Improving the Flavour of Enzymatically Hydrolysed Beef Liquid by Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
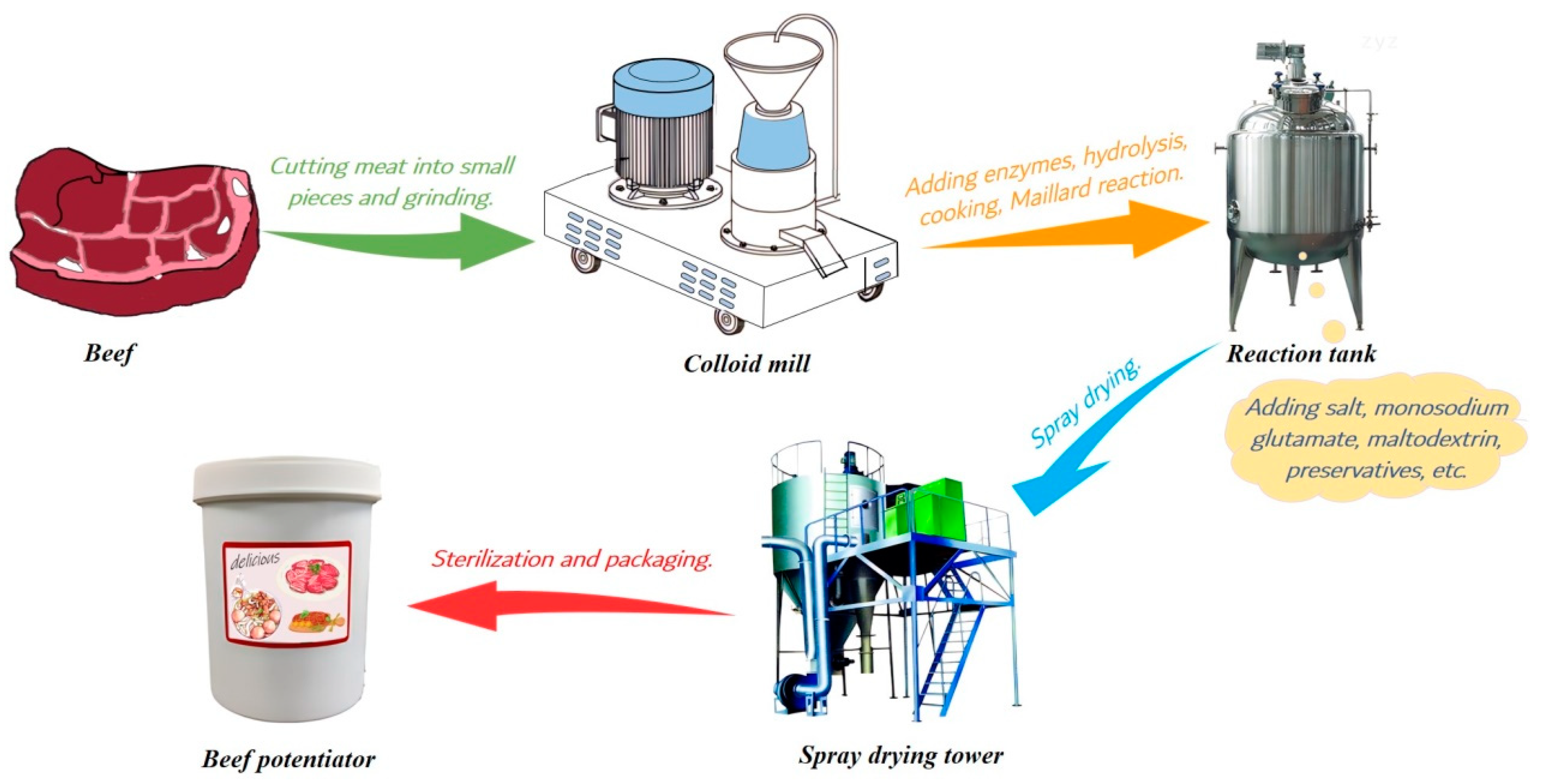
2.2. Beef Homogenate Preparation and Treatment
2.3. Sensory Evaluation
2.4. Analyses of Total Nitrogen, Formaldehyde Nitrogen, Hydrolysis Degree, Total Sugars and Reducing Sugars
2.5. Free Amino Acids Analysis
2.6. Thresholds and Taste Intensity of FAAs
2.7. Protease Activity Analysis during Sonication
2.8. Volatile Compound Extraction Using Solid-Phase Micro-Extraction (SPME)
2.9. Analyses of GC-Olfactometry and Flavour Dilution Factor
2.10. Qualitative Analysis of Volatile Compounds
2.11. Quantitative Analysis of Key Odorants
2.12. Odour Activity Value (OAV)
2.13. Statistical Analysis
3. Results and Discussion
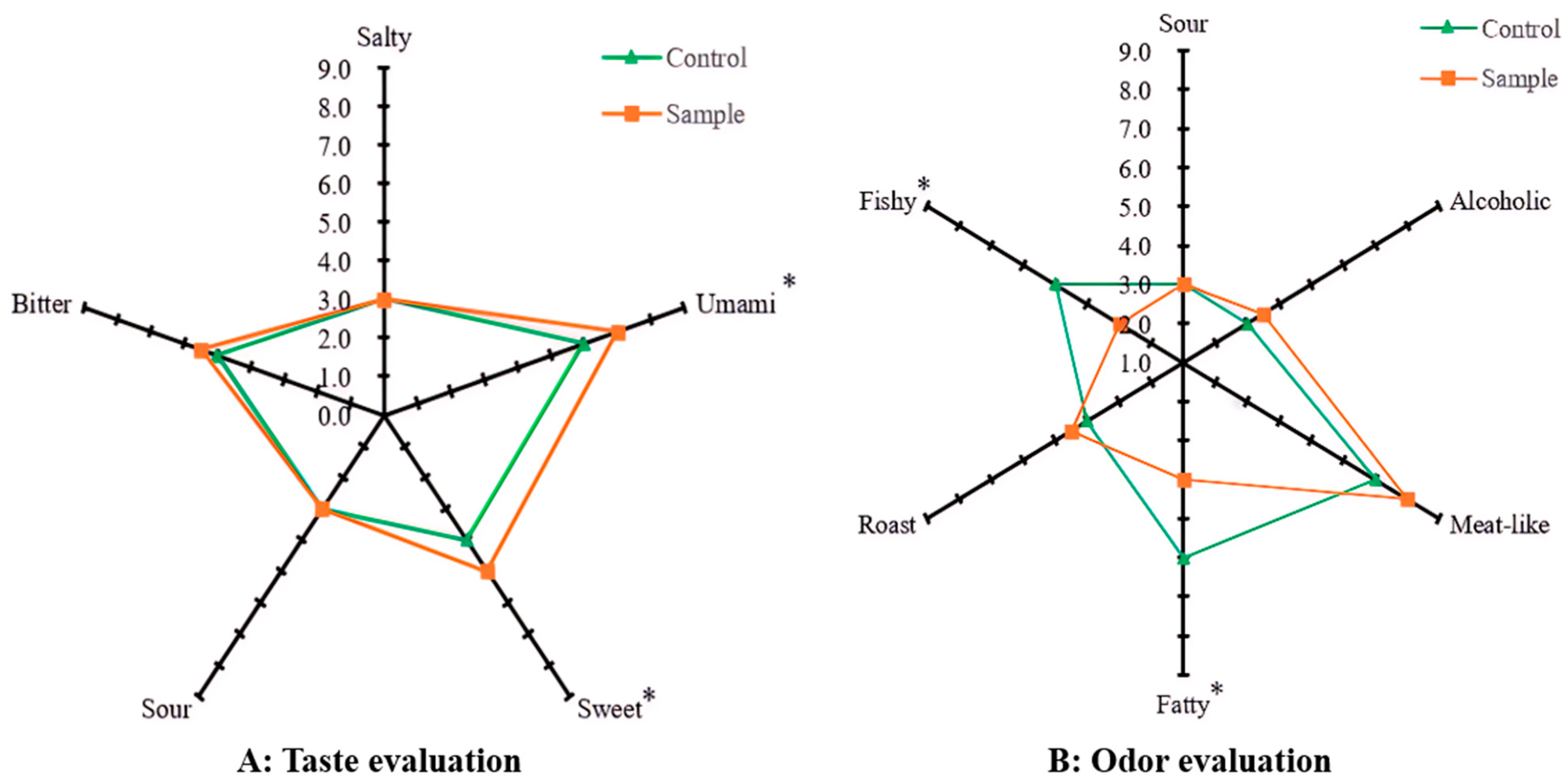
3.1. Sensory Evaluation
3.2. Analyses of Total Nitrogen, Formaldehyde Nitrogen, Hydrolysis Degree, Total Sugars and Reducing Sugars
3.3. Analysis of Free Amino Acids
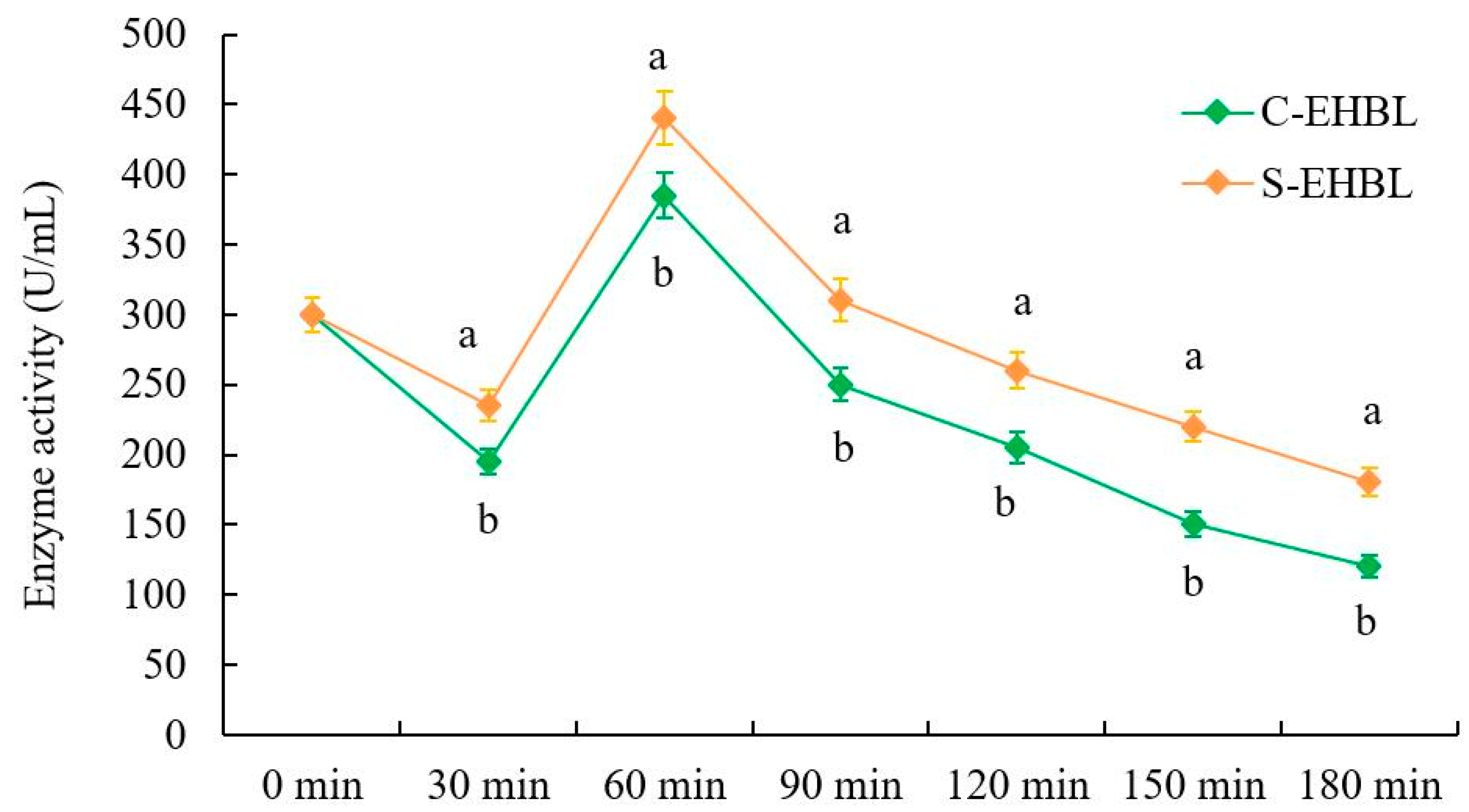
3.4. Analysis of Neutral Protease Activity
3.5. Characterization of Volatile Compounds
3.6. Key Odour-Active Compounds Identified by OAVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.L.; Yan, S.; Yang, B.; Lu, J.; Jin, Z. Comparison of physicochemical properties of beef potentiators prepared by synergistic fermentation and traditional method. Int. J. Food Sci. Technol. 2013, 48, 1932–1939. [Google Scholar] [CrossRef]
- Gao, X.L.; Yan, S.; Yang, B.; Lu, J.; Jin, Z. A novel method for beef potentiator preparation and identification of its characteristic aroma compounds. J. Sci. Food Agric. 2014, 94, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Toldra, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49 (Suppl. S1), S101–S110. [Google Scholar] [CrossRef]
- Adhikari, K.; Chambers Iv, E.; Miller, R.; VÁZquez-Araújo, L.; Bhumiratana, N.; Philip, C. Development of a lexicon for beef flavor in intact muscle. J. Sens. Stud. 2011, 26, 413–420. [Google Scholar] [CrossRef]
