Functional Fruit Trees from the Atlantic and Amazon Forests: Selection of Potential Chestnut Trees Rich in Antioxidants, Nutrients, and Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
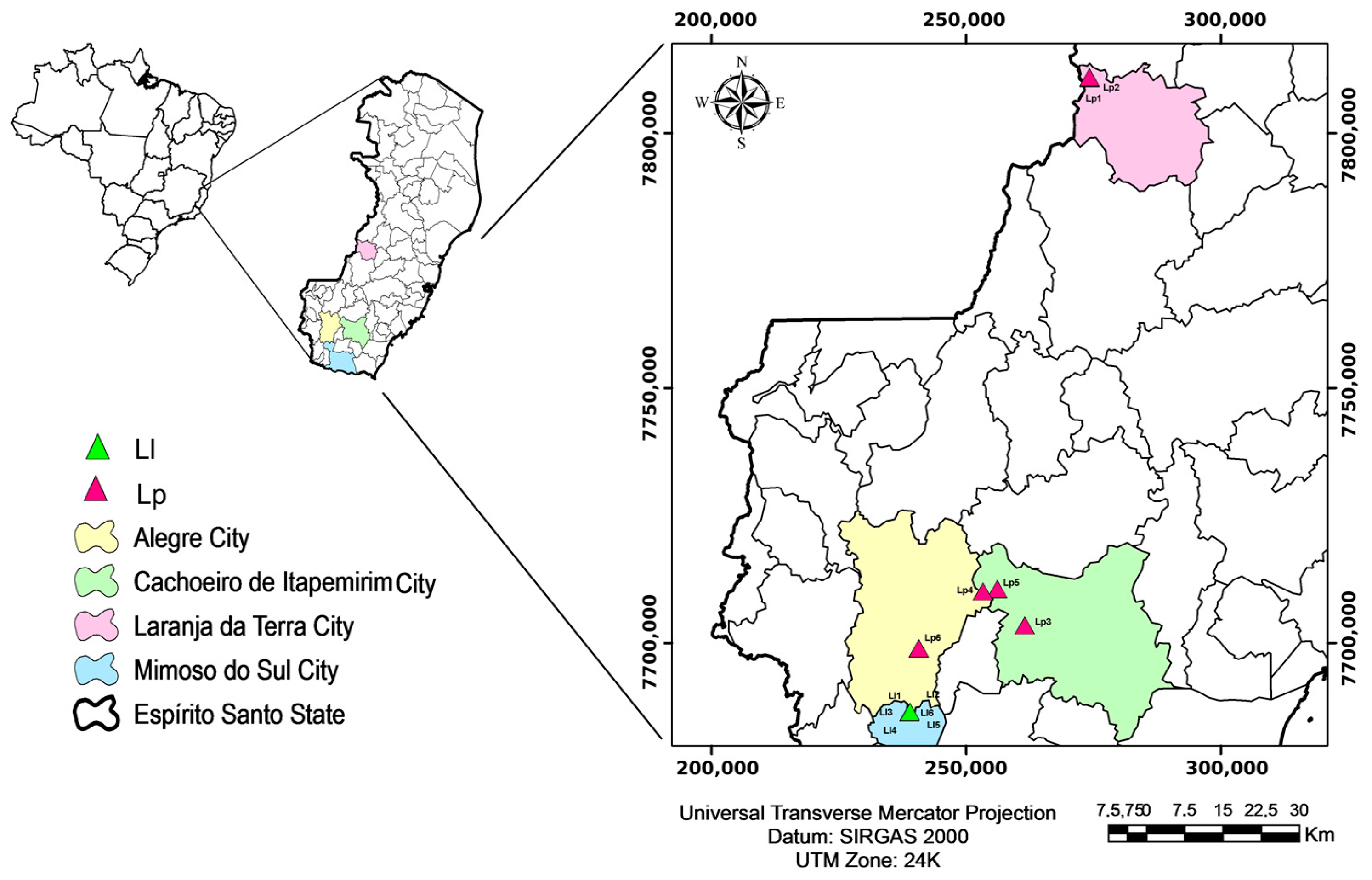
2.1. Location of L. pisonis and L. lanceolata Trees
2.2. Extraction of Minerals from Chestnuts and Leaves
2.3. Extraction of Minerals from the Soil
2.4. Biochemical Analyses
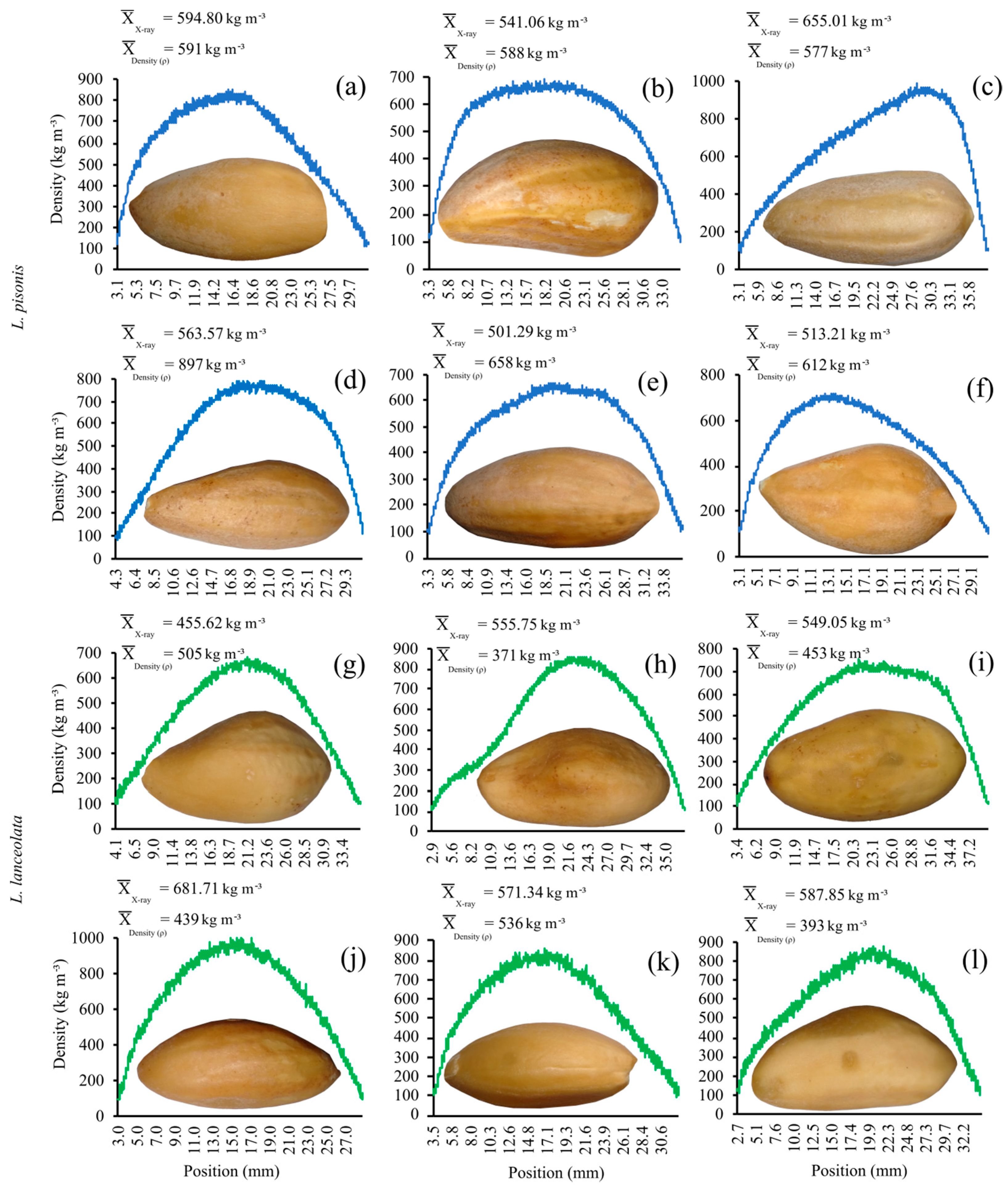
2.5. X-ray Densitometry
2.6. Extraction of the Oil
2.7. Transesterification Reaction
2.8. FAME Analysis
2.9. Statistical Analysis
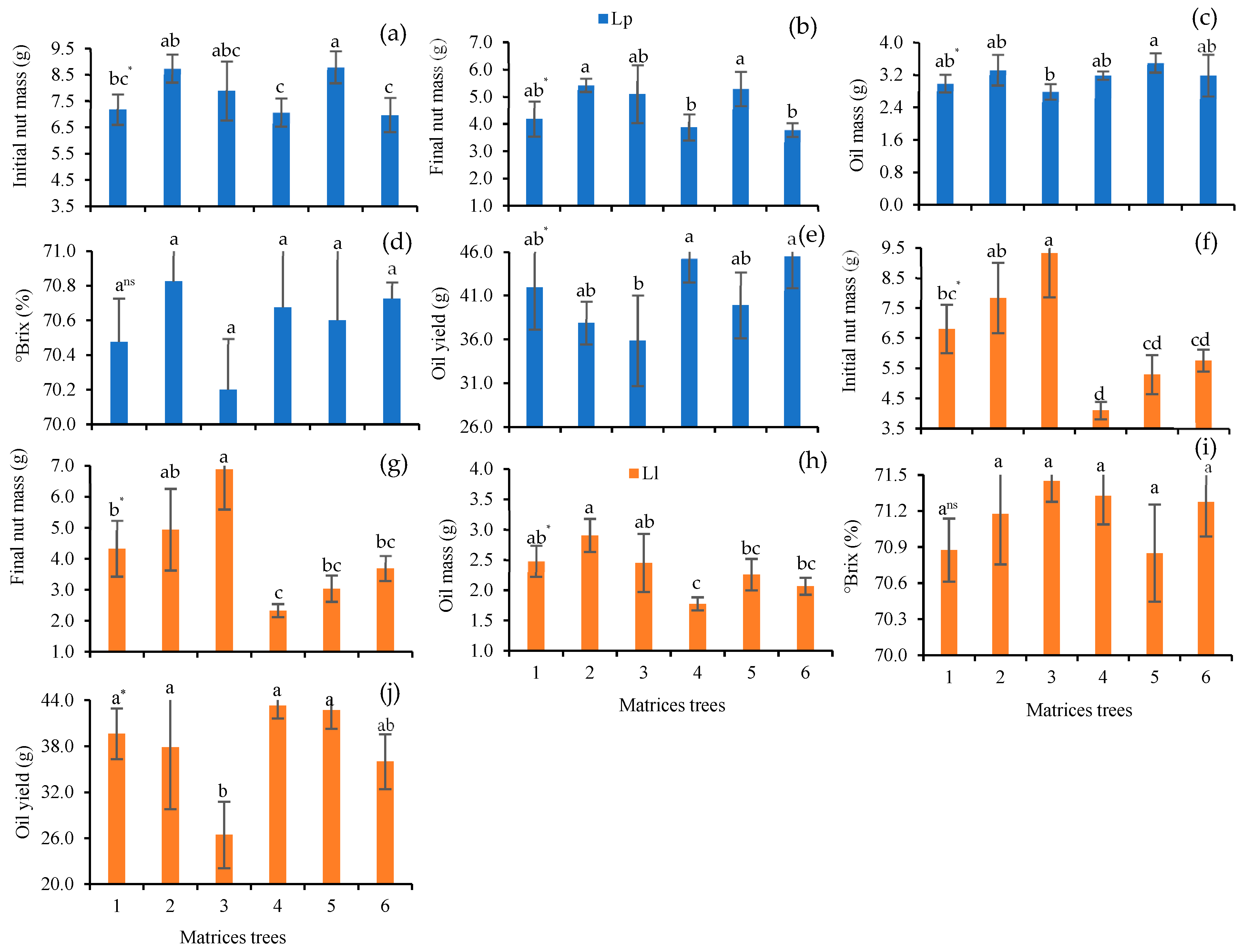
3. Results
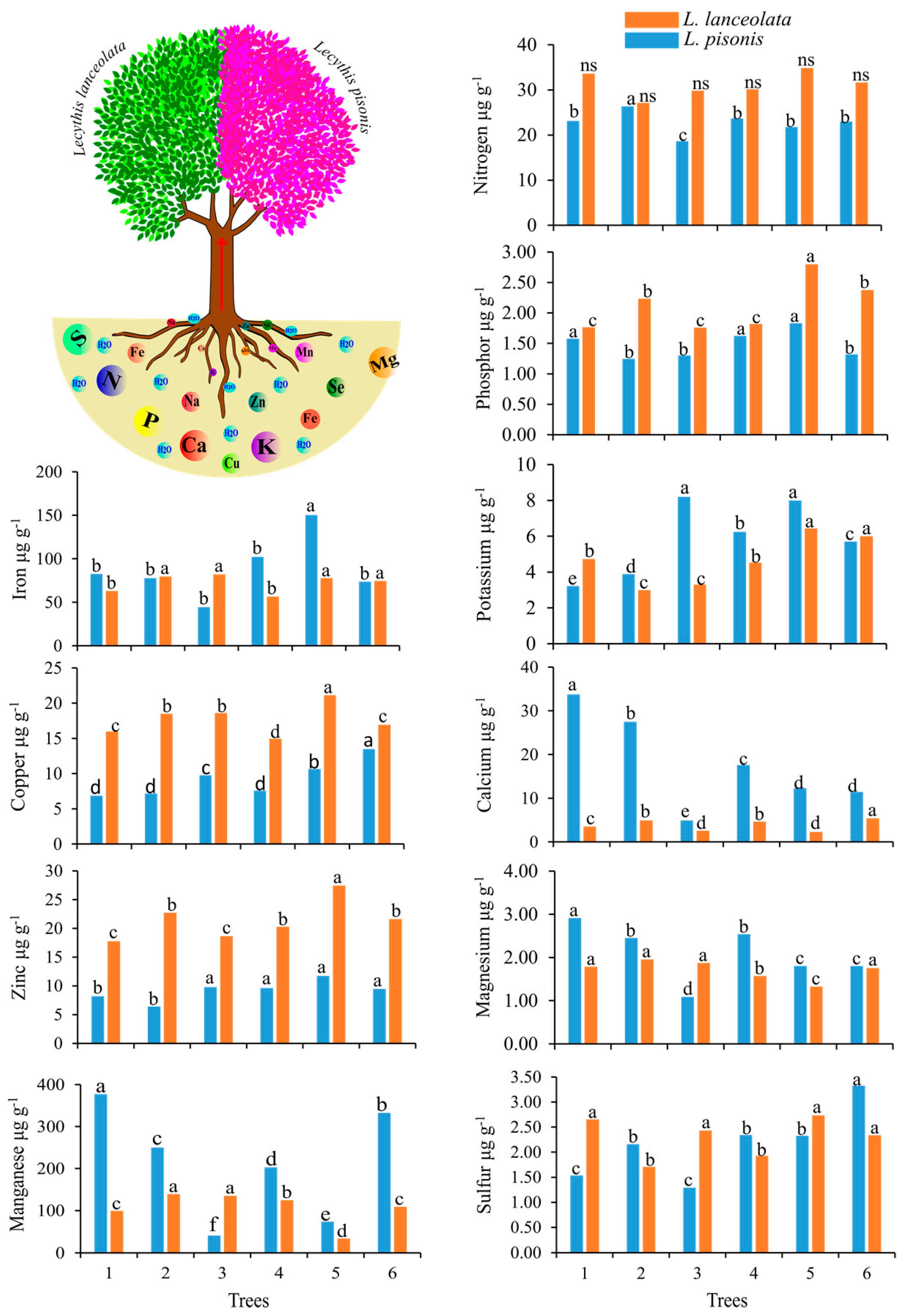
3.1. Extraction of Minerals from Leaves, Soil and Nuts
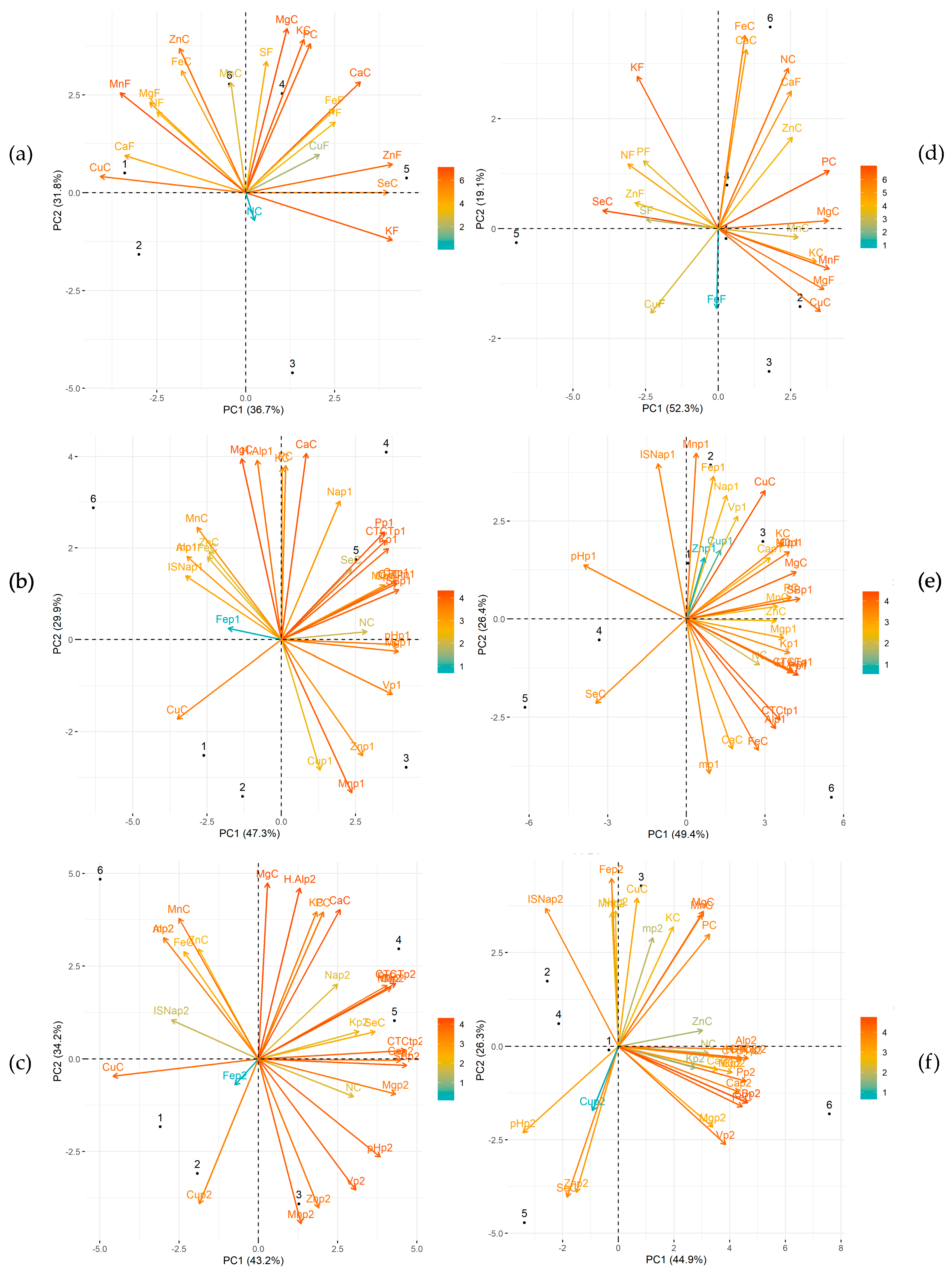
3.2. PCA Analysis
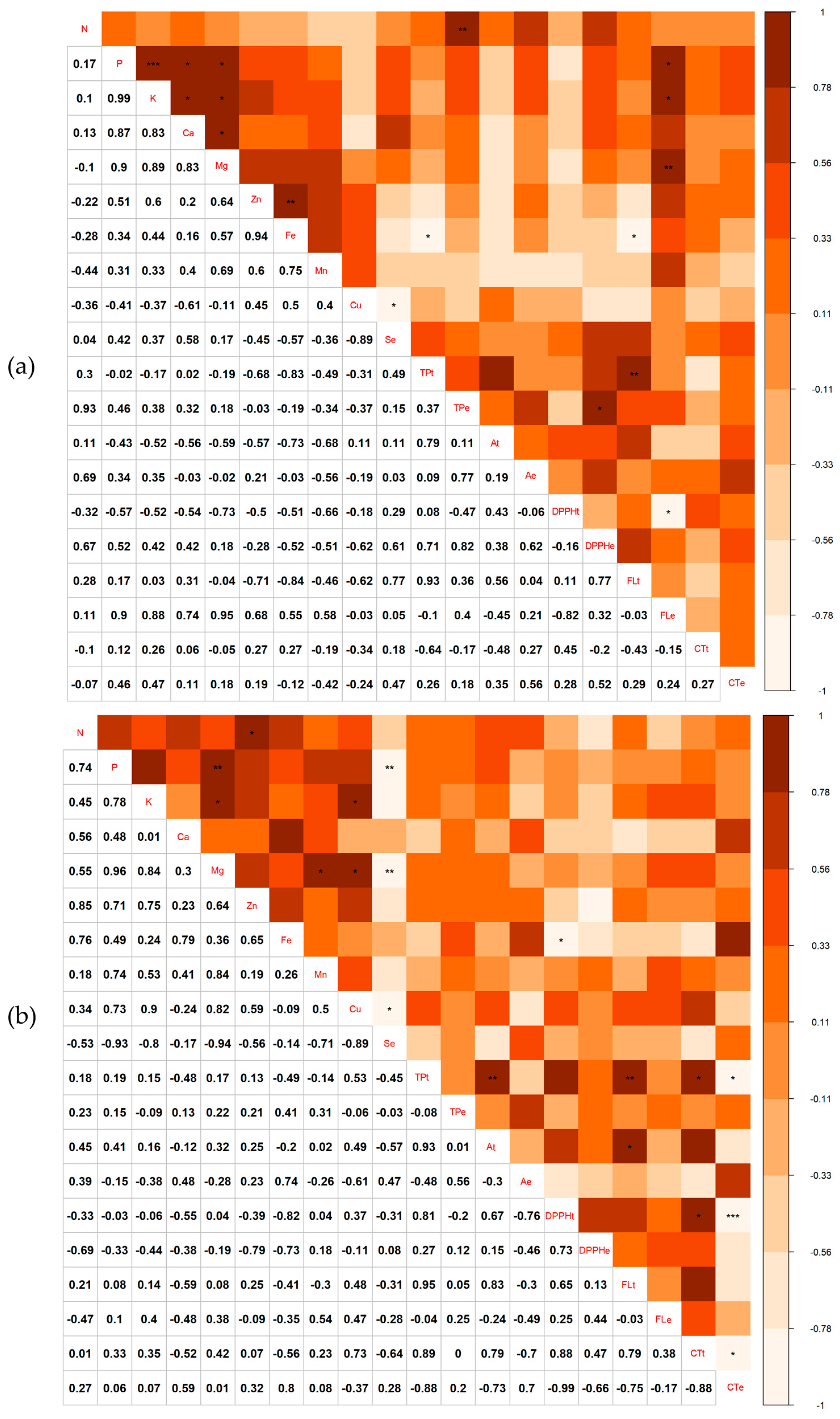
3.3. Minerals and Antioxidants in Chestnuts
3.4. X-ray Densitometry in Chestnuts
3.5. Brix and Fatty Acid Composition in Chestnut Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, N.; Melo, J.C.; Batista, L.F.; Paula-Souza, J.; Fronza, P.; Brandão, M.G. Edible fruits from Brazilian biodiversity: A review on their sensorial characteristics versus bioactivity as tool to select research. Food Res. Int. 2019, 119, 325–348. [Google Scholar] [PubMed]
- Lima, R.A.F.; Souza, V.C.; Siqueira, M.F.; Steege, H. Defining endemism levels for biodiversity conservation: Tree species in the Atlantic Forest hotspot. Biol. Conserv. 2020, 252, 108825. [Google Scholar]
- Demoliner, F.; Policarpi, P.d.B.; Ramos, J.C.; Bascuñan, V.L.A.F.; Ferrari, R.A.; Jachmanián, I.; Casas, A.d.F.d.; Vasconcelos, L.F.L.; Block, J.M. Sapucaia nut (Lecythis pisonis Cambess) and its by-products: A promising and underutilized source of bioactive compounds. Part I: Nutritional composition and lipid profile. Food Res. Int. 2018, 108, 27–34. [Google Scholar] [PubMed]
- Demoliner, F.; Policarpi, P.d.B.; Vasconcelos, L.F.L.; Vitali, L.; Micke, G.A.; Block, J.M. Sapucaia nut (Lecythis pisonis Cambess) and its by-products: A promising and underutilized source of bioactive compounds. Part II: Phenolic compounds profile. Food Res. Int. 2018, 112, 434–442. [Google Scholar]
- Rosa, T.L.M.; de Araujo, C.P.; Kamke, C.; Ferreira, A.; Ferreira, M.F.d.S.; de Oliveira, J.P.B.; Schmildt, E.R.; Lopes, J.C.; Mengarda, L.H.G.; Otoni, W.C.; et al. Sapucaia nut: Morphophysiology, minerals content, methodological validation in image analysis, phenotypic and molecular diversity in Lecythis pisonis Cambess. Food Res. Int. 2020, 137, 109383. [Google Scholar]
