Protection of Whey Polypeptide on the Lipid Oxidation, Color, and Textural Stability of Frozen–Thawed Spanish Mackerel Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of WPH
2.3. Preparation of Surimi
2.4. The Measurement of Thiobarbituric Acid Reactive Substances
2.5. The Measurement of Total Volatile Basic Nitrogen
2.6. The Measurement of Gumminess
2.7. Determination of Resilience
2.8. Determination of Adhesiveness
2.9. Determination of WPH on the Color of Surimi
2.10. Determination of Shear Force
2.11. Statistical Analysis
3. Results and Discussion
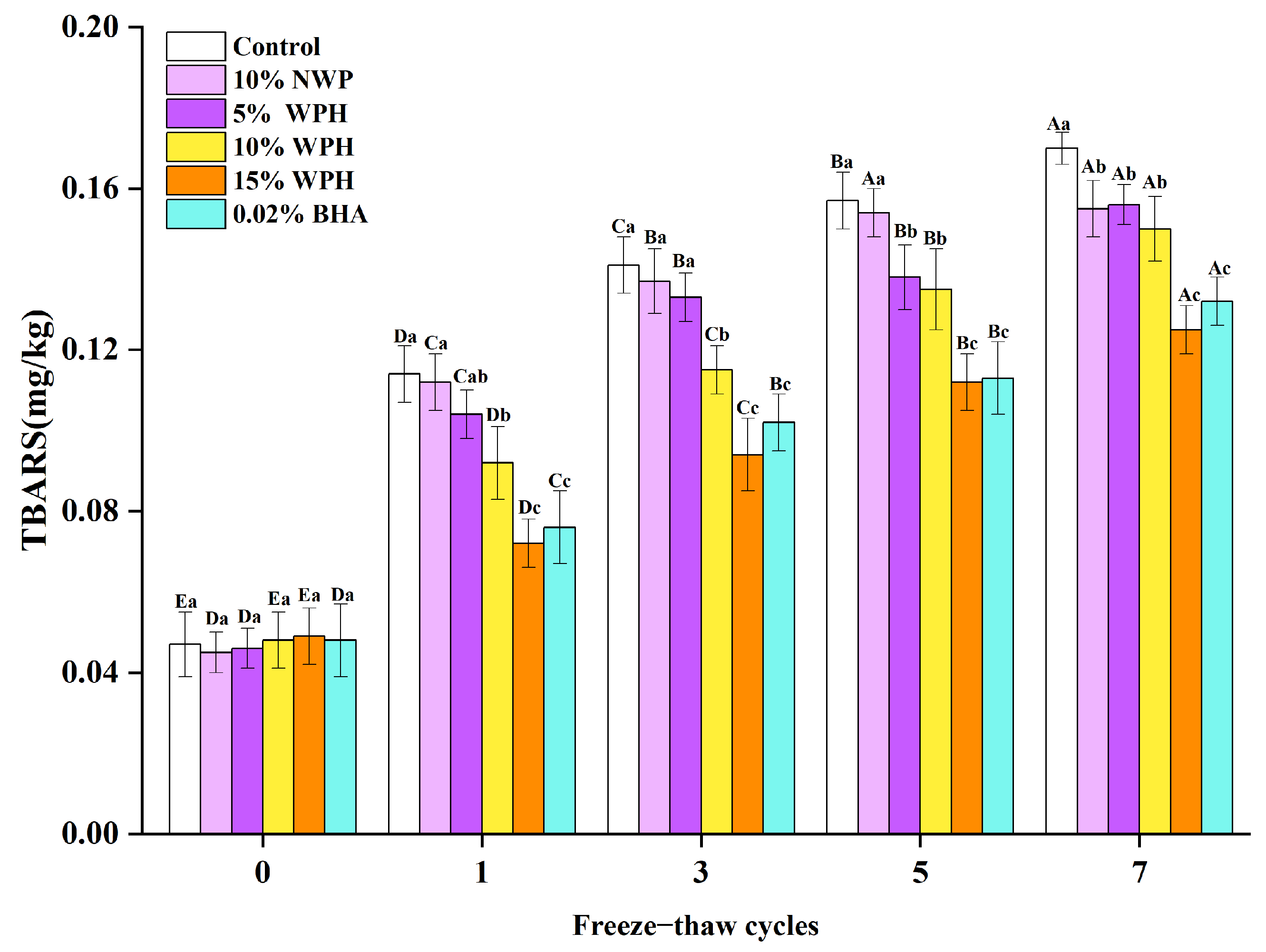
3.1. Effect of WPH on TBARS Values of Repeated Freeze–Thawing Surimi
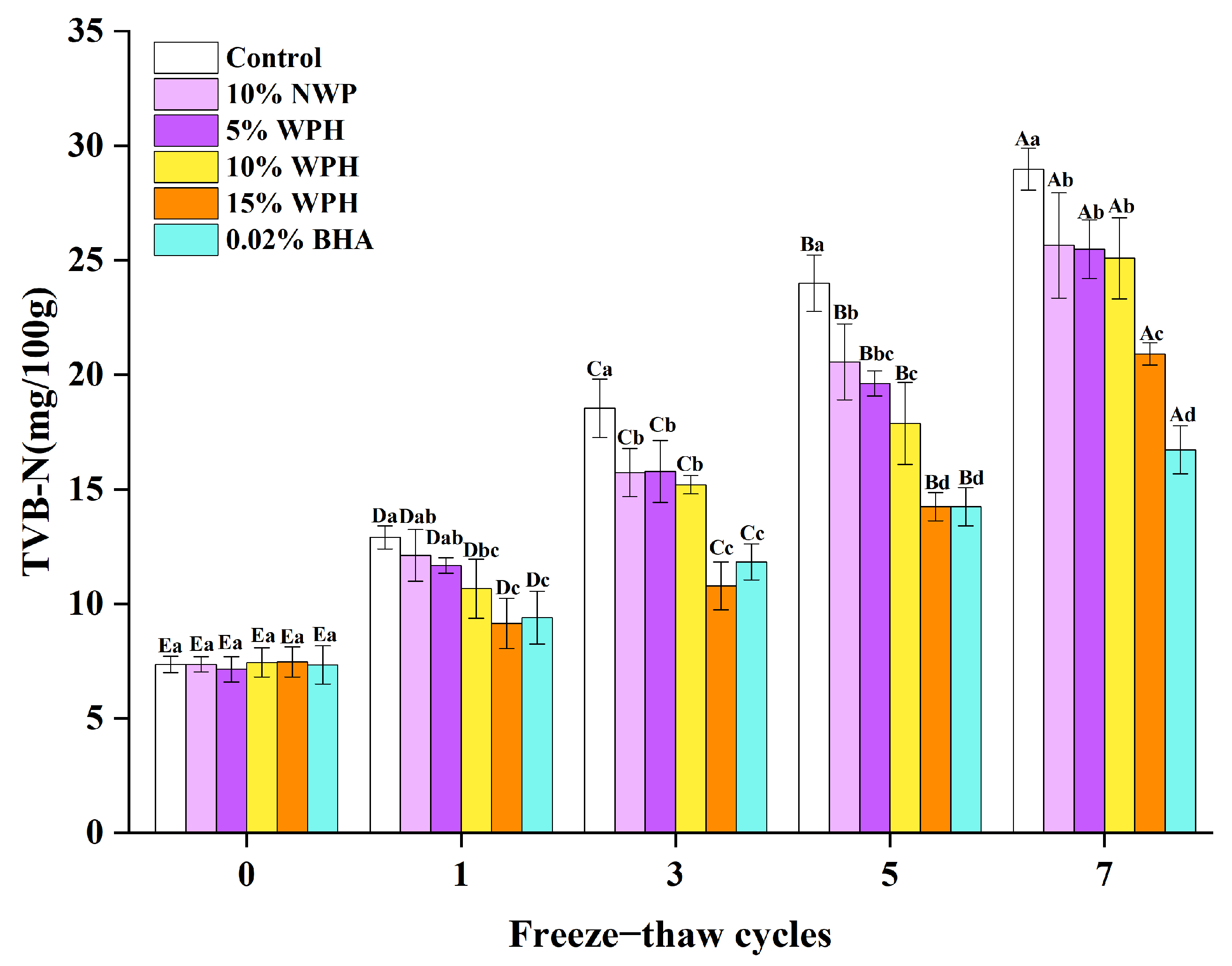
3.2. Effect of WPH on TVB-N Values of Repeated Freeze–Thawing Surimi
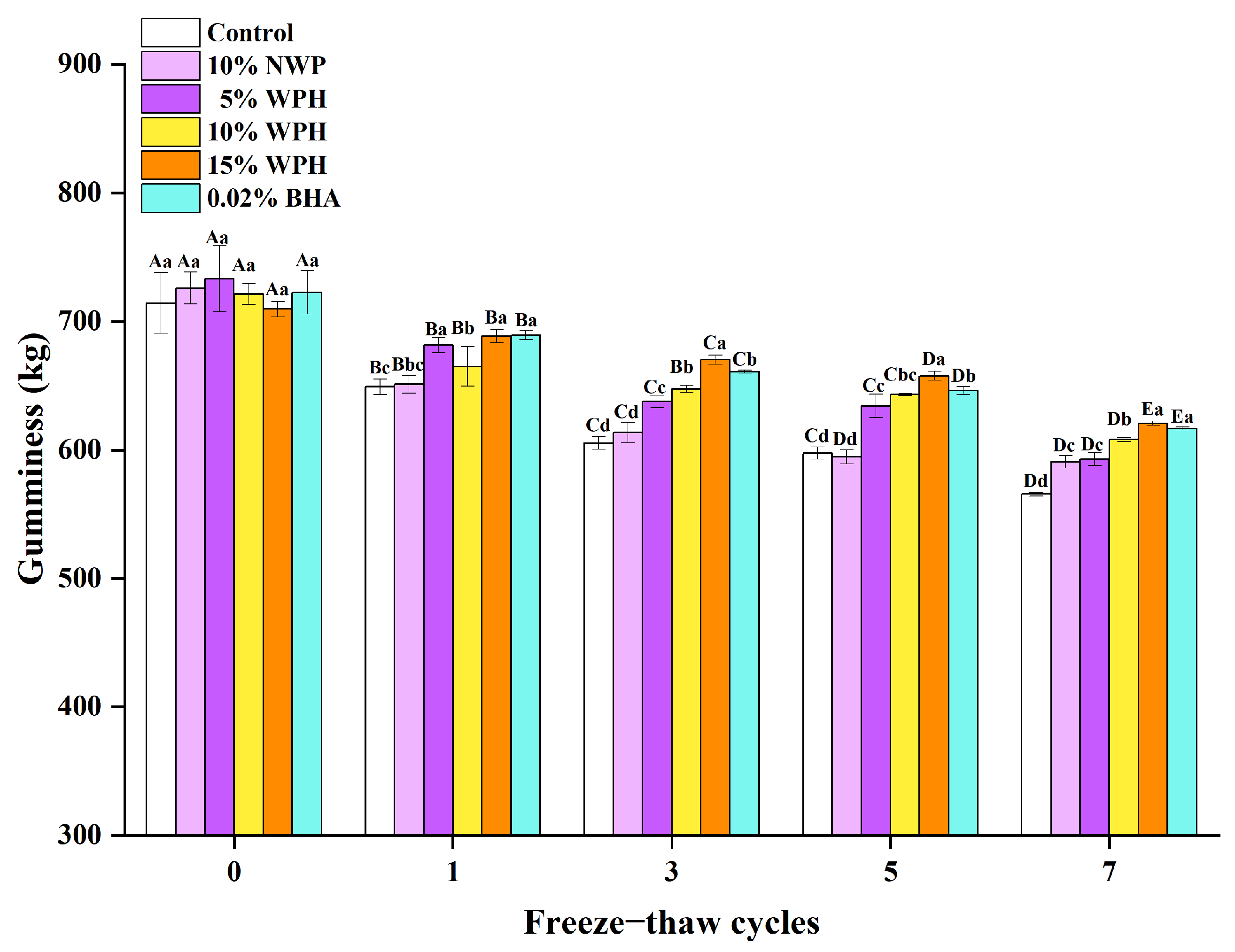
3.3. Effects of WPH on Gumminess Stability of Repeatedly Freeze–Thawing Surimi
3.4. Effects of WPH on the Resilience Stability of Repeatedly Freeze–Thawing Surimi
3.5. Effects of WPH on the Adhesiveness Stability of Repeatedly Freeze–Thawing Surimi
3.6. Effects of WPH on the L*, a*, and b* Stability of Repeatedly Freeze–Thawing Surimi
3.7. Effects of WPH on the Whiteness Stability of Repeatedly Freeze–Thawing Surimi
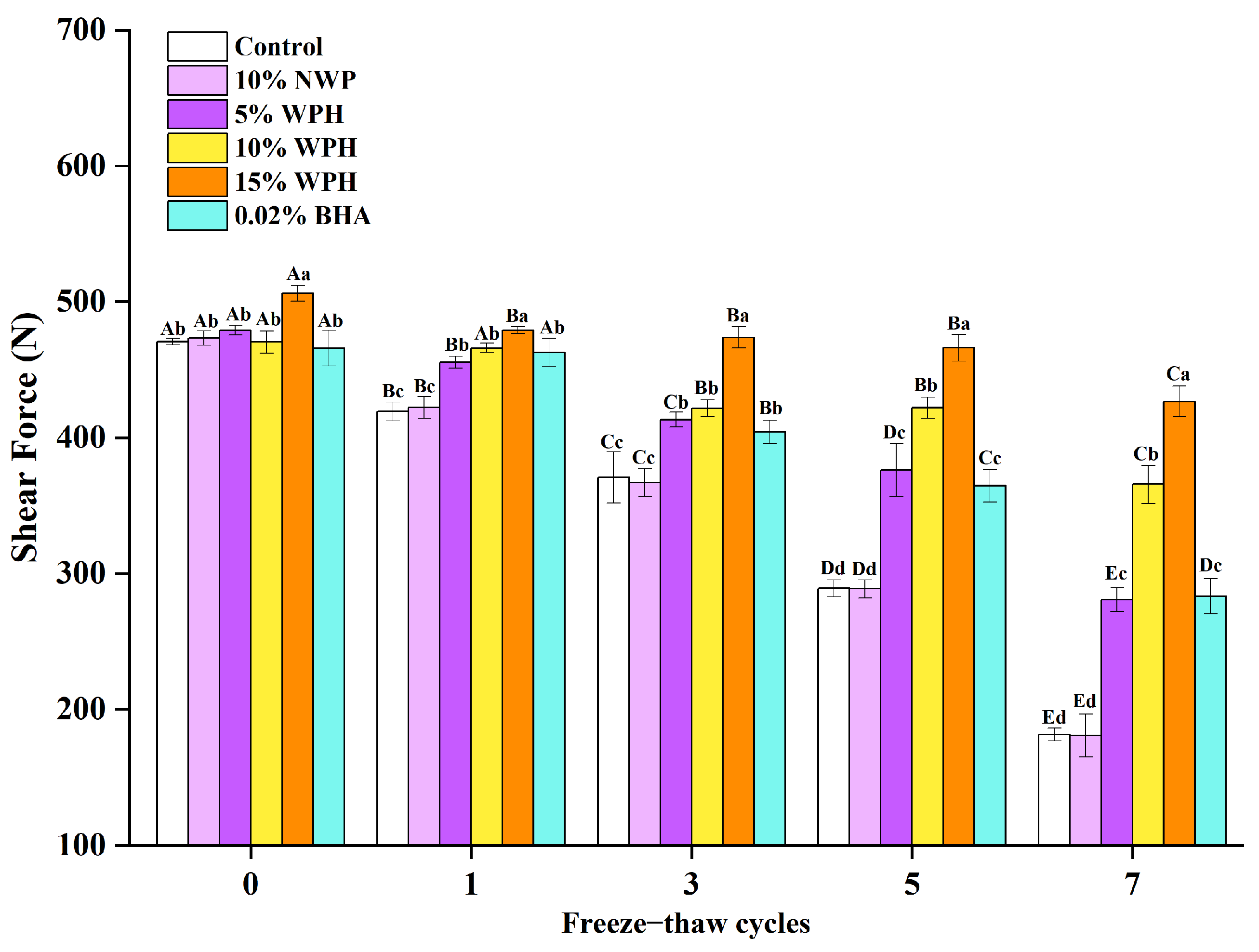
3.8. Effects of WPH on Shear Force Stability of Repeatedly Freeze–Thawing Surimi
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Zhu, Y.; Ye, T.; Nie, Y.; Jiang, S.; Lin, L.; Lu, J. Physicochemical properties and microstructure of composite surimi gels: The effects of ultrasonic treatment and olive oil concentration. Ultrason. Sonochemistry 2022, 88, 106065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lei, M.; Lin, J.; Zhao, W.; Zeng, X.; Bai, W. The roles of lipoxygenases and autoxidation during mackerel (Scomberomorus niphonius) dry-cured processing. Food Res. Int. 2023, 173, 113309. [Google Scholar] [CrossRef]