- Liu, X.F.; Sun, X.J.; Wei, Y.J.; Ma, Y.Y.; Sun, P.; Li, X.X. Effects of ultrasonic treatment on physico-chemical properties and structure of tuna (Thunnus tonggol) myofibrillar proteins. Ultrason. Sonochem. 2022, 108, 104438. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, M.; Fan, H.; Liu, Y. Effect of microwave combined with ultrasonic pretreatment on flavor and antioxidant activity of hydrolysates based on enzymatic hydrolysis of bovine bone. Food Biosci. 2021, 44, 101399. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Dong, Z.M.; Wen, R.W.; Kong, B.H.; Yu, W.H.; Wang, J.Z.; Liu, H.T.; Chen, Q. Combination of ultrasound treatment and starter culture for improving the quality of beef jerky. Meat Sci. 2023, 204, 109240. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, A.; Jebelli, J.A.; Hesarinejad, M.A.; Mahdavi, A.; Parsaeimehr, M. The combined effect of ultrasound treatment and leek (Allium ampeloprasum) extract on the quality properties of beef. Food Biosci. 2022, 47, 101622. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.L.; Li, S.T.; Ren, X.F.; Olayemi Oladejo, A.; Lu, F.; Ma, H.L. Effects and mechanism of ultrasound pretreatment of protein on the Maillard reaction of protein-hydrolysate from grass carp (Ctenopharyngodon idella). Ultrason. Sonochem. 2020, 64, 104964. [Google Scholar] [CrossRef]
- Bai, H.L.; Li, L.H.; Wu, Y.Y.; Chen, S.J.; Zhao, Y.Q.; Cai, Q.X.; Wang, Y.Q. Ultrasound improves the low-sodium salt curing of sea bass: Insights into the effects of ultrasound on texture, microstructure, and flavor characteristics. Ultrason. Sonochem. 2023, 100, 106597. [Google Scholar] [CrossRef]
- Gao, X.L.; Zhang, J.K.; Liu, E.M.; Yang, M.Q.; Chen, S.; Hu, F.; Ma, H.L.; Liu, Z.; Yu, X.T. Enhancing the taste of raw soy sauce using low intensity ultrasound treatment during moromi fermentation. Food Chem. 2019, 298, 124928. [Google Scholar] [CrossRef]