- Polmann, G.; Badia, V.; Danielski, R.; Ferreira, S.R.S.; Block, J.M. Non-conventional nuts: An overview of reported composition and bioactivity and new approaches for its consumption and valorization of co-products. Future Foods 2021, 4, 100099. [Google Scholar]
- Araujo, N.M.P.; Arruda, H.S.; Marques, D.R.P.; Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and nutritional properties of selected Amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating progress of chestnut quality: A review of recent developments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar]
- Vieira, M.E.B.; Vasconcelos, I.M.; Machado, O.L.T. Isolation, characterization and mechanism of action of an antimicrobial peptide from Lecythis pisonis seeds with inhibitory activity against Candida albicans. Acta Biochim. Biophys. Sin. 2015, 47, 716–729. [Google Scholar]
- Carvalho, R.F.; Huguenin, G.V.B.; Luiz, R.R.; Moreira, A.S.B.; Oliveira, G.M.M.; Rosa, G. Intake of partially defatted Brazil nut flour reduces serum cholesterol in hypercholesterolemic patients-a randomized controlled trial. Nutr. J. 2015, 14, 59. [Google Scholar]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Usta-Gorgun, B.; Keser, G.; Ciniviz, M.; Demiray-Teymuroglu, M. The probiotic chestnut-based dairy matrix: Influence on the metabolomic formation and the nutritional quality. Int. J. Dairy Technol. 2023. [Google Scholar] [CrossRef]
- Mas, A.L.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel cookie formulation with defatted sesame flour: Evaluation of its technological and sensory properties. Changes in phenolic profile, antioxidant activity, and gut microbiota after simulated gastrointestinal digestion. Food Chem. 2022, 389, 133122. [Google Scholar]
- Pinto, D.; Moreira, M.M.; Vieira, E.F.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and characterization of functional cookies enriched with chestnut shells extract as source of bioactive phenolic compounds. Foods 2023, 12, 640. [Google Scholar]
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional foods modulating inflammation and metabolism in chronic diseases: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4371–4392. [Google Scholar]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdedú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays–A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Marshall, T.M.; Church, D.D.; Jajtner, A.R.; Esposito, R. Foundational Nutrition: Implications for Human Health. Nutrients 2023, 15, 2837. [Google Scholar] [PubMed]
