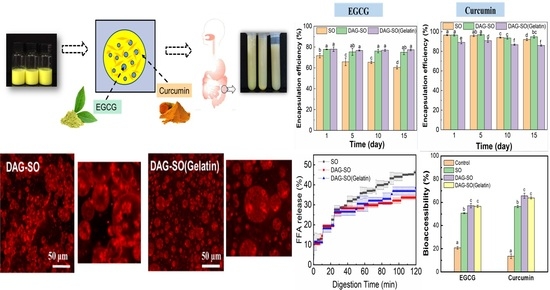
Effect of Diacylglycerol Crystallization on W/O/W Emulsion Stability, Controlled Release Properties and In Vitro Digestibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DAG Synthesis
2.3. Fabrication of W/O/W Emulsion
Effect of Osmotic Stress on the Stability of Double Emulsion
2.4. Microscopy Analysis
2.5. Confocal Laser Light Scanning Microscopy (CLSM)
2.6. Viscosity Measurement
2.7. Droplet Size Analysis
2.8. Salt Release Behavior
2.9. Encapsulation Efficiency of EGCG and Curcumin
2.10. In Vitro Digestion Profile of Emulsions
2.11. Bioaccessibility Calculation
2.12. EGCG and Curcumin Determination
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of W/O/W Emulsion
3.1.1. Effect of PGPR Concentration
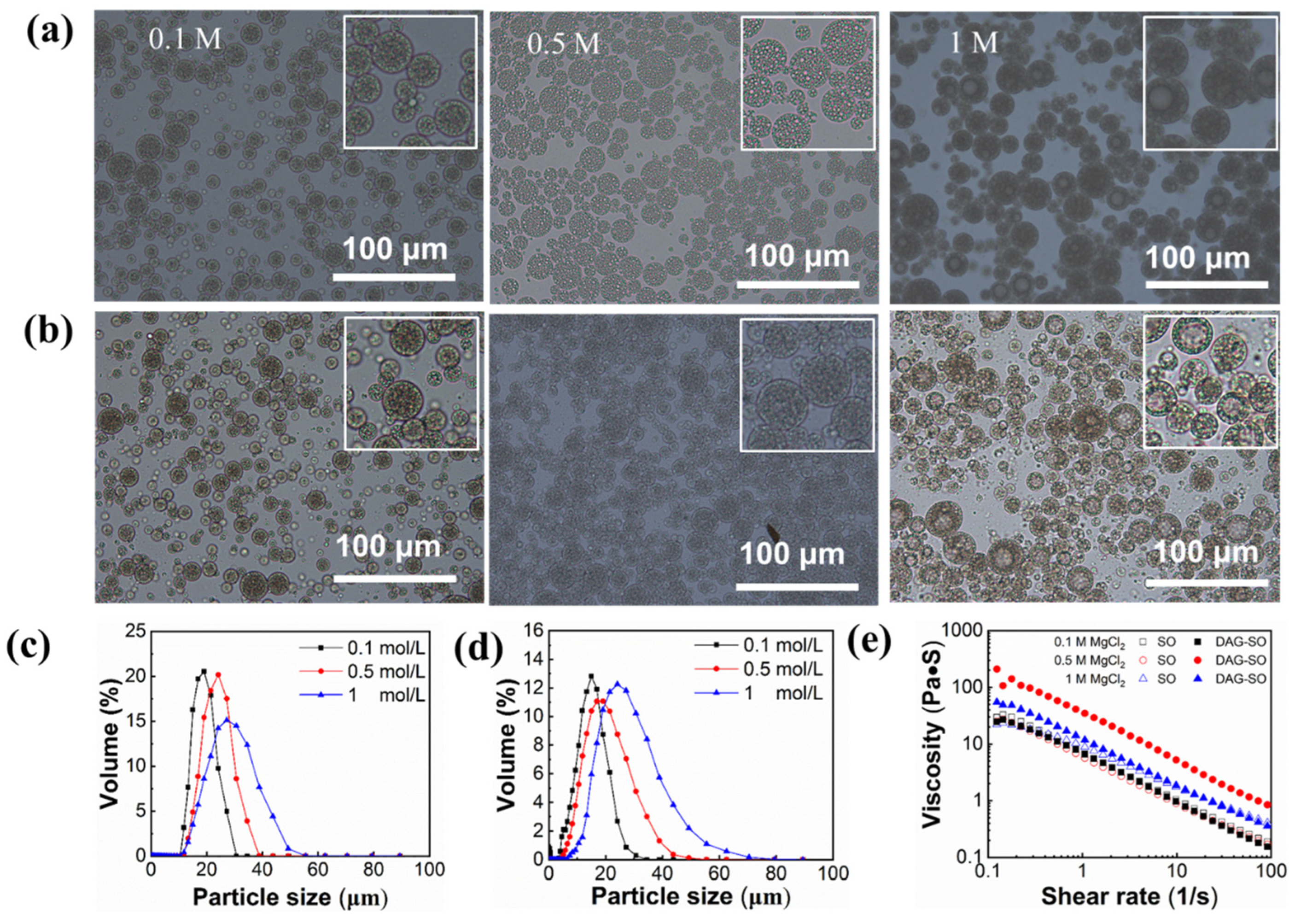
3.1.2. Effect of Salt Concentration
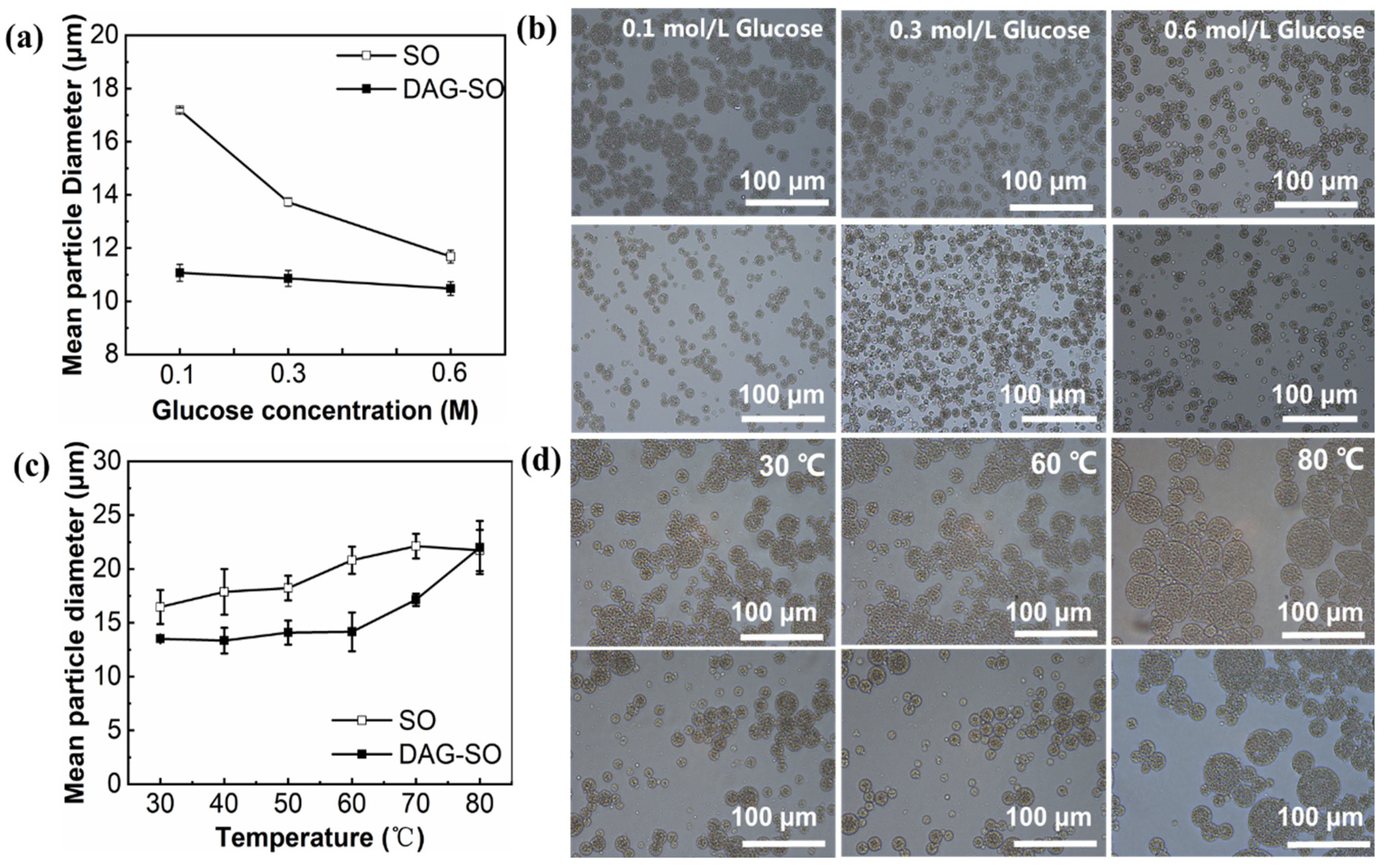
3.1.3. Effect of Osmotic Pressure
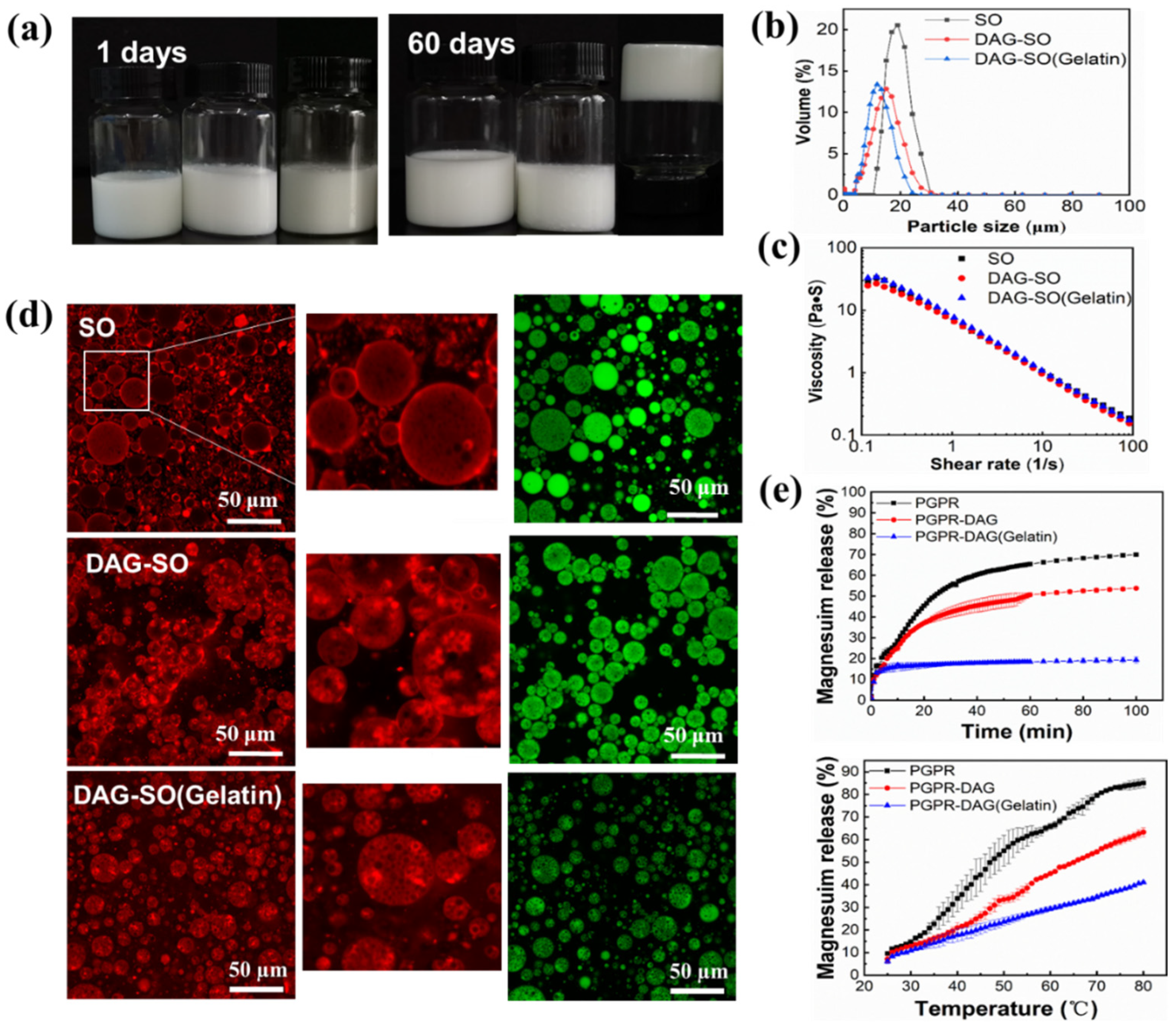
3.2. Effect of Heating on W/O/W Emulsion Stability
3.3. Effect of Gelatin in the Inner Water Phase
3.4. Salt Release Profiles
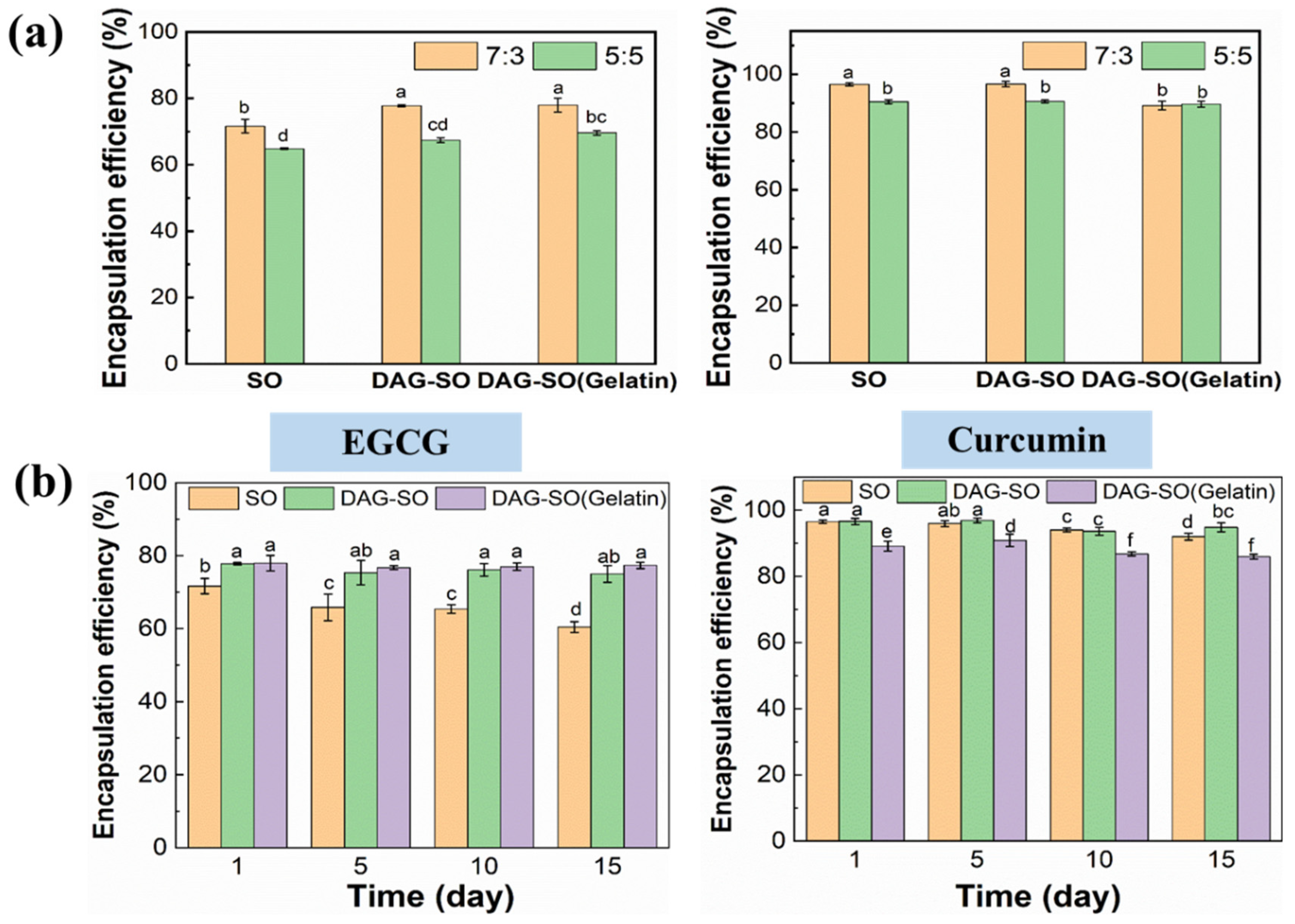
3.5. Encapsulation Efficiency (EE) of EGCG and Curcumin in the W/O/W Emulsions
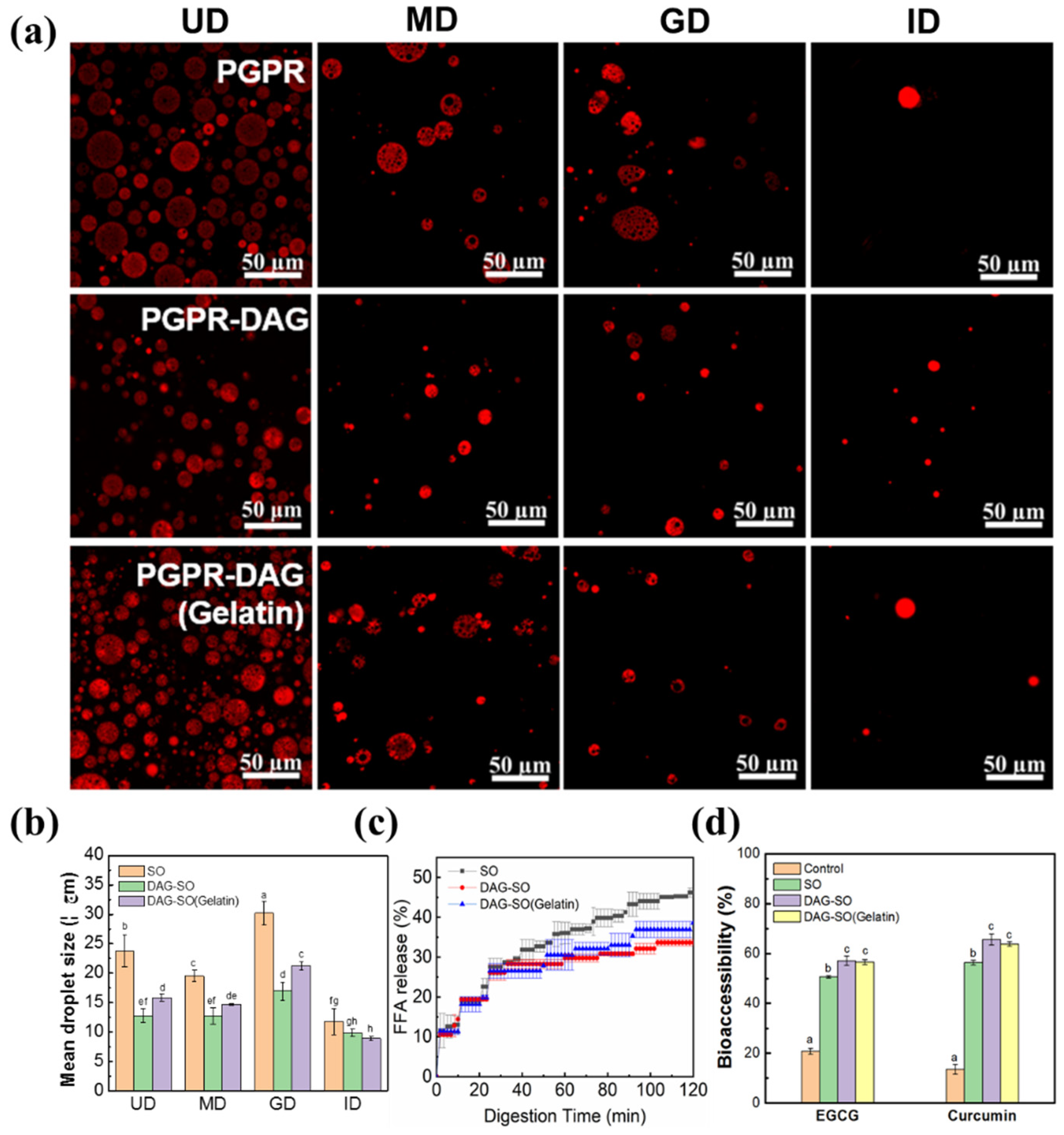
3.6. In Vitro Digestion Profile of W/O/W Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buyukkestelli, H.I.; El, S.N. Development and characterization of double emulsion to encapsulate iron. J. Food Eng. 2019, 263, 446–453. [Google Scholar] [CrossRef]