- Goh, K.M.; Lai, O.M.; Abas, F.; Tan, C.P. Effects of sonication on the extraction of free-amino acids from moromi and application to the laboratory scale rapid fermentation of soy sauce. Food Chem. 2017, 215, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.P.; Chen, S.W.; Dai, C.H.; Sun, L.; Sun, W.L.; Tang, Y.X.; Xiong, F.; He, R.H.; Ma, H.L. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, H.; Yu, X.; Liu, B.; Yagoub, Y.A.A.; Pan, Z. Pretreatment of defatted wheat germ proteins (by-products of flour mill industry) using ultrasonic horn and bath reactors: Effect on structure and preparation of ACE-inhibitory peptides. Ultrason. Sonochem. 2013, 20, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Zeng, Q.X.; Zhu, Z.W.; Zhang, L.Y. Chemical and sensory changes associated Yu-lu fermentation process–A traditional Chinese fish sauce. Food Chem. 2007, 104, 1629–1634. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Yarmand, M.S.; Ghanbarzadeh, B.; Mirzaee, H. Generation of bioactive peptides from lentil protein: Degree of hydrolysis, antioxidant activity, phenol content, ACE-inhibitory activity, molecular weight, sensory, and functional properties. J. Food Meas. Charact. 2021, 15, 5021–5035. [Google Scholar] [CrossRef]
- Yue, F.F.; Zhang, J.R.; Xu, J.X.; Niu, T.F.; Lu, X.; Liu, M.S. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef]
- Gao, X.L.; Liu, E.M.; Zhang, J.K.; Yang, L.X.; Huang, Q.R.; Chen, S.; Ma, H.L.; Ho, C.T.; Liao, L. Accelerating aroma maturation of raw soy sauce using low intensity sonication. Food Chem. 2020, 329, 127118. [Google Scholar] [CrossRef] [PubMed]
- Ozkara, K.T.; Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. GC-MS-Olfactometric differentiation of aroma-active compounds in turkish heat-treated sausages by application of aroma extract dilution analysis. Food Anal. Method. 2018, 12, 729–741. [Google Scholar] [CrossRef]
- Takakura, Y.; Sakamoto, T.; Hirai, S.; Masuzawa, T.; Wakabayashi, H.; Nishimura, T. Characterization of the key aroma compounds in beef extract using aroma extract dilution analysis. Meat Sci. 2014, 97, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Zhang, Z.H.; He, R.H.; Xu, R.Y.; Zhang, L.; Gao, X.L. Improving soy sauce aroma using high hydrostatic pressure and the preliminary mechanism. Foods 2022, 11, 2190. [Google Scholar] [CrossRef]