- Yeum, K.-J.; Beretta, G.; Krinsky, N.I.; Russell, R.M.; Aldini, G. Synergistic interactions of antioxidant nutrients in a biological model system. Nutrition 2009, 25, 839–846. [Google Scholar] [PubMed]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar]
- World Health Organization. Fact Sheet: Malnutrition; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Junior, E.S.; Wadt, L.; Silva, K.; Lima, R.; Batista, K.; Guedes, M.; Carvalho, G.; Reis, A.; Lopes, G.; Guilherme, L. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere 2017, 188, 650–658. [Google Scholar]
- Junior, E.C.d.S.; Wadt, L.H.d.O.; da Silva, K.E.; de Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Junior, R.C.d.O.; dos Reis, A.R.; Lopes, G.; Broadley, M.R.; et al. Geochemistry of selenium, barium, and iodine in representative soils of the Brazilian Amazon rainforest. Sci. Total Environ. 2022, 828, 154426. [Google Scholar]
- Statwick, J.; Sher, A.A. Selenium in soils of western Colorado. J. Arid. Environ. 2017, 137, 1–6. [Google Scholar]
- Gunstone, F.D. Fatty acids—Nomenclature, structure, isolation and structure determination, biosynthesis and chemical synthesis. In Fatty Acid and Lipid Chemistry; Blackie Academic & Profesional: Glasgow, UK, 1996; pp. 1–33. [Google Scholar]
- Haast, R.A.; Kiliaan, A.J. Impact of fatty acids on brain circulation, structure and function. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 3–14. [Google Scholar]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.d.C.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar]
- Brosolo, G.; Da Porto, A.; Marcante, S.; Picci, A.; Capilupi, F.; Capilupi, P.; Bertin, N.; Vvarelli, C.; Bulfone, L.; Vacca, A.; et al. Omega-3 fatty acids in arterial hypertension: Is there any good news? Int. J. Mol. Sci. 2023, 24, 9520. [Google Scholar] [PubMed]
- Silva, F.C. Manual de Análises Químicas de Solos, Plantas E Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Rio de Janeiro, Brazil, 2009; p. 627. [Google Scholar]
- Olson, O.E.; Palmer, I.S.; Cary, E.E. Modification of the Official Fluorometric Method for Selenium in Plants. J. AOAC Int. 1975, 58, 117–121. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa Solos: Brasília, Brazil, 2017. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 182–205. [Google Scholar]
- Hagerman, A.E. Radial diffusion method for determining tannin in plant extracts. J. Chem. Ecol. 1987, 13, 437–449. [Google Scholar]