- Wu, S. Effect of pullulan on gel properties of Scomberomorus niphonius surimi. Int. J. Biol. Macromol. 2016, 93, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; Thavaraj, P.; Yang, X.; Guo, Y. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017, 224, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Dong, Y.; Ding, H.; Chen, K.; Lu, T.; Dai, Z. Study on the mechanism of protein hydrolysate delaying quality deterioration of frozen surimi. LWT 2022, 167, 113767. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of Cryoprotectants in Surimi and Factors Affecting Surimi Gel Properties: A Review. Food Rev. Int. 2022, 38, 1103–1122. [Google Scholar] [CrossRef]
- Minj, S.; Anand, S. Whey Proteins and Its Derivatives: Bioactivity, Functionality, and Current Applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Master, P.B.Z.; Macedo, R.C.O. Effects of dietary supplementation in sport and exercise: A review of evidence on milk proteins and amino acids. Crit. Rev. Food Sci. Nutr. 2020, 61, 1225–1239. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, X.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Antioxidant stability and in vitro digestion of β-carotene-loaded oil-in-water emulsions stabilized by whey protein isolate-Mesona chinensis polysaccharide conjugates. Food Res. Int. 2023, 174, 113584. [Google Scholar] [CrossRef]
- Jafar, S.; Kamal, H.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel whey protein hydrolysates displayed enhanced cholesteryl esterase and lipase inhibitory, anti-hypertensive and anti-haemolytic properties. LWT 2018, 98, 212–218. [Google Scholar] [CrossRef]
- Moro, T.; Brightwell, C.R.; Velarde, B.; Fry, C.S.; Nakayama, K.; Sanbongi, C.; Volpi, E.; Rasmussen, B.B. Whey Protein Hydrolysate Increases Amino Acid Uptake, mTORC1 Signaling, and Protein Synthesis in Skeletal Muscle of Healthy Young Men in a Randomized Crossover Trial. J. Nutr. 2019, 149, 1149–1158. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Zaitoun, B.J.; Palmer, N.; Amamcharla, J.K. Characterization of a Commercial Whey Protein Hydrolysate and Its Use as a Binding Agent in the Whey Protein Isolate Agglomeration Process. Foods 2022, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.C.; Udechukwu, M.C.; Mohan, A.; Anderson, D.M.; Udenigwe, C.C.; Colombo, S.M.; Collins, S.A. Whey protein hydrolysate as a multi-functional ingredient in diets for Arctic charr: Effect on growth response and hepatic antioxidative status. Anim. Feed. Sci. Technol. 2020, 270, 114698. [Google Scholar] [CrossRef]