- Chouaibi, M.; Mejri, J.; Rezig, L.; Abdelli, K.; Hamdi, S. Experimental study of quercetin microencapsulation using water-in-oil-in-water (W1/O/W2) double emulsion. J. Mol. Liq. 2019, 273, 183–191. [Google Scholar] [CrossRef]
- Ying, X.; Gao, J.; Lu, J.; Ma, C.; Lv, J.; Adhikari, B.; Wang, B. Preparation and drying of water-in-oil-in-water (W/O/W) double emulsion to encapsulate soy peptides. Food Res. Int. 2021, 141, 110148. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chu, H.; Hou, Z.; Wang, C.; Zhang, G.; Liu, L.; Ma, X.; Li, C.; He, J. W/O/W emulsions stabilized with whey protein concentrate and pectin: Effects on storage, pasteurization, and gastrointestinal viability of Lacticaseibacillus rhamnosus. Int. J. Biol. Macromol. 2023, 232, 123477. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Ning, X.-Y.; Zou, Y.; Liu, X.; Yang, X.-Q. Multicompartment emulsion droplets for programmed release of hydrophobic cargoes. Food Funct. 2019, 10, 4522–4532. [Google Scholar] [CrossRef]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-delivery of curcumin and epigallocatechin gallate in W/O/W emulsions stabilized by protein fibril-cellulose complexes. Colloid Surf. B-Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Aalim, H.; Li, L.; Lin, X.; Liu, S.; Ma, C.; Li, Q.; Zou, Y.; Luo, Z. Protein-polysaccharide complex coated W/O/W emulsion as secondary microcapsule for hydrophilic arbutin and hydrophobic coumaric acid. Food Chem. 2019, 300, 125171. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, R.; Kumar, V.; Kumar, S.; Gehlot, R.; Aggarwal, P. New insights into water-in-oil-in-water (W/O/W) double emulsions: Properties, fabrication, instability mechanism, and food applications. Trends Food Sci. Technol. 2022, 128, 22–37. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, W.J.; Tan, C.P.; Lai, O.M.; Wang, Y.; Qiu, C. W/O high internal phase emulsion featuring by interfacial crystallization of diacylglycerol and different internal compositions. Food Chem. 2022, 372, 131305. [Google Scholar] [CrossRef]
- Nelis, V.; Declerck, A.; Vermeir, L.; Balcaen, M.; Dewettinck, K.; Van der Meeren, P. Fat crystals: A tool to inhibit molecular transport in W/O/W double emulsions. Magn. Reson. Chem. 2019, 57, 707–718. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wang, Z.-M.; Meng, H.-C.; Lin, J.-W.; Guo, X.-M.; Zhang, T.; Chen, H.-L.; Lei, C.-Y.; Yu, S.-J. Robust W/O/W emulsion stabilized by genipin-cross-linked sugar beet pectin-bovine serum albumin nanoparticles: Co-encapsulation of betanin and curcumin. J. Agric. Food Chem. 2021, 69, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-Epigallocatechin-3-gallate and quercetin in particle-stabilized