- Ye, Y.K.; Ye, S.S.; Wanyan, Z.X.; Ping, H.; Xu, Z.X.; He, S.D.; Cao, X.D.; Chen, X.Y.; Hu, W.W.; Wei, Z.J. Producing beef flavors in hydrolyzed soybean meal-based Maillard reaction products participated with beef tallow hydrolysates. Food Chem. 2022, 378, 132119. [Google Scholar] [CrossRef]
- Li, X.P.; Liu, Y.M.; Wang, Y.Y.; Wang, J.X.; Xu, Y.X.; Yi, S.M.; Zhu, W.H.; Mi, H.B.; Li, T.T.; Li, J.R. Combined ultrasound and heat pretreatment improve the enzymatic hydrolysis of clam (Aloididae aloidi) and the flavor of hydrolysates. Innov. Food Sci. Emerg. 2021, 67, 102596. [Google Scholar] [CrossRef]
- Song, S.; Zheng, F.; Tian, X.; Feng, T.; Yao, L.; Sun, M.; Shi, L. Evolution analysis of free fatty acids and aroma-active compounds during tallow oxidation. Molecules 2022, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Luo, Y.J.; Qi, B.K.; Luo, J.Q.; Wan, Y.H. Improving the hydrolysis efficiency of soy sauce residue using ultrasonic probe-assisted enzymolysis technology. Ultrason. Sonochem. 2017, 35, 351–358. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhang, X.Y.; Tang, S.T.; Mi, S.J.; Lu, L.Z.; Zeng, Q.; Xia, M.Q.; Cai, Z.X. Improved effect of ultrasound-assisted enzymolysis on egg yolk powder: Structural properties, hydration properties and stability characteristics. Food Chem. 2022, 382, 132549. [Google Scholar] [CrossRef]
- Guo, Z.L.; Ge, X.Z.; Yang, L.H.; Ma, G.Y.; Ma, J.B.; Yu, Q.L.; Han, L. Ultrasound-assisted thawing of frozen white yak meat: Effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021, 70, 105345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Xia, Q.; He, J.; Sun, Y.Y.; Dang, Y.L.; Zhou, G.H.; Geng, F.; Pan, D.D.; Cao, J.X. Insights into ultrasonic treatment on the mechanism of proteolysis and taste improvement of defective dry-cured ham. Food Chem. 2022, 388, 133059. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.C.; Zhao, M.Y.; Hou, L.; Liang, J.J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef]