- Peng, X.; Ruan, S.; Liu, Y.; Huang, L.; Zhang, C. The addition of hydrolyzed whey protein fractions to raw pork patties with subsequent chilled storage and its effect on oxidation and gel properties. CyTA J. Food 2018, 16, 553–560. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Ding, N.; Liang, S.; Mubango, E.; Zheng, Y.; Yu, Q.; Dai, R.; Tan, Y.; Luo, Y.; et al. Cryoprotective effect of fistular onion stalk polysaccharide on frozen surimi derived from bighead carp: Physicochemical properties and gel quality during storage. Food Hydrocoll. 2023, 148, 109404. [Google Scholar] [CrossRef]
- Ghimire, A.; Paudel, N.; Poudel, R. Effect of pomegranate peel extract on the storage stability of ground buffalo (Bubalus bubalis) meat. LWT 2022, 154, 112690. [Google Scholar] [CrossRef]
- Gan, S.; Zhang, M.; Mujumdar, A.S.; Jiang, Q. Effects of different thawing methods on quality of unfrozen meats. Int. J. Refrig. 2022, 134, 168–175. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Chien, H.-I.; Lee, Y.-C.; Kao, J.-C.; Huang, Y.-R.; Huang, Y.-L.; Huang, C.-Y.; Tsai, Y.-H. Physicochemical Quality Retention during Cold Storage of Prepackaged Barramundi Meat Processed with a New Microwave-Assisted Induction Heating Technology. Foods 2023, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ye, G.; Yang, D.; Xie, J.; Huo, Y. Observation on the ice crystal formation process of large yellow croaker (Pseudosciaena crocea) and the effect of multiple cryoprotectants pre-soaking treatments on frozen quality. Cryobiology 2023, 113, 104580. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, F.; Pan, N.; Kong, B.; Xia, X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021, 172, 108335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, M.; Fang, F.; Fu, C.; Xing, S.; Qian, C.; Liu, J.; Kan, J.; Jin, C. Effect of sous vide cooking treatment on the quality, structural properties and flavor profile of duck meat. Int. J. Gastron. Food Sci. 2022, 29, 100565. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Tong, Y.; Zhong, Q.; Zhuang, Y.; Yang, H. Physicochemical properties of surimi gel from silver carp as affected by ultrasonically emulsified vegetable oils. Int. J. Food Prop. 2023, 26, 1214–1229. [Google Scholar] [CrossRef]