W/O/W emulsion gels: Controlled release and bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Mundo, J.L.M.; Tan, Y.; Pham, H.; McClements, D.J. Fabrication and characterization of W/O/W emulsions with crystalline lipid phase. J. Food Eng. 2020, 273, 109826. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton, I.T. W1/O/W2 double emulsions stabilised by fat crystals–Formulation, stability and salt release. J. Colloid Interface Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, M.; Xia, Q. Improved stability of (W1/O/W2) double emulsions based on dual gelation: Oleogels and hydrogels. J. Food Process Eng. 2019, 42, e13186. [Google Scholar] [CrossRef]
- Herzi, S.; Essafi, W. Crystallizable W/O/W double emulsions made with milk fat: Formulation, stability and release properties. Food Res. Int. 2019, 116, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Herzi, S.; Essafi, W. Different magnesium release profiles from W/O/W emulsions based on crystallized oils. J. Colloid Interface Sci. 2018, 509, 178–188. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Food. 2020, 65, 103732. [Google Scholar] [CrossRef]
- Eom, D.-W.; Lee, J.H.; Kim, Y.-J.; Hwang, G.S.; Kim, S.-N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.-J.; Ham, J. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015, 48, 461. [Google Scholar] [CrossRef]
- Wang, S.; Lee, W.J.; Wang, Y.; Tan, C.P.; Lai, O.M.; Wang, Y. Effect of purification methods on the physicochemical and thermodynamic properties and crystallization kinetics of medium-chain, medium–long-chain, and long-chain diacylglycerols. J. Agric. Food Chem. 2020, 68, 8391–8403. [Google Scholar] [CrossRef]
- Fernández-Martín, F.; Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Olive oil based edible W/O/W emulsions stability as affected by addition of some acylglycerides. J. Food Eng. 2017, 196, 18–26. [Google Scholar] [CrossRef]
- Zhu, J.-P.; Liang, M.-Y.; Ma, Y.-R.; White, L.V.; Banwell, M.G.; Teng, Y.; Lan, P. Enzymatic synthesis of an homologous series of long-and very long-chain sucrose esters and evaluation of their emulsifying and biological properties. Food Hydrocoll. 2022, 124, 107149. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, L.; Zhang, H.; Saito, M.; Yin, L. Impact of the release rate of magnesium ions in multiple emulsions (water-in-oil-in-water) containing BSA on the resulting physical properties and microstructure of soy protein gel. Food Chem. 2017, 220, 452–459. [Google Scholar] [CrossRef]
- Nadin, M.; Rousseau, D.; Ghosh, S. Fat crystal-stabilized water-in-oil emulsions as controlled release systems. LWT-Food Sci. Technol. 2014, 56, 248–255. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef]
