Isothermal Amplification and CRISPR/Cas12a-System-Based Assay for Rapid, Sensitive and Visual Detection of Staphylococcus aureus
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Genomic DNA Extraction and Primer Design
2.3. Construction of ICS
2.4. LAMP, RPA and PCR Assay for S. aureus
2.5. LAMP, RPA-CRISPR/Cas12a-ICS and the LAMP, RPA-CRISPR/Cas12a-Flu Assay
2.6. Optimization of Reaction Conditions for LAMP and RPA Based on the CRISPR/Cas12a-Flu Platform
2.7. Specificity and Sensitivity of LAMP, RPA-CRISPR/Cas12a Platform
2.8. Sample Validation
2.9. Statistical Analysis
3. Results
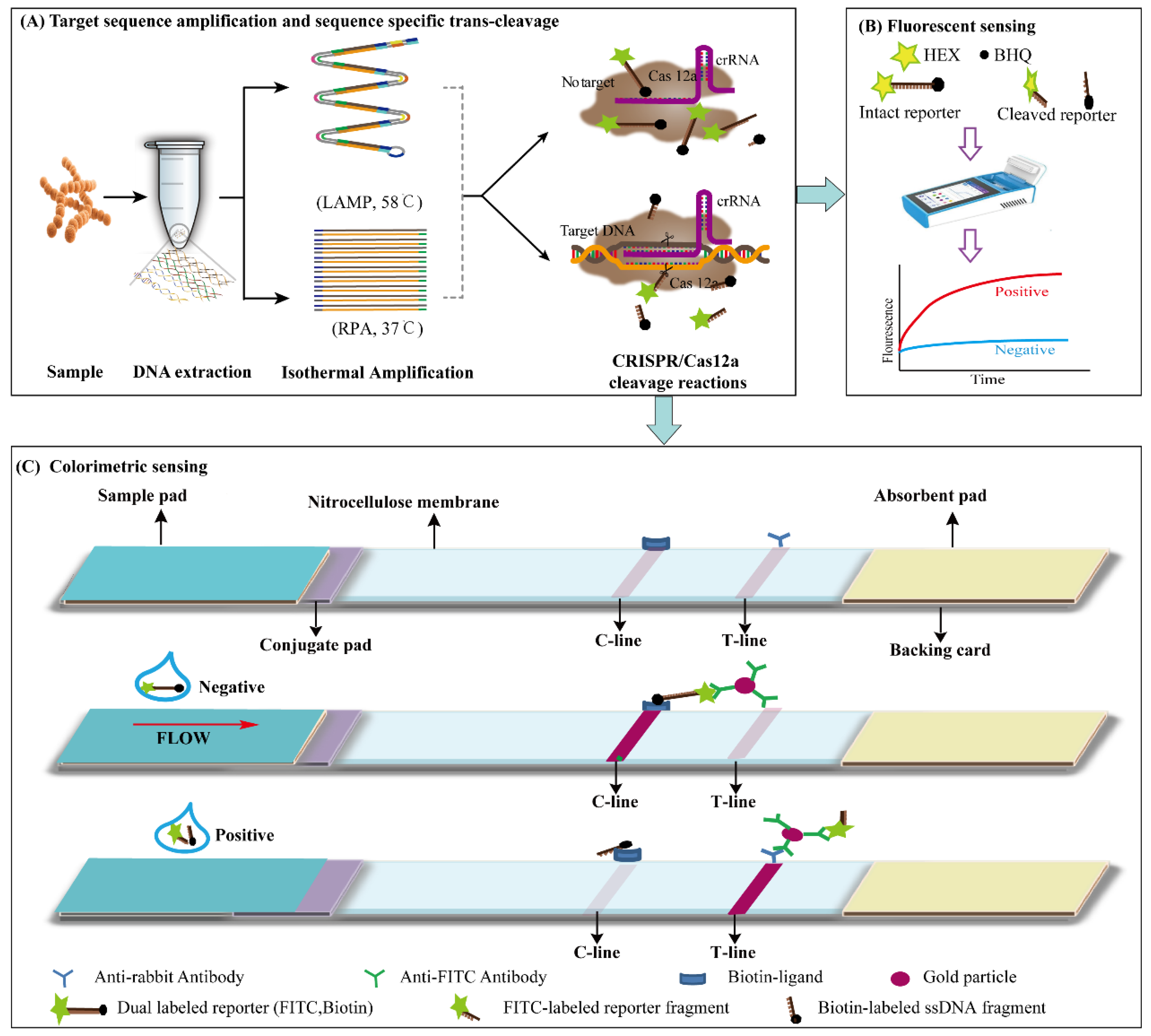
3.1. Principle of LAMP, RPA-CRISPR/Cas12a Platform
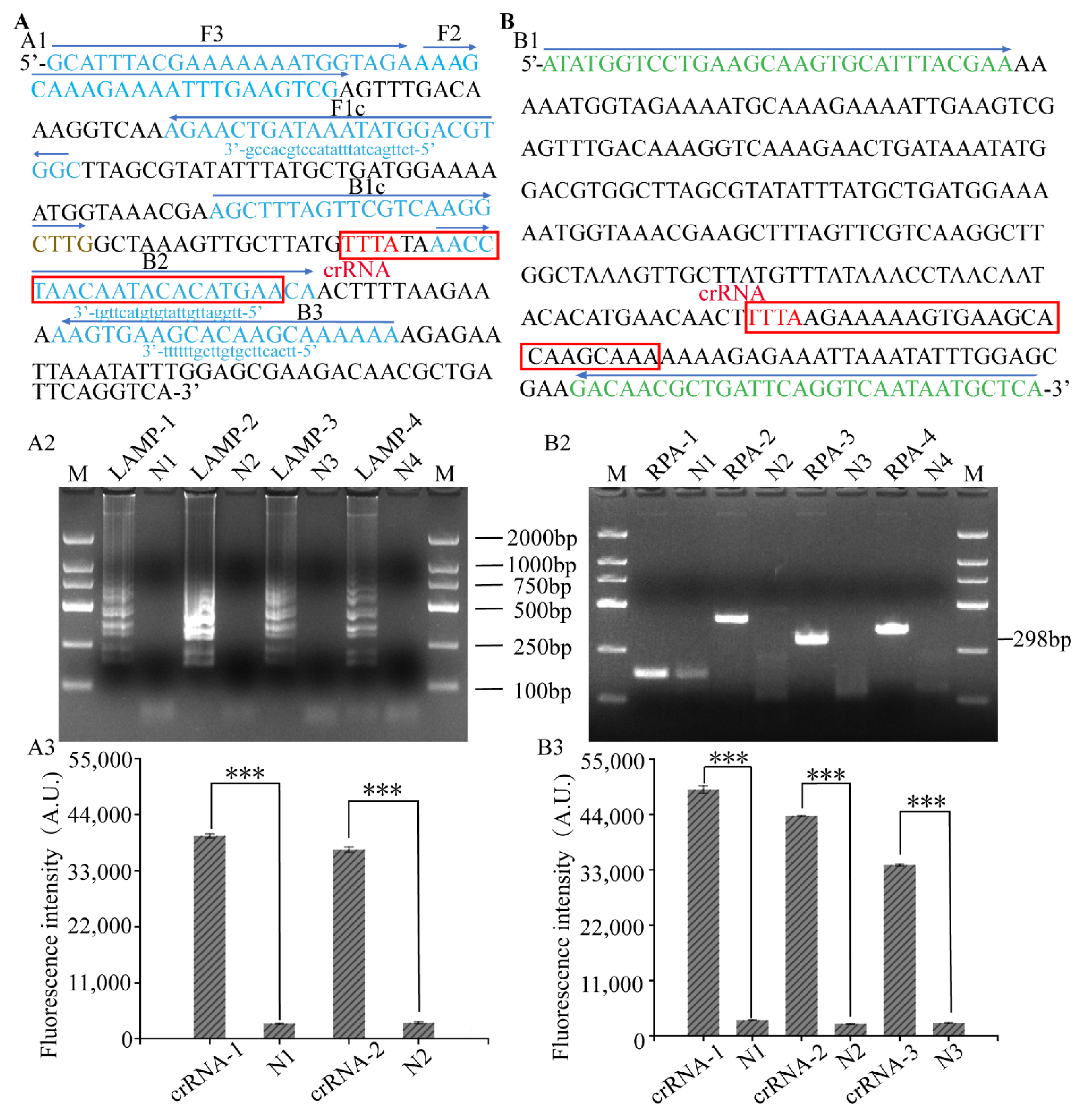
3.2. Primer Screening of LAMP, RPA-CRISPR/Cas12a Platform
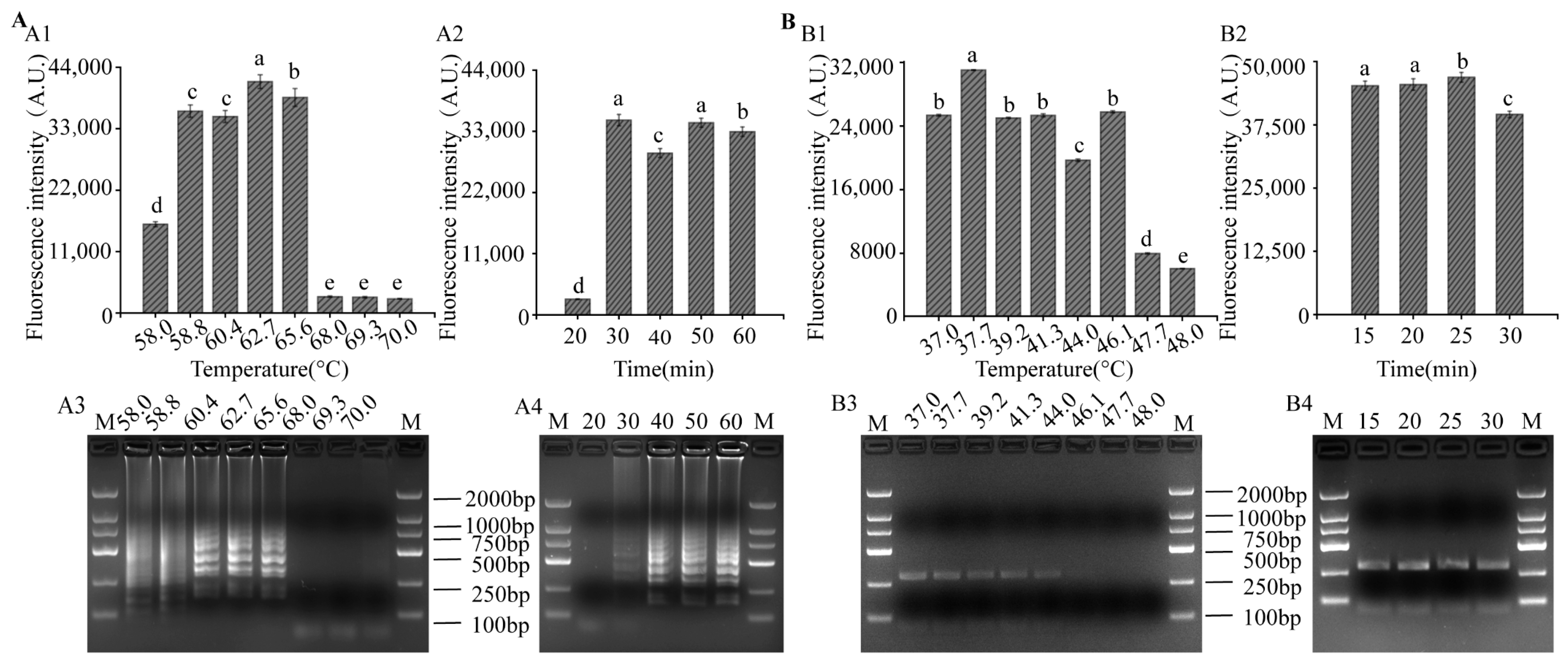
3.3. Optimization of LAMP and RPA Reaction Conditions for LAMP-CRISPR/Cas12a System
3.4. Specificity of LAMP, RPA-CRISPR/Cas12a Platform
3.5. Sensitivity of LAMP, RPA-CRISPR/Cas12a Platform
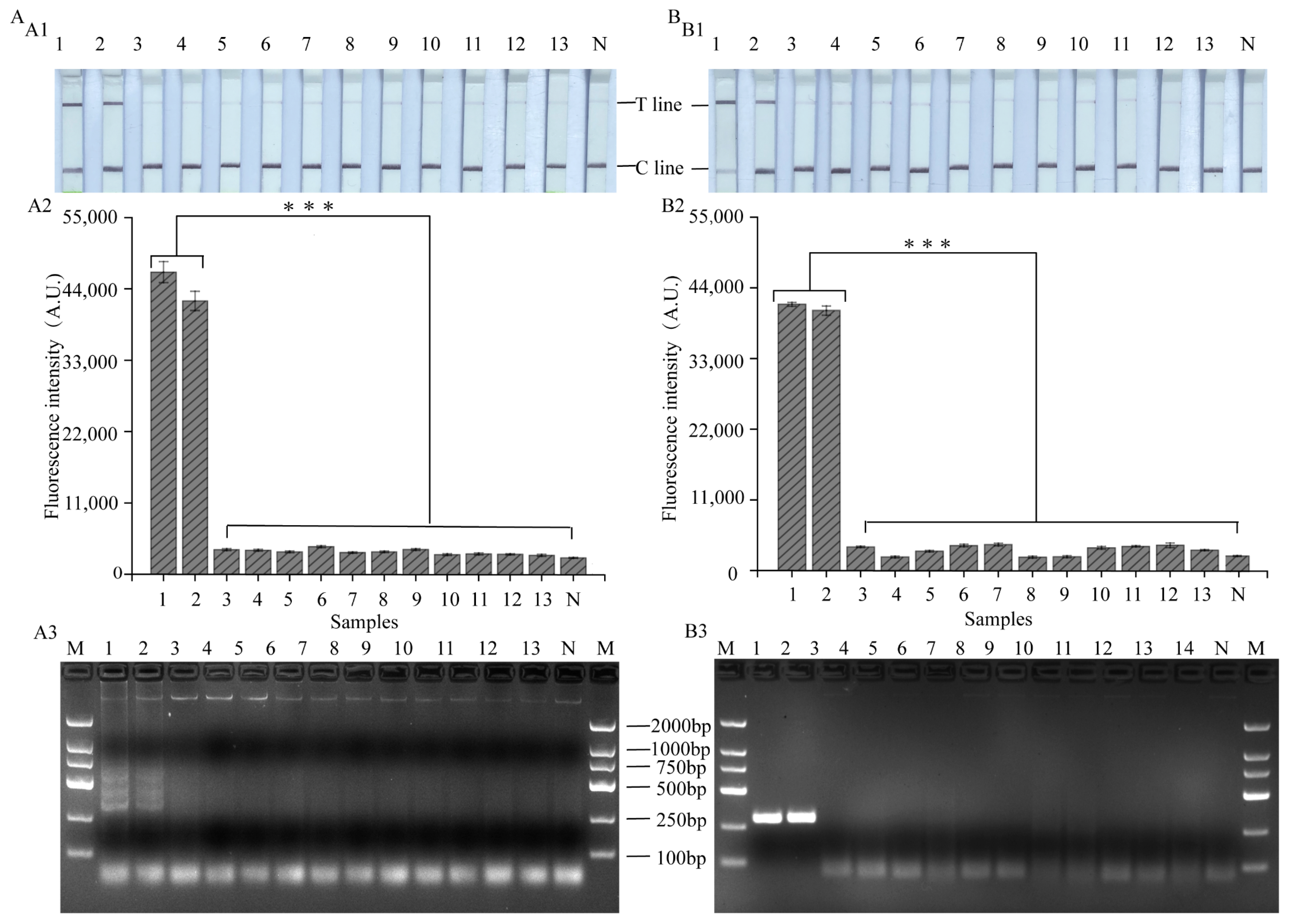
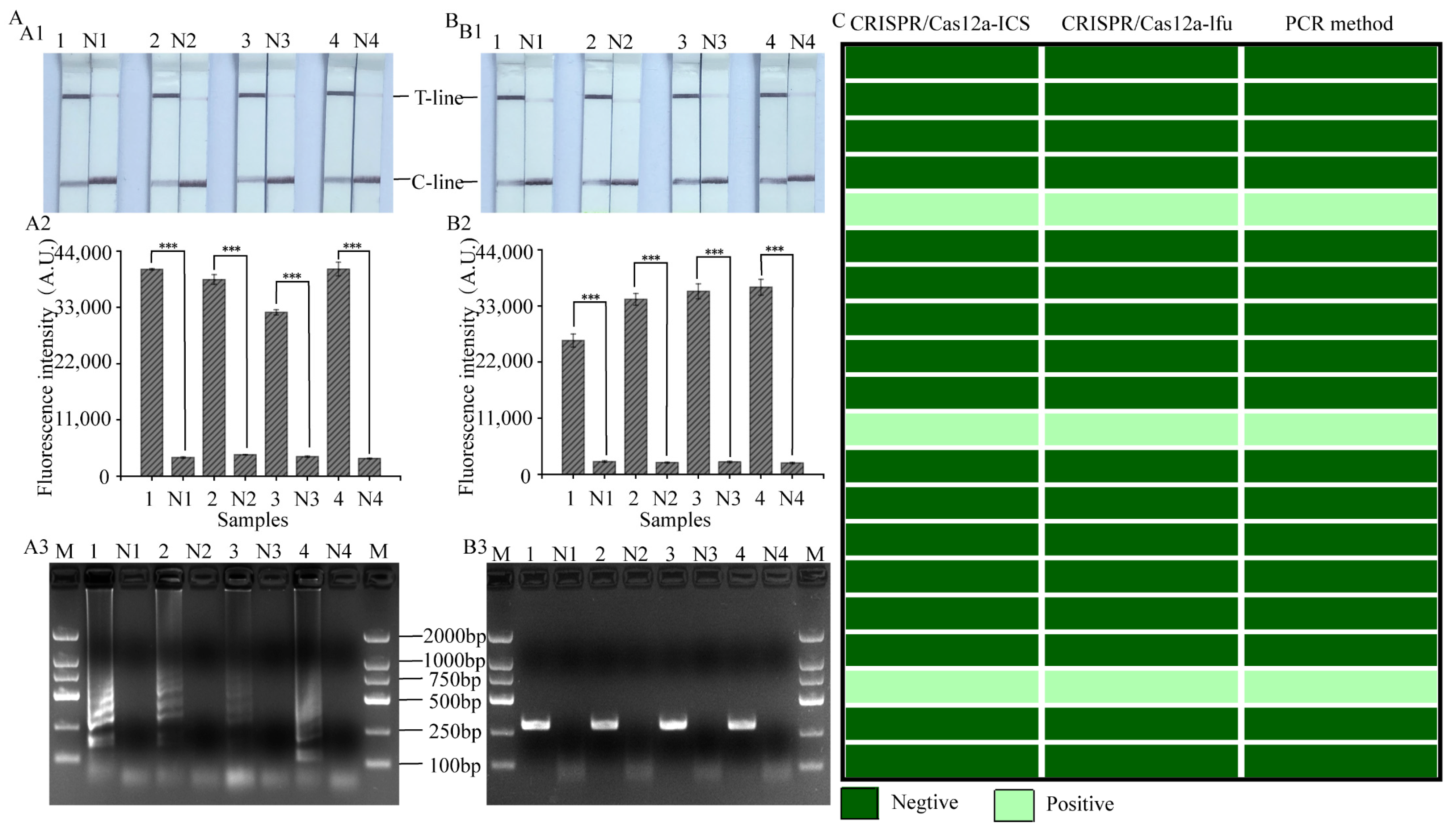
3.6. Evaluation of the Practical Value of LAMP, RPA-CRISPR/Cas12a Platform