- Kawamura, K.; Ma, D.; Pereira, A.; Ahn, D.; Kim, D.; Kang, I. Subzero Saline Chilling with or without Prechilling in Icy Water Improved Chilling Efficiency and Meat Tenderness of Broiler Carcasses. Poult. Sci. 2023, 102, 103070. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhao, M.; Li, Y.; Kong, B.; Liu, Q.; Sun, F.; Yu, W.; Xia, X. Effect of hot air gradient drying on quality and appearance of beef jerky. LWT 2021, 150, 111974. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, J.; Li, X.; Wang, J.; Yi, S.; Zhu, W.; Xu, Y.; Li, J. Prediction of the freshness of horse mackerel (Trachurus japonicus) using E-nose, E-tongue, and colorimeter based on biochemical indexes analyzed during frozen storage of whole fish. Food Chem. 2023, 402, 134325. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef]
- Rahmanifarah, K.; Regenstein, J.M.; Nikoo, M. Physicochemical properties of silver carp (Hypophthalmichthys molitrix) mince sausages as influenced by washing and frozen storage. Aquac. Fish. 2023, 8, 403–409. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Xia, X.; Liu, Q.; Diao, X. Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process. Biochem. 2013, 48, 863–870. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Y. Inhibition of Oxidant-Induced Biochemical Changes of Pork Myofibrillar Protein by Hydrolyzed Potato Protein. J. Food Sci. 2008, 73, C482–C487. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Shi, H.; Zhang, X.; Dong, P.; Luo, X.; Qin, H.; Zhang, Y.; Mao, Y.; Holman, B.W. Influence of low-energy electron beam irradiation on the quality and shelf-life of vacuum-packaged pork stored under chilled and superchilled conditions. Meat Sci. 2023, 195, 109019. [Google Scholar] [CrossRef]