- Ma, D.; Tu, Z.-C.; Wang, H.; Zhang, Z.; McClements, D.J. Microgel-in-microgel biopolymer delivery systems: Controlled digestion of encapsulated lipid droplets under simulated gastrointestinal conditions. J. Agric. Food Chem. 2018, 66, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Amid, B.T.; Kazemi, M.; Hedayatnia, S. Encapsulation properties, release behavior and physicochemical characteristics of water-in-oil-in-water (W/O/W) emulsion stabilized with pectin–pea protein isolate conjugate and Tween 80. Food Hydrocoll. 2016, 61, 599–608. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef]
- Liu, J.; Kharat, M.; Tan, Y.; Zhou, H.; Mundo, J.L.M.; McClements, D.J. Impact of fat crystallization on the resistance of W/O/W emulsions to osmotic stress: Potential for temperature-triggered release. Food Res. Int. 2020, 134, 109273. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.; Deng, C.; Tang, C.; Liu, C.; Hu, X. Fabrication and characterization of the W/O/W multiple emulsion through oleogelation of oil. Food Chem. 2021, 358, 129856. [Google Scholar] [CrossRef]
- Brizzi, S.; Funiciello, F.; Corbi, F.; Di Giuseppe, E.; Mojoli, G. Salt matters: How salt affects the rheological and physical properties of gelatine for analogue modelling. Tectonophysics 2016, 679, 88–101. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; Wall-Medrano, A.; González-Aguilar, G.A.; Martín-Belloso, O. In vitro digestibility and release of a mango peel extract encapsulated within water-in-oil-in-water (W1/O/W2) emulsions containing sodium carboxymethyl cellulose. Food Funct. 2019, 10, 6110–6120. [Google Scholar] [CrossRef] [PubMed]