- Kerth, C.R.; Miller, R.K. Beef flavor: A review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography-mass spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kitabayashi, K.; Fukusima, C.; Sugiyama, S.; Hashimoto, T. Effect of storage period before reheating on the volatile compound composition and lipid oxidation of steamed meat of yellowtail Seriola quinqueradiata. Fisheries Sci. 2015, 81, 1145–1155. [Google Scholar] [CrossRef]
- Wang, H.L.; Yang, P.; Liu, C.; Song, H.L.; Pan, W.Q.; Gong, L. Characterization of key odor-active compounds in thermal reaction beef flavoring by SGC×GC-O-MS, AEDA, DHDA, OAV and quantitative measurements. J. Food Compos. Anal. 2022, 114, 104805. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC-MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef]
- Zhuang, J.D.; Xiao, Q.; Feng, T.; Huang, Q.R.; Ho, C.T.; Song, S.Q. Comparative flavor profile analysis of four different varieties of Boletus mushrooms by instrumental and sensory techniques. Food Res. Int. 2020, 136, 109485. [Google Scholar] [CrossRef]
- Duan, Z.L.; Dong, S.L.; Sun, Y.X.; Dong, Y.W.; Gao, Q.F. Response of Atlantic salmon (Salmo salar) flavor to environmental salinity while culturing between freshwater and seawater. Aquaculture 2021, 530, 735953. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Blank, I.; Wang, B.; Cao, Y.P. Changes in odorants and flavor profile of heat-processed beef flavor during storage. J. Food Sci. 2022, 87, 5208–5224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Pu, D.D.; Sun, B.G.; Ren, F.Z.; Zhang, Y.Y.; Chen, H.T. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef] [PubMed]
| Total Nitrogen (g/L) | Formaldehyde Nitrogen (g/L) | Hydrolysis Degree (%) | Total Sugars (g/L) | Reducing Sugars (g/L) | |
|---|---|---|---|---|---|
| Control | 8.83 ± 0.21 b | 1.01 ± 0.01 b | 16.33 ± 0.48 b | 0.93 ± 0.02 b | 0.84 ± 0.02 b |
| Sample | 10.53 ± 0.15 a | 1.21 ± 0.02 a | 19.67 ± 0.51 a | 1.04 ± 0.01 a | 0.92 ± 0.03 a |