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.Y.; Xia, D.D.; Ma, P.P.; Gao, X.F.; Kang, W.Y.; Wei, J.F. Advances in the detection of virulence genes of Staphylococcus aureus originate from food. Food Sci. Hum. Wellness 2020, 9, 40–44. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, H.Y.; Liu, X.X.; Zhang, M.F.; Han, R.P.; Yin, C.H.; Liu, X.C.; Li, H.; Li, J.; Song, X.L. Visual detection of Staphylococcus aureus based on immunomagnetic separation and polymerase spiral reaction. Food Control 2023, 147, 109621. [Google Scholar] [CrossRef]
- Lin, L.Y.; Zha, G.G.; Wei, H.G.; Zheng, Y.Z.; Yang, P.K.; Liu, Y.Q.; Liu, M.Q.; Wang, Z.H.; Zou, X.H.; Zhu, H.; et al. Rapid detection of Staphylococcus aureus in food safety using an RPA-CRISPR-Cas12a assay. Food Control 2023, 145, 109505. [Google Scholar] [CrossRef]
- Ziegler, I.; Cajander, S.; Rasmussen, G.; Ennefors, T.; Mölling, P.; Strålin, K. High nuc DNA load in whole blood is associated with sepsis, mortality and immune dysregulation in Staphylococcus aureus bacteraemia. Infect. Dis. 2019, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Huang, C.; Chen, M.; Wang, J.; Shao, Y.; Wang, X. Rapid and simultaneous detection of viable S. aureus and its penicillin susceptibility by phage amplification techniques in different food matrices. LWT 2023, 176, 114526. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Zhang, Y.; Li, H.; Yang, H.; Wei, H. Sensitive and rapid detection of Staphylococcus aureus in milk via cell binding domain of lysin. Biosens. Bioelectron. 2016, 77, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.L.; Soko, W.C.; Jing, X.; Bi, H.Y. Microchip coupled with MALDI-TOF MS for the investigation of bacterial contamination of fish muscle products. Food Chem. 2022, 396, 133658. [Google Scholar] [CrossRef]
- Xing, J.; Chang, Y.H.; Tang, X.Q.; Sheng, X.Z.; Zhan, W.B. Separation of haemocyte subpopulations in shrimp Fenneropenaeus chinensis by immunomagnetic bead using monoclonal antibody against granulocytes. Fish Shellfish Immunol. 2017, 60, 114–118. [Google Scholar] [CrossRef]
- Nouri, A.; Ahari, H.; Shahbazzadeh, D. Designing a direct ELISA kit for the detection of Staphylococcus aureus enterotoxin A in raw milk samples. Int. J. Biol. Macromol. 2018, 107, 1732–1737. [Google Scholar] [CrossRef]
- Necidová, L.; Bursová, Š.; Haruštiaková, D.; Bogdanovičová, K.; Lačanin, I. Effect of heat treatment on activity of staphylococcal enterotoxins of type A, B, and C in milk. J. Dairy Sci. 2019, 102, 3924–3932. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.B.; Zhong, Z.T.; Li, C.Q.; Chen, W.; Liu, B.; Zhao, Y.D. A smartphone-based rapid quantitative detection platform for lateral flow strip of human chorionic gonadotropin with optimized image algorithm. Microchem. J. 2020, 157, 105038. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Yi, J.; Song, M.H.; Li, D.D.; Wu, Y.J.; Liu, Y.J.; Yang, M.C.; Qiao, L. Highly efficient enrichment and identification of pathogens using a herringbone microfluidic chip and by MALDI-TOF mass spectrometry. Analyst 2021, 146, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.F.; Qi, X.Y.; Wang, H.; Zhao, B.B.; Xu, L.X.; Zhang, Y.T.; Wang, X.L.; Zhou, N.D. A surface-enhanced Raman scattering aptasensor for Escherichia coli detection based on high-performance 3D substrate and hot spot effect. Anal. Chim. Acta 2022, 1221, 340141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.H.; Wang, X.F.; Xu, R.; Yan, H.J.; Li, X.R.; Liu, S.J.; Yin, S.R.; Yao, H.; Yang, Y.; et