- Frasch-Melnik, S.; Norton, I.T.; Spyropoulos, F. Fat-crystal stabilised w/o emulsions for controlled salt release. J. Food Eng. 2010, 98, 437–442. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Frasch-Melnik, S.; Norton, I.T. W/O/W emulsions stabilized by fat crystals-Their formulation, stability and ability to retain salt. Procedia Food Sci. 2011, 1, 1700–1708. [Google Scholar] [CrossRef][Green Version]
- Phan, S.; Salentinig, S.; Gilbert, E.; Darwish, T.A.; Hawley, A.; Nixon-Luke, R.; Bryant, G.; Boyd, B.J. Disposition and crystallization of saturated fatty acid in mixed micelles of relevance to lipid digestion. J. Colloid Interface Sci. 2015, 449, 160–166. [Google Scholar] [CrossRef]
- Ortega, N.; Reguant, J.; Romero, M.-P.; Macia, A.; Motilva, M.-J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- Zhuang, H.; Li, X.; Wu, S.; Wang, B.; Yan, H. Fabrication of grape seed proanthocyanidin-loaded W/O/W emulsion gels stabilized by polyglycerol polyricinoleate and whey protein isolate with konjac glucomannan: Structure, stability, and in vitro digestion. Food Chem. 2023, 418, 135975. [Google Scholar] [CrossRef]
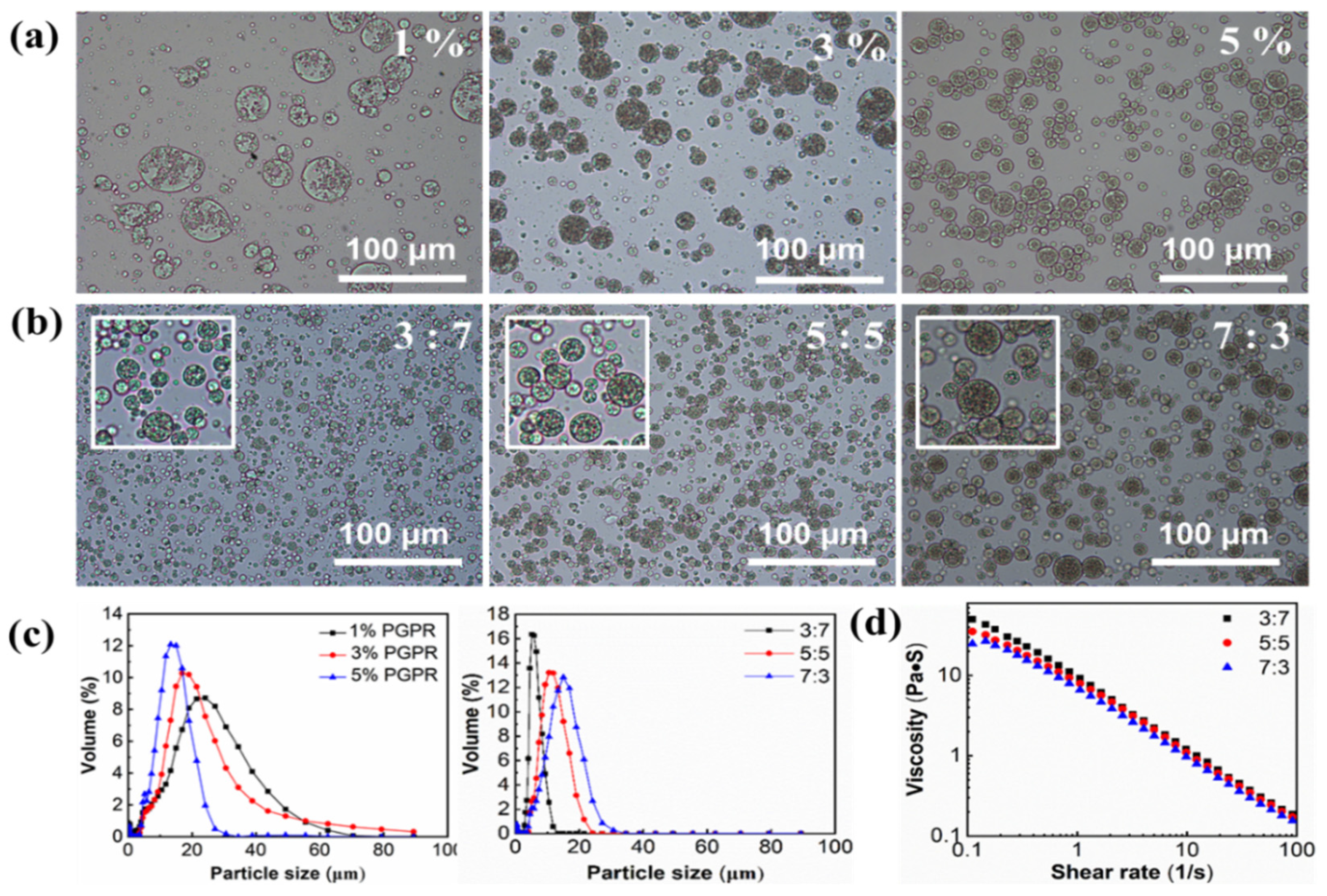
| Samples | PGPR (w/w) | W1:O Ratios | ||||
|---|---|---|---|---|---|---|