- Zhou, P.-C.; Xie, J. Effect of different thawing methods on the quality of mackerel (Pneumatophorus japonicus). Food Sci. Biotechnol. 2021, 30, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Gao, H.; Chen, C.; Xie, J. Effects of starch/polyvinyl alcohol active film containing cinnamaldehyde on the quality of large yellow croaker (Pseudosciaena crocea) proteins during frozen storage. Food Chem. 2022, 389, 133065. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wan, J.; Li, X.; Li, J. Effects of different thawing methods on physicochemical properties and structure of largemouth bass (Micropterus salmoides). J. Food Sci. 2020, 85, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Qu, Z.; Liu, M.; Wang, J.; Zhu, M.; Liu, Z.; Li, J.; Zhan, X.; Jia, F. Effect of curdlan on the quality of frozen-cooked noodles during frozen storage. J. Cereal Sci. 2020, 95, 103019. [Google Scholar] [CrossRef]
- Fan, L.; Ruan, D.; Shen, J.; Hu, Z.; Liu, C.; Chen, X.; Xia, W.; Xu, Y. The role of water and oil migration in juiciness loss of stuffed fish ball with the fillings of pig fat/meat as affected by freeze-thaw cycles and cooking process. LWT 2022, 159, 113244. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Sun, Y.; Tan, H.; Zhang, Y. Effect of drying methods on the solubility and amphiphilicity of room temperature soluble gelatin extracted by microwave-rapid freezing-thawing coupling. Food Chem. 2021, 351, 129226. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yin, T.; Xiong, S.; You, J.; Hu, Y.; Huang, Q. Gelling properties of silver carp surimi incorporated with konjac glucomannan: Effects of deacetylation degree. Int. J. Biol. Macromol. 2021, 191, 925–933. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.J.H.; Kang, Y.; Cui, H.; Yang, H. Effects of acid and alkaline treatments on physicochemical and rheological properties of tilapia surimi prepared by pH shift method during cold storage. Food Res. Int. 2021, 145, 110424. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.C.d.A.; Silva, E.L.L.; Ferreira, V.C.d.S.; Madruga, M.S.; da Silva, F.A.P. Oxidative stability of green weakfish (Cynoscion virescens) by-product surimi and surimi gel enhanced with a Spondias mombin L. waste phenolic-rich extract during cold storage. Food Biosci. 2022, 50, 102021. [Google Scholar] [CrossRef]