| FAAs | Control (g/L) | Sample (g/L) | Taste Attributes | Thresholds (g/L) | Taste Intensities | |
|---|---|---|---|---|---|---|
| Control | Sample | |||||
| Aspartic acid | 0.14 ± 0.01 b | 0.25 ± 0.01 a | Umami | 0.17 | 0.82 | 1.47 |
| Glutamic acid | 0.43 ± 0.01 a | 0.40 ± 0.01 a | Umami | 0.075 | 5.73 | 5.33 |
| Umami FAAs | 0.57 | 0.65 | 6.55 | 6.80 | ||
| Serine | 0.35 ± 0.02 a | 0.33 ± 0.01 a | Sweet | 1.50 | 0.23 | 0.22 |
| Glycine | 0.48 ± 0.03 a | 0.52 ± 0.01 a | Sweet | 1.30 | 0.37 | 0.40 |
| Threonine | 0.12 ± 0.01 b | 0.27 ± 0.02 a | Sweet | 5.20 | 0.02 | 0.05 |
| Alanine | 0.75 ± 0.03 a | 0.73 ± 0.01 a | Sweet | 0.60 | 1.25 | 1.21 |
| Proline | 0.09 ± 0.01 b | 0.30 ± 0.01 a | Sweet | 3.00 | 0.03 | 0.10 |
| Lysine | 1.16 ± 0.04 b | 1.28 ± 0.01 a | Sweet | 0.50 | 2.32 | 2.56 |
| Sweet FAAs | 2.95 | 3.53 | 4.22 | 4.54 | ||
| Histidine | 0.16 ± 0.01 a | 0.16 ± 0.01 a | Bitter | 0.20 | 0.80 | 0.80 |
| Arginine | 0.03 ± 0.01 b | 0.12 ± 0.01 a | Bitter | 0.50 | 0.06 | 0.24 |
| Valine | 0.37 ± 0.02 a | 0.34 ± 0.02 a | Bitter | 0.10 | 3.70 | 3.40 |
| Tyrosine | 0.29 ± 0.01 b | 0.34 ± 0.01 a | Bitter | 0.46 | 0.63 | 0.74 |
| Methionine | 0.37 ± 0.02 a | 0.39 ± 0.02 a | Bitter | 0.30 | 1.23 | 1.30 |
| Leucine | 1.04 ± 0.05 b | 1.15 ± 0.06 a | Bitter | 0.95 | 1.09 | 1.21 |
| Isoleucine | 0.32 ± 0.02 a | 0.25 ± 0.02 b | Bitter | 0.90 | 0.36 | 0.28 |
| Phenylalanine | 0.47 ± 0.04 b | 0.59 ± 0.04 a | Bitter | 0.23 | 2.04 | 2.57 |
| Bitter FAAs | 3.05 | 3.33 | 9.91 | 10.54 | ||
| Cysteine | 0.39 ± 0.03 b | 0.45 ± 0.01 a | Tasteless | |||
| Total | 6.96 | 7.96 | 20.68 | 21.88 | ||
| Compounds | CAS | RI (DB-Wax) | Aroma Description | Identification | |
|---|---|---|---|---|---|
| Control | Sample | ||||
| 1-Pentanol | 71-41-0 | 1257 | Balsamic | nd | MS/RI/O |
| 3-Ethyl-2-pentanol | 609-27-8 | 1452 | un | nd | MS/RI |
| 1-Hexanol | 111-27-3 | 1365 | Resin, flower, green | MS/RI/O | MS/RI/O |
| Heptaldehyde | 111-71-7 | 1178 | Fat, citrus, rancid | MS/RI/O | MS/RI/O |
| (E)-Hept-2-enal | 18829-55-5 | 1245 | Soap, fat, almond | MS/RI/O | MS/RI/O |