- Nascimento, J.C.; Lage, L.F.O.; Camargos, C.R.D.; Amaral, J.C.; Costa, L.M.; Sousa, A.D.; Oliveira, F.Q. Determinação da atividade antioxidante pelo método DPPH e doseamento de flavonóides totais em extratos de folhas da Bauhinia variegata L. Rev. Bras. Farmácia 2011, 92, 327–332. [Google Scholar]
- Mello, T.; Zanardo, T.É.C.; dos Santos Taliuli, Y.; Simões, I.M.; Baptista, J.O.; Gonçalves, F.G.; Hegedus, C.E.N.; Schmildt, E.R.; Ferreira, A.; Santos, H.O.; et al. Maturation and quality of seeds of an endangered tropical palm species (Euterpe edulis Martius) assessed by imaging and X-ray densitometry. 2023. [Google Scholar] [CrossRef]
- Zeng, A.X.; Chin, S.-T.; Nolvachai, Y.; Kulsing, C.; Sidisky, L.M.; Marriott, P.J. Characterisation of capillary ionic liquid columns for gas chromatography–mass spectrometry analysis of fatty acid methyl esters. Anal. Chim. Acta 2013, 803, 166–173. [Google Scholar]
- International Olive Council. Determination of Fatty Acid Methyl Esters by Gas Chromatography. COI/T. 20/DOC. No 33/Ver. 1. 2007. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf (accessed on 29 May 2022).
- Rosa, T.L.M.; de Araujo, C.P.; Alexandre, R.S.; Schmildt, E.R.; Lopes, J.C. Biometry and genetic diversity of paradise nut genotypes (Lecythidaceae). Pesqui. Agropecu Bras. 2019, 54, e00240. [Google Scholar]
- Barker, A.V.; Stratton, M.L. Nutrient density of fruit crops as a function of soil fertility. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Oxford, UK, 2020; pp. 13–31. [Google Scholar]
- Maity, A.; Babu, K.D.; Sarkar, A.; Pal, R.K. Seasonality of nutrients vis-à-vis fruit quality of pomegranate cv. Bhagwa on vertisol. J. Plant Nutr. 2017, 40, 1351–1363. [Google Scholar]
- de Figueiredo, M.A.; Boldrin, P.F.; Hart, J.J.; de Andrade, M.J.; Guilherme, L.R.; Glahn, R.P.; Li, L. Zinc and selenium accumulation and their effect on iron bioavailability in common bean seeds. Plant Physiol. Biochem. 2017, 111, 193–202. [Google Scholar] [PubMed]
- Zuo, Q.S.; Zhou, G.S.; Yang, S.F.; Yang, Y.; Wu, L.R.; Leng, S.H.; Yang, G.; Wu, J.S. Effects of nitrogen rate and genotype on seed protein and amino acid content in canola. J. Agric. Sci. 2016, 154, 438–455. [Google Scholar]
- Vallilo, M.I.; Tavares, M.; Pimentel, S.A.; Badolato, E.S.G.; Inomata, E.I. Caracterização química parcial das sementes de Lecythis pisonis Camb. (Sapucaia). Acta Amaz. 1998, 28, 131–140. [Google Scholar]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet Assoc. 2000, 100, 637–640. [Google Scholar]