- Chan, J.T.; Omana, D.A.; Betti, M. Application of high pressure processing to improve the functional properties of pale, soft, and exudative (PSE)-like turkey meat. Innov. Food Sci. Emerg. Technol. 2011, 12, 216–225. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, F.; Wu, J.; Huang, D.; Huang, J.; Wang, S. Effects and mechanism of antifreeze peptides from silver carp scales on the freeze-thaw stability of frozen surimi. Food Chem. 2022, 396, 133717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L.; Wang, J.; Li, X.; Li, J.; Cui, F.; Yi, S.; Xu, Y.; Zhu, W.; Mi, H. Effects of ultrasound–assisted freezing on the quality of large yellow croaker (Pseudosciaena crocea) subjected to multiple freeze–thaw cycles. Food Chem. 2023, 404, 134530. [Google Scholar] [CrossRef]
- Cui, B.; Mao, Y.; Liang, H.; Li, Y.; Li, J.; Ye, S.; Chen, W.; Li, B. Properties of soybean protein isolate/curdlan based emulsion gel for fat analogue: Comparison with pork backfat. Int. J. Biol. Macromol. 2022, 206, 481–488. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, H.; Song, L.; Xue, Y.; Xue, C. Impact of O/W emulsion stabilized by different soybean phospholipid/sodium caseinate ratios on the physicochemical, rheological and gel properties of surimi sausage. LWT 2023, 175, 114461. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, Y.; Puolanne, E.; Ertbjerg, P. Role of freezing-induced myofibrillar protein denaturation in the generation of thaw loss: A review. Meat Sci. 2022, 190, 108841. [Google Scholar] [CrossRef]
- Wan, W.; Feng, J.; Wang, H.; Du, X.; Wang, B.; Yu, G.; Xia, X. Influence of repeated freeze-thaw treatments on the oxidation and degradation of muscle proteins from mirror carp (Cyprinus carpio L.), based on myofibrillar protein structural changes. Int. J. Biol. Macromol. 2023, 226, 454–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Shu, Y.; Zhang, X.; Yang, T.; Qi, W.; Xu, H.-N. Improving the freeze-thaw stability of pork sausage with oleogel-in-water Pickering emulsion used for pork backfat substitution. LWT 2023, 180, 114698. [Google Scholar] [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on the effect of the freeze–thawing process on the physicochemical properties and microstructures of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) muscle. Food Chem. 2007, 104, 113–121. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Xiong, Y.L.; Feng, J.; Ramirez-Suarez, J.C.; Webster, C.D.; Thompson, K.R.; Muzinic, L.A. Quality Changes in Australian Red Claw Crayfish (Cherax Quadricarinatus) Subjected To Multiple Freezing-Thawing Cycles. J. Food Qual. 2003, 26, 285–298. [Google Scholar] [CrossRef]
- Xu, C.; Zang, M.; Qiao, X.; Wang, S.; Zhao, B.; Shi, Y.; Bai, J.; Wu, J. Effects of ultrasound-assisted thawing on lamb meat quality and oxidative stability during refrigerated storage using non-targeted metabolomics. Ultrason. Sonochemistry 2022, 90, 106211. [Google Scholar] [CrossRef]
- Pan, N.; Dong, C.; Du, X.; Kong, B.; Sun, J.; Xia, X. Effect of freeze-thaw cycles on the quality of quick-frozen pork patty with different fat content by consumer assessment and instrument-based detection. Meat Sci. 2020, 172, 108313. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Y.; Yin, Y.; Zhao, H.; Li, X.; Yi, S.; Li, J. Improvement of the gel properties and flavor adsorption capacity of fish myosin upon yeast β-glucan incorporation. Food Chem. 2022, 397, 133766. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Niaz, N.; Ahmad, M.N.; Hassan, A.; Nawaz, A.; Ahmad, I.; Wang, P.-K. Cryoprotective effect of egg white proteins and xylooligosaccharides mixture on oxidative and structural changes in myofibrillar proteins of Culter alburnus during frozen storage. Int. J. Biol. Macromol. 2020, 158, 865–874. [Google Scholar] [CrossRef]
- Chen, H.; Kong, B.; Guo, Y.; Xia, X.; Diao, X.; Li, P. The Effectiveness of Cryoprotectants in Inhibiting Multiple Freeze-Thaw-Induced Functional and Rheological Changes in the Myofibrillar Proteins of Common Carp (Cyprinus carpio) Surimi. Food Biophys. 2013, 8, 302–310. [Google Scholar] [CrossRef]