| Hexanoic acid | 142-62-1 | 1832 | Sweat | MS/RI/O | MS/RI/O |
| Heptanol | 111-70-6 | 1470 | Fruity, green | MS/RI/O | MS/RI/O |
| 1-Octen-3-ol | 3391-86-4 | 1397 | Mushroom, fishy, grass, fatty | MS/RI/O | MS/RI/O |
| 2-Pentylfuran | 3777-69-3 | 1241 | Green bean, butter, pungent | MS/RI/O | MS/RI/O |
| Dodecane | 112-40-3 | 1210 | Alkane | MS/RI/O | MS/RI/O |
| Octanal | 124-13-0 | 1282 | Fat, soap, lemon, green | nd | MS/RI/O |
| (E)-2-Octenal | 2548-87-0 | 1350 | Meaty, fatty, green, nut, fat | MS/RI/O | MS/RI/O |
| 1-Octanol | 111-87-5 | 1555 | Fruity | MS/RI/O | MS/RI/O |
| 4-Methyl-5-decanol | 213547-15-0 | 1955 | un | nd | MS/RI |
| 1-Nonanal | 124-19-6 | 1387 | Fat, citrus | MS/RI/O | MS/RI/O |
| (2E)-2-Nonenal | 18829-56-6 | 1530 | Cucumber, fat, green | MS/RI/O | MS/RI/O |
| 1-Nonano | 143-08-8 | 1504 | Fat, green | MS/RI/O | MS/RI/O |
| Decanal | 112-31-2 | 1485 | Soap, orange peel, tallow | MS/RI/O | MS/RI/O |
| Trans-2-Decenal | 3913-81-3 | 1592 | Tallow, mushroom | MS/RI/O | MS/RI/O |
| Nonadecane | 629-92-5 | 1911 | Alkane | MS/RI/O | MS/RI/O |
| Undecanal | 112-44-7 | 1649 | Oil, pungent, sweet | MS/RI/O | MS/RI/O |
| (E,E)-2,4-Decadien-1-al | 25152-84-5 | 1715 | Fried, wax, fat | MS/RI/O | MS/RI/O |
| (E)-2-Hexadecenal | 22644-96-8 | 2655 | un | MS/RI | nd |
| Pentadecanal | 2765-11-9 | 2060 | Fresh | nd | MS/RI/O |
| Diethyl phthalate | 84-66-2 | 1711 | un | MS/RI/O | MS/RI/O |
| Docosanal | 57402-36-5 | 2754 | un | nd | MS/RI |
| Hexadecanal | 629-80-1 | 2156 | Strawberry and bayberry like aroma | nd | MS/RI/O |
| 2,4-Dimethylhexane | 589-43-5 | 820 | un | MS/RI | nd |
| 3-Hydroxy-2-butanone | 513-86-0 | 1290 | Butter, cream, milky | MS/RI/O | nd |
| Undecane | 1120-21-4 | 1110 | Alkane | MS/RI/O | nd |
| 3-Ethyl-2-methyl-1,3-hexadiene | 61142-36-7 | 1030 | un | MS/RI | nd |
| 1-Nonen-4-ol | 35192-73-5 | 1657 | un | MS/RI | nd |
| N-heneicosanal | 51227-32-8 | 2846 | un | MS/RI | nd |
| No. | Compounds | Threshold (μg/kg) | Concentration (μg/kg) | OAV | FD | |||
|---|---|---|---|---|---|---|---|---|