- Esteve-Llorens, X.; Darriba, C.; Moreira, M.T.; Feijoo, G.; González-García, S. Towards an environmentally sustainable and healthy Atlantic dietary pattern: Life cycle carbon footprint and nutritional quality. Sci. Total Environ. 2019, 646, 704–715. [Google Scholar]
- Combet, E.; Buckton, C. Micronutrient deficiencies, vitamin pills and nutritional supplements. Med. Baltim. 2019, 47, 145–151. [Google Scholar]
- Liu, E.A.; Lieberman, A.P. The intersection of lysosomal and endoplasmic reticulum calcium with autophagy defects in lysosomal diseases. Neurosci. Lett. 2019, 697, 10–16. [Google Scholar] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [PubMed]
- de Brito, R.C.M.; Junior, J.B.P.; Dantas, K.d.G.F. Quantification of inorganic constituents in Brazil nuts and their products by inductively coupled plasma optical emission spectrometry. LWT-Food Sci. Technol. 2019, 116, 108383. [Google Scholar]
- Krikorian, R.; Kalt, W.; McDonald, J.E.; Shidler, M.D.; Summer, S.S.; Stein, A.L. Cognitive performance in relation to urinary anthocyanins and their flavonoid-based products following blueberry supplementation in older adults at risk for dementia. J. Funct. Foods 2020, 64, 103667. [Google Scholar]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating c-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT-Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar]
- Zhang, M.; Steele, M.A.; Yi, X. Reconsidering the effects of tannin on seed dispersal by rodents: Evidence from enclosure and field experiments with artificial seeds. Behav. Process. 2013, 100, 200–207. [Google Scholar]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Relationships between DNA damage protection activity, total phenolic content, condensed tannin content and antioxidant potential among Indian barley cultivars. Biocatal. Agric. Biotechnol. 2017, 11, 201–206. [Google Scholar]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods 2010, 2, 196–209. [Google Scholar]
- Aliakbarkhani, S.T.; Farajpour, M.; Asadian, A.H.; Aalifar, M.; Ahmadi, S.; Akbari, M. Variation of nutrients and antioxidant activity in seed and exocarp layer of some Persian pistachio genotypes. Ann. Agric. Sci. 2017, 62, 39–44. [Google Scholar]
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of phenolic compounds on physicochemical and functional properties of protein isolate from Cinnamomum camphora seed kernel. Food Hydrocoll. 2020, 102, 105612. [Google Scholar] [CrossRef]
- Xavier, A.D.S.; Furtado, D.Z.S.; Assunção, N.A.; Nascimento, A.N. Bioacessibility of Fe and Zn (associated to proteins) in cashew nut. J. Food Compos. Anal. 2019, 83, 103259. [Google Scholar]