| 1% | 3% | 5% | 3:7 | 5:5 | 7:3 | |
| Mean droplet size (μm) | 14.71 ± 0.40 | 13.63 ± 0.42 | 10.53 ± 0.28 | 5.53 ± 0.12 | 9.86 ± 0.15 | 10.86 ± 0.30 |
| MgCl2 Concentration | 0.1 M | 0.5 M | 1 M | |
|---|---|---|---|---|
| Mean droplet size (μm) | SO | 13.73 ± 0.15 | 21.18 ± 0.10 | 24.30 ± 0.13 |
| DAG-SO | 10.86 ± 0.30 | 15.87 ± 0.20 | 22.73 ± 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Liu, Y.; Chen, C.; Lee, Y.Y.; Wang, Y. Effect of Diacylglycerol Crystallization on W/O/W Emulsion Stability, Controlled Release Properties and In Vitro Digestibility. Foods 2023, 12, 4431. https://doi.org/10.3390/foods12244431
Qiu C, Liu Y, Chen C, Lee YY, Wang Y. Effect of Diacylglycerol Crystallization on W/O/W Emulsion Stability, Controlled Release Properties and In Vitro Digestibility. Foods. 2023; 12(24):4431. https://doi.org/10.3390/foods12244431
Chicago/Turabian StyleQiu, Chaoying, Yingwei Liu, Canfeng Chen, Yee Ying Lee, and Yong Wang. 2023. "Effect of Diacylglycerol Crystallization on W/O/W Emulsion Stability, Controlled Release Properties and In Vitro Digestibility" Foods 12, no. 24: 4431. https://doi.org/10.3390/foods12244431
APA StyleQiu, C., Liu, Y., Chen, C., Lee, Y. Y., & Wang, Y. (2023). Effect of Diacylglycerol Crystallization on W/O/W Emulsion Stability, Controlled Release Properties and In Vitro Digestibility. Foods, 12(24), 4431. https://doi.org/10.3390/foods12244431