| Control | Sample | Control | Sample | Control | Sample | |||
| 1 | Octanal | 0.70 | 50.25 ± 2.65 a | 48.55 ± 1.35 b | 71.79 | 69.36 | 32 | 32 |
| 2 | Hexanoic acid | 0.08 | 1.87 ± 0.11 a | 1.21 ± 0.10 b | 23.38 | 15.13 | 32 | 16 |
| 3 | 1-Hexanol | 5.60 | 3.63 ± 0.1 b | 41.32 ± 2.25 a | 0.65 | 7.38 | — | 4 |
| 4 | Heptaldehyde | 2.80 | 25.02 ± 1.32 b | 32.90 ± 2.51 a | 8.94 | 11.75 | 4 | 4 |
| 5 | Nonanal | 1.00 | 78.65 ± 2.3 a | 58.18 ± 2.1 b | 78.65 | 58.18 | 32 | 16 |
| 6 | Decanal | 0.10 | 2.59 ± 0.04 a | 2.78 ± 0.05 a | 25.9 | 27.8 | 16 | 16 |
| 7 | Hexadecanal | 0.91 | nd | 2.13 ± 0.11 | nd | 2.34 | nd | 1 |
| 8 | 2-Pentylfuran | 5.80 | 12.56 ± 1.51 a | 10.60 ± 1.41 a | 2.17 | 1.83 | 1 | 1 |
| 9 | 1-Octen-3-ol | 1.50 | 28.55 ± 1.08 a | 28.15 ± 0.95 a | 19.03 | 18.77 | 8 | 8 |
| 10 | (E)-2-Octenal | 3.00 | 14.96 ± 0.21 a | 14.36 ± 0.18 a | 4.99 | 4.79 | 2 | 2 |
| 11 | (2E)-2-Nonenal | 0.08 | 18.54 ± 1.4 a | 20.02 ± 1.5 a | 231.75 | 250.25 | 128 | 128 |
| 12 | (E,E)-2,4-Decadien-1-al | 0.07 | 7.56 ± 0.2 a | 8.11 ± 0.3 a | 108.00 | 115.86 | 64 | 64 |
| 13 | trans-2-Decenal | 0.30 | 27.54 ± 1.8 b | 31.25 ± 2.2 a | 91.80 | 104.17 | 32 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Zhang, Z.; Zhang, Z.-H.; He, R.; Zhao, X.; Gao, X. Improving the Flavour of Enzymatically Hydrolysed Beef Liquid by Sonication. Foods 2023, 12, 4460. https://doi.org/10.3390/foods12244460
Ye C, Zhang Z, Zhang Z-H, He R, Zhao X, Gao X. Improving the Flavour of Enzymatically Hydrolysed Beef Liquid by Sonication. Foods. 2023; 12(24):4460. https://doi.org/10.3390/foods12244460
Chicago/Turabian StyleYe, Chao, Zhankai Zhang, Zhi-Hong Zhang, Ronghai He, Xue Zhao, and Xianli Gao. 2023. "Improving the Flavour of Enzymatically Hydrolysed Beef Liquid by Sonication" Foods 12, no. 24: 4460. https://doi.org/10.3390/foods12244460
APA StyleYe, C., Zhang, Z., Zhang, Z.-H., He, R., Zhao, X., & Gao, X. (2023). Improving the Flavour of Enzymatically Hydrolysed Beef Liquid by Sonication. Foods, 12(24), 4460. https://doi.org/10.3390/foods12244460