al. Use of nano titanium hydroxide and nano zirconium hydroxide fixed filter paper for rapid detection of Staphylococcus aureus in dairy products by PCR without pre-enrichment. Food Control 2023, 148, 109664. [Google Scholar] [CrossRef]
- Chamchoy, T.; Okello, E.; Williams, D.R.; Tonooka, K.; Glenn, K.; Maehana, K.; Gardner, I.A.; Aly, S.S. Bayesian estimation of sensitivity and specificity of a rapid mastitis test kit, bacterial culture, and PCR for detection of Staphylococcus aureus, Streptococcus species, and coliforms in bovine milk samples. J. Dairy Sci. 2022, 105, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Wakabayashi, Y.; Seto, K.; Lee, K.; Iyoda, S.; Kawatsu, K. Real-time PCR assays to detect 10 Shiga toxin subtype (Stx1a, Stx1c, Stx1d, Stx2a, Stx2b, Stx2c, Stx2d, Stx2e, Stx2f, and Stx2g) genes. Diagn. Microbiol. Infect. Dis. 2023, 105, 115874. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Q.; Liu, Z. Simultaneous enumeration of Cronobacter sakazakii and Staphylococcus aureus in powdered infant foods through duplex TaqMan real-time PCR. Int. Dairy J. 2021, 117, 105019. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Wei, S.; Oh, D.-H. A highly selective enrichment broth combined with real-time PCR for detection of Staphylococcus aureus in food samples. LWT-Food Sci. Technol. 2018, 94, 103–110. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J.W.; Li, Z.Y.; Ma, Y.; Wang, J.F. Sensitive detection of foodborne pathogens based on CRISPR-Cas13a. J. Food Sci. 2021, 86, 2615–2625. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, S.W. Filtration-based LAMP-CRISPR/Cas12a system for the rapid, sensitive and visualized detection of Escherichia coli O157:H7. Talanta 2022, 241, 123186. [Google Scholar] [CrossRef]
- Martzy, R.; Kolm, C.; Krska, R.; Mach, R.L.; Farnleitner, A.H.; Reischer, G.H. Challenges and perspectives in the application of isothermal DNA amplification methods for food and water analysis. Anal. Bioanal. Chem. 2019, 411, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Oh, S.W. Lateral flow biosensor based on LAMP-CRISPR/Cas12a for sensitive and visualized detection of Salmonella spp. Food Control 2023, 145, 109494. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovic, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.B.; Harrington, L.B.; Da Costa, M.; Tian, X.R.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Fernandez, J.P.; Vejnar, C.E.; Giraldez, A.J.; Rouet, R.; Moreno-Mateos, M.A. Optimized CRISPR-Cpf1 system for genome editing in zebrafish. Methods 2018, 150, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, Q.X.; Liu, J.K.; Nie, X.Q.; Zhao, G.P.; Wang, J. CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 2018, 28, 491–493. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef]
- Chaijarasphong, T.; Thammachai, T.; Itsathitphaisarn, O.; Sritunyalucksana, K.; Suebsing, R. Potential application of CRISPR-Cas12a fluorescence assay coupled with rapid nucleic acid amplification for detection of white spot syndrome virus in shrimp. Aquaculture 2019, 512, 734340. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Hu, X.; Tang, X.; Zhang, C. A rapid and high-throughput Helicobacter pylori RPA-CRISPR/Cas12a-based nucleic acid detection system. Clin. Chim. Acta 2023, 540, 117201. [Google Scholar] [CrossRef]
- GB 29921-2021; National Standard for Food Safety, the Limit of Pathogenic Bacteria in Pre-Packaged Food. Ministry of Health: Beijing, China, 2021.