- Zaghbib, I.; Felix, M.; Romero, A.; Arafa, S.; Hassouna, M. Effects of Whitening Agents and Frozen Storage on the Quality of Sardine (Sardina pilchardus) Surimi: Physicochemical and Mechanical Properties. J. Aquat. Food Prod. Technol. 2016, 26, 29–42. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhou, X.; Zang, H.; Zhang, R.; Yang, H.; Jin, S.; Feng, X.; Shan, A. Oxidative stability and gelation properties of myofibrillar protein from chicken breast after post-mortem frozen storage as influenced by phenolic compound-pterostilbene. Int. J. Biol. Macromol. 2022, 221, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Carne, A.; Ha, M.; Franks, P. Physical Interventions to Manipulate Texture and Tenderness of Fresh Meat: A Review. Int. J. Food Prop. 2013, 17, 433–453. [Google Scholar] [CrossRef]
- Peng, X.; Liu, C.; Wang, B.; Kong, L.; Wen, R.; Zhang, H.; Yu, X.; Bai, Y.; Jang, A. Hygroscopic properties of whey protein hydrolysates and their effects on water retention in pork patties during repeated freeze–thaw cycles. LWT 2023, 184, 114984. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.A.; Rajput, N.; Naeem, M.; Zhang, W.; Li, C.-B.; Zhou, G. Desmin as molecular chaperone for myofibrillar degradation during freeze-thaw cycles. Food Chem. 2022, 386, 132691. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kong, L.; Zhang, X.; Wen, R.; Peng, X. Protection of Whey Polypeptide on the Lipid Oxidation, Color, and Textural Stability of Frozen–Thawed Spanish Mackerel Surimi. Foods 2023, 12, 4464. https://doi.org/10.3390/foods12244464
Li Y, Kong L, Zhang X, Wen R, Peng X. Protection of Whey Polypeptide on the Lipid Oxidation, Color, and Textural Stability of Frozen–Thawed Spanish Mackerel Surimi. Foods. 2023; 12(24):4464. https://doi.org/10.3390/foods12244464
Chicago/Turabian StyleLi, Yunying, Lingru Kong, Xiaotong Zhang, Rongxin Wen, and Xinyan Peng. 2023. "Protection of Whey Polypeptide on the Lipid Oxidation, Color, and Textural Stability of Frozen–Thawed Spanish Mackerel Surimi" Foods 12, no. 24: 4464. https://doi.org/10.3390/foods12244464
APA StyleLi, Y., Kong, L., Zhang, X., Wen, R., & Peng, X. (2023). Protection of Whey Polypeptide on the Lipid Oxidation, Color, and Textural Stability of Frozen–Thawed Spanish Mackerel Surimi. Foods, 12(24), 4464. https://doi.org/10.3390/foods12244464




