu, X.; Jiang, J.; Tang, X.; Tian, Z.; Zhang, Z.; Li, P. Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay. Foods 2023, 12, 431. https://doi.org/10.3390/foods12030431
Tian Y, Hu X, Jiang J, Tang X, Tian Z, Zhang Z, Li P. Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay. Foods. 2023; 12(3):431. https://doi.org/10.3390/foods12030431
Chicago/Turabian StyleTian, Yunxin, Xiaofeng Hu, Jun Jiang, Xiaoqian Tang, Zhiquan Tian, Zhaowei Zhang, and Peiwu Li. 2023. "Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay" Foods 12, no. 3: 431. https://doi.org/10.3390/foods12030431
APA StyleTian, Y., Hu, X., Jiang, J., Tang, X., Tian, Z., Zhang, Z., & Li, P. (2023). Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay. Foods, 12(3), 431. https://doi.org/10.3390/foods12030431