- Liu, X.; Yang, Y.; Deng, X.; Li, M.; Zhang, W.; Zhao, Z. Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ. Exp. Bot. 2017, 135, 13–20. [Google Scholar]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Trippe, R.C., III; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- Gomes-Junior, F.; Yagushi, J.; Belini, U.; Cicero, S.; Tomazello-Filho, M. X-ray densitometry to assess internal seed morphology and quality. Seed Sci. Technol. 2012, 40, 102–107. [Google Scholar] [CrossRef]
- Colpo, E.; Vilanova, C.D.D.; Reetz, L.G.B.; Duarte, M.M.; Farias, I.L.G.; Meinerz, D.F.; Mariano, D.O.C.; Vendrusculo, R.G.; Boligon, A.A.; Corte, C.L.D.; et al. Brazilian nut consumption by healthy volunteers improves inflammatory parameters. Nutrition 2014, 30, 459–465. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Carrero, J.J.; Weide, L.D.C.C. Effect of selenium supplementation via Brazil nut (Bertholletia excelsa, HBK) on thyroid hormones levels in hemodialysis patients: A pilot study. Nutr. Hosp. 2015, 32, 1808–1812. [Google Scholar]
- Zanqui, A.B.; Silva, C.M.D.; Ressutte, J.B.; Morais, D.R.D. Brazil nut oil extraction using subcritical n-propane: Advantages and chemical composition. J. Braz. Chem. Soc. 2020, 31, 603–612. [Google Scholar]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [PubMed]
- Aglietti, C.; Cappelli, A.; Andreani, A. From chestnut tree (Castanea sativa) to flour and foods: A systematic review of the main criticalities and control strategies towards the relaunch of chestnut production chain. Sustainability 2022, 14, 12181. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Alves, T.O.; Ferreira, M.S.; Scherf, K.A. Recent progress in analytical method development to ensure the safety of gluten-free foods for celiac disease patients. J. Cereal Sci. 2020, 96, 103114. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Piazza, S.; Vrhovsek, U.; Fumagalli, M.; Khalilpour, S.; Masuero, D.; Di Lorenzo, C.; Colombo, L.; Mattivi, F.; De Fabiani, E.; et al. A bio-guided approach for the development of a chestnut-based proanthocyanidin-enriched nutraceutical with potential anti-gastritis properties. Pharmacol. Res. 2018, 134, 145–155. [Google Scholar] [CrossRef] [PubMed]
| tR (min) | Fatty acid | L. pisonis | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 15.59 | Myristic acid (C14:0) | 11.01 ± 0.23 | 11.50 ± 0.34 | 12.10 ± 0.40 | 12.70 ± 0.03 | 12.00 ± 0.22 | 13.00 ± 0.08 |
| 19.88 | Palmitic acid (C16:0) | 0.24 ± 0.03 | 0.20 ± 0.01 | 0.60 ± 0.34 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.40 ± 0.01 |