- GB 4789.10-2010; National Food Safety Standard Food Microbiological Examination: Staphylococcus aureus. Ministry of Health: Beijing, China, 2010.
- Misawa, N.; Kawashima, K.; Kawamoto, H.; Kondo, F. Development of a combined filtration-enrichment culture followed by a one-step duplex PCR technique for the rapid detection of Campylobacter jejuni and C. coli in human faecal samples. J. Med. Microbiol. 2002, 51, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Wang, Y.; Hu, X.W.; Yang, Q.W.; Chen, Y.F.; Jiang, W.; Liu, X.F.; Liu, H.; Zeng, H.J. The development of RPA and CRISPR-Cas12a based immunoassay strip for sensitive detection of genetically modified crops. Food Control 2022, 139, 109048. [Google Scholar] [CrossRef]
- Hu, J.Q.; Wang, Y.; Ding, H.M.; Jiang, C.P.; Geng, Y.; Sun, X.C.; Jing, J.Z.; Gao, H.; Wang, Z.C.; Dong, C.W. Recombinase polymerase amplification with polymer flocculation sedimentation for rapid detection of Staphylococcus aureus in food samples. Int. J. Food Microbiol. 2020, 331, 108691. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, P.; Monistero, V.; Moroni, P.; Barberio, A.; Almeida, R.; Latorre, A.A.; Castiglioni, B. Detection Methods. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 457–468. [Google Scholar]
- Fu, X.R.; Sun, J.D.; Ye, Y.L.; Zhang, Y.Z.; Sun, X.L. A rapid and ultrasensitive dual detection platform based on Cas12a for simultaneous detection of virulence and resistance genes of drug-resistant Salmonella. Biosens. Bioelectron. 2022, 195, 113682. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, L.; He, F.; Chen, G.H.; Bai, L.L.; He, K.Y.; Zhang, F.; Xu, X.H. Label-Free Colorimetric Method for Detection of Vibrio parahaemolyticus by Trimming the G-Quadruplex DNAzyme with CRISPR/Cas12a. Anal. Chem. 2021, 93, 14300–14306. [Google Scholar] [CrossRef]
- Cao, X.; Chang, Y.; Tao, C.; Chen, S.; Lin, Q.; Ling, C.; Huang, S.; Zhang, H. Cas12a/Guide RNA-Based Platforms for Rapidly and Accurately Identifying Staphylococcus aureus and Methicillin-Resistant S. aureus. Microbiol. Spectr. 2023, 11, e04870-22. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Hu, A.; Cui, J.; Yang, K.; Liu, Y.; Deng, G.; Zhu, C.; Zhu, L. Rapid One-Tube RPA-CRISPR/Cas12 Detection Platform for Methicillin-Resistant Staphylococcus aureus. Diagnostics 2022, 12, 829. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Zeng, H.; Wu, W.; Liu, H.; Wang, J. Isothermal Amplification and CRISPR/Cas12a-System-Based Assay for Rapid, Sensitive and Visual Detection of Staphylococcus aureus. Foods 2023, 12, 4432. https://doi.org/10.3390/foods12244432
Xu D, Zeng H, Wu W, Liu H, Wang J. Isothermal Amplification and CRISPR/Cas12a-System-Based Assay for Rapid, Sensitive and Visual Detection of Staphylococcus aureus. Foods. 2023; 12(24):4432. https://doi.org/10.3390/foods12244432
Chicago/Turabian StyleXu, Danhong, Haijuan Zeng, Wenhui Wu, Hua Liu, and Jinbin Wang. 2023. "Isothermal Amplification and CRISPR/Cas12a-System-Based Assay for Rapid, Sensitive and Visual Detection of Staphylococcus aureus" Foods 12, no. 24: 4432. https://doi.org/10.3390/foods12244432
APA StyleXu, D., Zeng, H., Wu, W., Liu, H., & Wang, J. (2023). Isothermal Amplification and CRISPR/Cas12a-System-Based Assay for Rapid, Sensitive and Visual Detection of Staphylococcus aureus. Foods, 12(24), 4432. https://doi.org/10.3390/foods12244432