| 20.41 | Palmitoleic acid (C16:1) | 0.10 ± 0.01 | - | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | - |
| 22.19 | Margaric acid (C17:0) | - | - | 0.10 ± 0.01 | - | - | - |
| 22.71 | Heptadec-10-enoic acid (17:1) | 12.80 ± 0.08 | 6.50 ± 0.15 | 8.40 ± 0.06 | 11.70 ± 0.01 | 13.00 ± 0.11 | 7.60 ± 0.04 |
| 24.75 | Stearic acid (C18:0) | 36.50 ± 0.16 | 41.60 ± 0.25 | 52.40 ± 0.77 | 38.90 ± 0.05 | 40.20 ± 0.16 | 44.80 ± 0.02 |
| 25.22 | Oleic acid (C18:1 n9c) | 37.70 ± 0.19 | 38.50 ± 0.28 | 24.20 ± 0.07 | 34.40 ± 0.02 | 32.90 ± 0.07 | 32.10 ± 0.01 |
| 25.46 | Vaccenic acid (C18:1 n11c) | 1.30 ± 0.05 | 1.30 ± 0.01 | 1.70 ± 0.09 | 1.30 ± 0.02 | 1.10 ± 0.01 | 1.60 ± 0.03 |
| 26.39 | Linoleic acid (C18:2 n6c) | 0.10 ± 0.03 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.10 ± 0.01 | 0.20 ± 0.02 |
| 27.85 | Linolenic acid (C18:3) | 0.20 ± 0.05 | 0.20 ± 0.01 | 0.20 ± 0.03 | 0.40 ± 0.03 | 0.30 ± 0.01 | 0.30 ± 0.01 |
| Total saturated fatty acids | 47.70 ± 0.20 | 53.20 ± 0.10 | 65.30 ± 0.32 | 51.90 ± 0.25 | 52.40 ± 0.08 | 58.20 ± 0.07 | |
| Total monounsaturated fatty acids | 51.80 ± 0.15 | 46.40 ± 0.12 | 34.40 ± 0.28 | 47.50 ± 0.40 | 47.10 ± 0.07 | 41.30 ± 0.08 | |
| Total polyunsaturated fatty acids | 0.40 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.03 | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.50 ± 0.02 | |
| S/U * | 0.91 ± 0.04 | 1.14 ± 0.01 | 1.88 ± 0.04 | 1.08 ± 0.01 | 1.10 ± 0.01 | 1.39 ± 0.03 | |
| tR (min) | Fatty acid | L. lanceolata | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 15.59 | Myristic acid (C14:0) | 9.30 ± 0.16 | 9.70 ± 0.23 | 10.30 ± 0.03 | 11.60 ± 0.15 | 9.80 ± 0.18 | 8.20 ± 0.15 |
| 19.88 | Palmitic acid (C16:0) | 0.10 ± 0.01 | 0.10 ± 0.03 | 0.10 ± 0.01 | - | 0.10 ± 0.01 | 0.10 ± 0.03 |
| 20.41 | Palmitoleic acid (C16:1) | - | - | - | - | 0.10 ± 0.01 | - |
| 22.19 | Margaric acid (C17:0) | - | - | - | - | - | - |
| 22.71 | Heptadec-10-enoic acid (17:1) | 3.70 ± 0.20 | 4.70 ± 0.05 | 5.60 ± 0.01 | 5.10 ± 0.03 | 6.10 ± 0.05 | 5.30 ± 0.07 |
| 24.75 | Stearic acid (C18:0) | 45.90 ± 0.17 | 45.10 ± 0.21 | 32.70 ± 0.05 | 31.80 ± 0.08 | 44.00 ± 0.11 | 50.50 ± 0.16 |
| 25.22 | Oleic acid (C18:1 n9c) | 39.40 ± 0.32 | 38.60 ± 0.01 | 49.50 ± 0.06 | 50.50 ± 0.10 | 38.50 ± 0.13 | 34.40 ± 0.02 |
| 25.46 | Vaccenic acid (C18:1 n11c) | 1.40 ± 0.01 | 1.40 ± 0.01 | 1.40 ± 0.05 | 1.00 ± 0.03 | 1.20 ± 0.02 | 1.00 ± 0.01 |
| 26.39 | Linoleic acid (C18:2 n6c) | - | 0.10 ± 0.03 | 0.10 ± 0.02 | - | 0.10 ± 0.01 | 0.10 ± 0.01 |
| 27.85 | Linolenic acid (C18:3) | 0.10 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.02 | - | 0.20 ± 0.05 | 0.20 ± 0.03 |
| Total saturated fatty acids | 55.40 ± 0.01 | 54.90 ± 0.01 | 43.10 ± 0.02 | 43.40 ± 0.10 | 53.96 ± 0.08 | 58.80 ± 0.04 | |
| Total monounsaturated fatty acids | 44.50 ± 0.05 | 44.80 ± 0.01 | 56.60 ± 0.03 | 56.60 ± 0.10 | 45.80 ± 0.09 | 40.80 ± 0.03 | |
| Total polyunsaturated fatty acids | 0.10 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0 | 0.30 ± 0.01 | 0.30 ± 0.01 | |
| S/U * | 1.24 ± 0.01 | 1.22 ± 0.02 | 0.76 ± 0.01 | 0.77 ± 0.07 | 1.17 ± 0.01 | 1.43 ± 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacio de Araujo, C.; Medeiros Simões, I.; Lins Monteiro Rosa, T.; de Mello, T.; Bravim Canal, G.; Ferreira, A.; Bestete de Oliveira, J.P.; Romais Schmildt, E.; Lopes, J.C.; da Silva de Souza, T.; et al. Functional Fruit Trees from the Atlantic and Amazon Forests: Selection of Potential Chestnut Trees Rich in Antioxidants, Nutrients, and Fatty Acids. Foods 2023, 12, 4422. https://doi.org/10.3390/foods12244422
Palacio de Araujo C, Medeiros Simões I, Lins Monteiro Rosa T, de Mello T, Bravim Canal G, Ferreira A, Bestete de Oliveira JP, Romais Schmildt E, Lopes JC, da Silva de Souza T, et al. Functional Fruit Trees from the Atlantic and Amazon Forests: Selection of Potential Chestnut Trees Rich in Antioxidants, Nutrients, and Fatty Acids. Foods. 2023; 12(24):4422. https://doi.org/10.3390/foods12244422
Chicago/Turabian StylePalacio de Araujo, Caroline, Ingridh Medeiros Simões, Thuanny Lins Monteiro Rosa, Tamyris de Mello, Guilherme Bravim Canal, Adésio Ferreira, João Paulo Bestete de Oliveira, Edilson Romais Schmildt, José Carlos Lopes, Tércio da Silva de Souza, and et al. 2023. "Functional Fruit Trees from the Atlantic and Amazon Forests: Selection of Potential Chestnut Trees Rich in Antioxidants, Nutrients, and Fatty Acids" Foods 12, no. 24: 4422. https://doi.org/10.3390/foods12244422
APA StylePalacio de Araujo, C., Medeiros Simões, I., Lins Monteiro Rosa, T., de Mello, T., Bravim Canal, G., Ferreira, A., Bestete de Oliveira, J. P., Romais Schmildt, E., Lopes, J. C., da Silva de Souza, T., Otoni, W. C., Pinheiro, P. F., Moreira Novaes, F. J., Gonçalves, F. G., Santos, A. R. d., & Sobreira Alexandre, R. (2023). Functional Fruit Trees from the Atlantic and Amazon Forests: Selection of Potential Chestnut Trees Rich in Antioxidants, Nutrients, and Fatty Acids. Foods, 12(24), 4422. https://doi.org/10.3390/foods12244422








