Exploration of Postharvest Conditions for Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen Roots Based on Sensory Quality, Active Components, Antioxidant Capacity and Physiological Changes at Different Storage Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Freezing Point Temperature of C. pilosula Roots
2.3. Storage Effect and Quality
2.3.1. Sensory Analysis
2.3.2. Weight Loss and Respiratory Intensity
2.3.3. O2−∙ Production Rate, H2O2 Content and MDA Content
2.3.4. SOD, CAT, POD and PPO Activity
2.3.5. PME, PG, Cx and β-Glu Activity
2.3.6. Lobetyolin Content, Syringin Content and Atractylenolide III Content
2.3.7. Total Flavonoid Content, Total Phenol Content and DPPH Radical Scavenging Rate
2.4. Statistical Analysis
3. Results
3.1. The Freezing Point Curve of Fresh C. pilosula Roots
3.2. Storage Quality of C. pilosula Roots
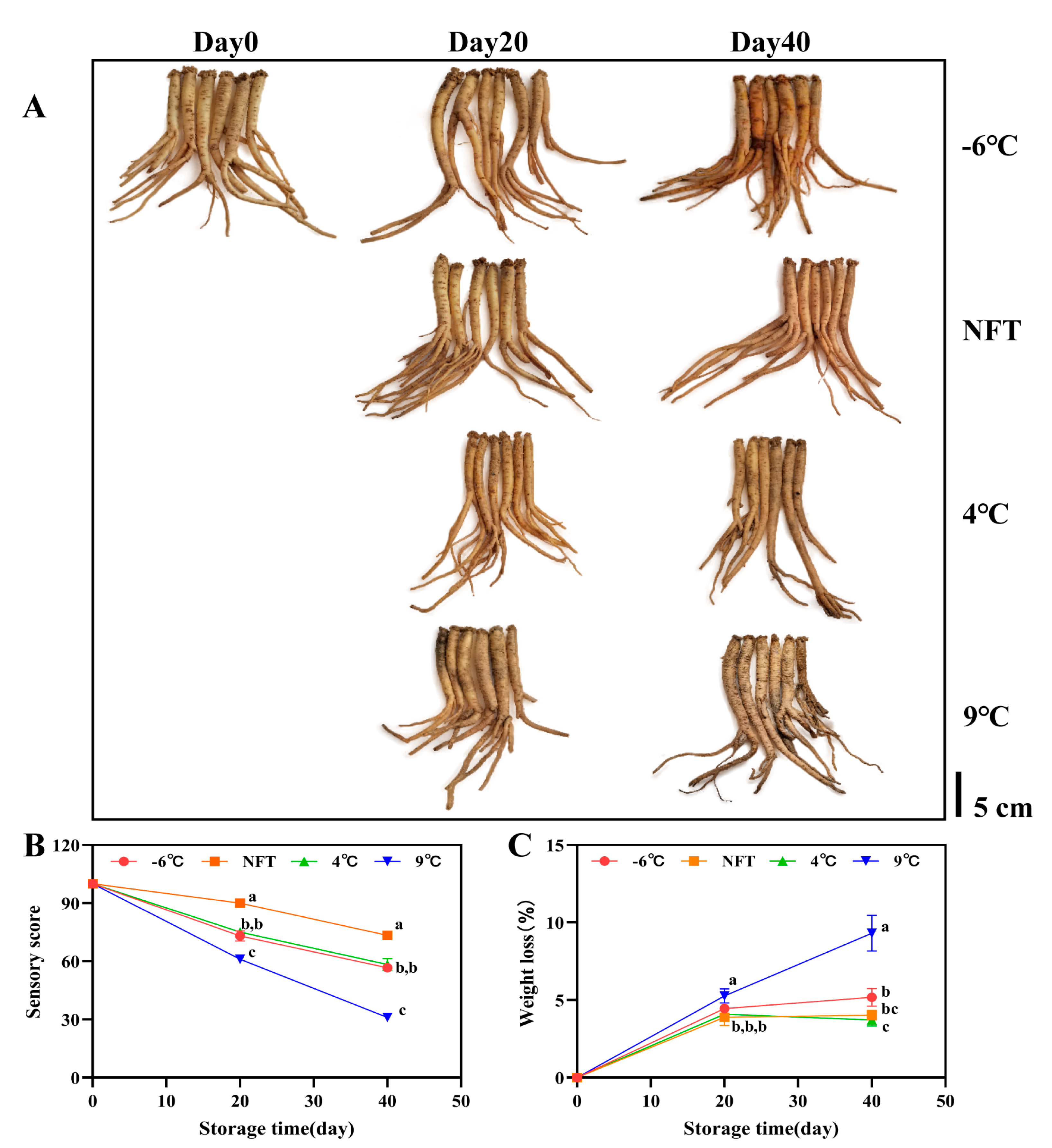
3.2.1. Changes in Appearance, Sensory Scores and Weight Loss
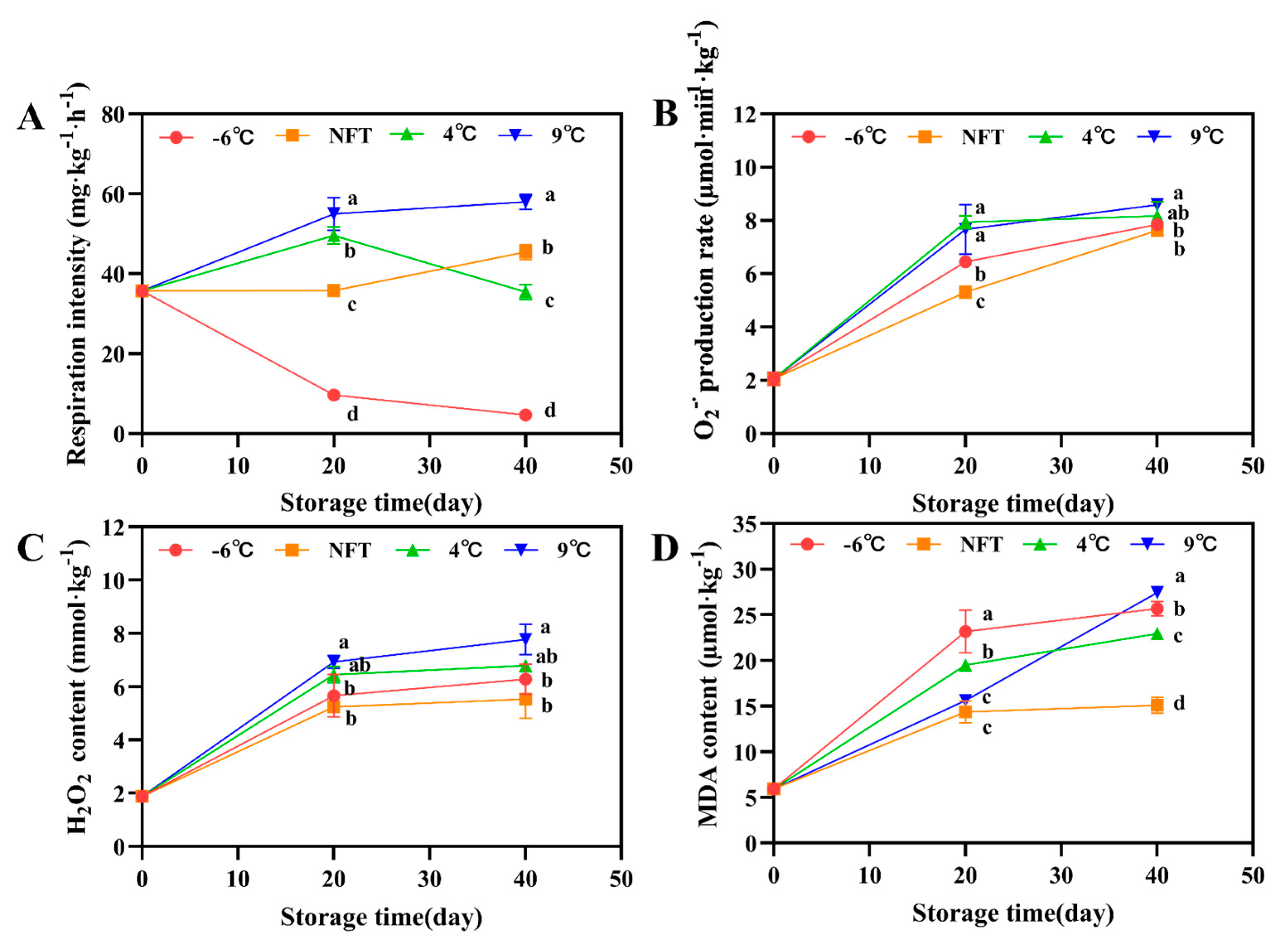
3.2.2. Changes in Respiratory Intensity, O2−∙ Production Rate, H2O2 Content and MDA Content
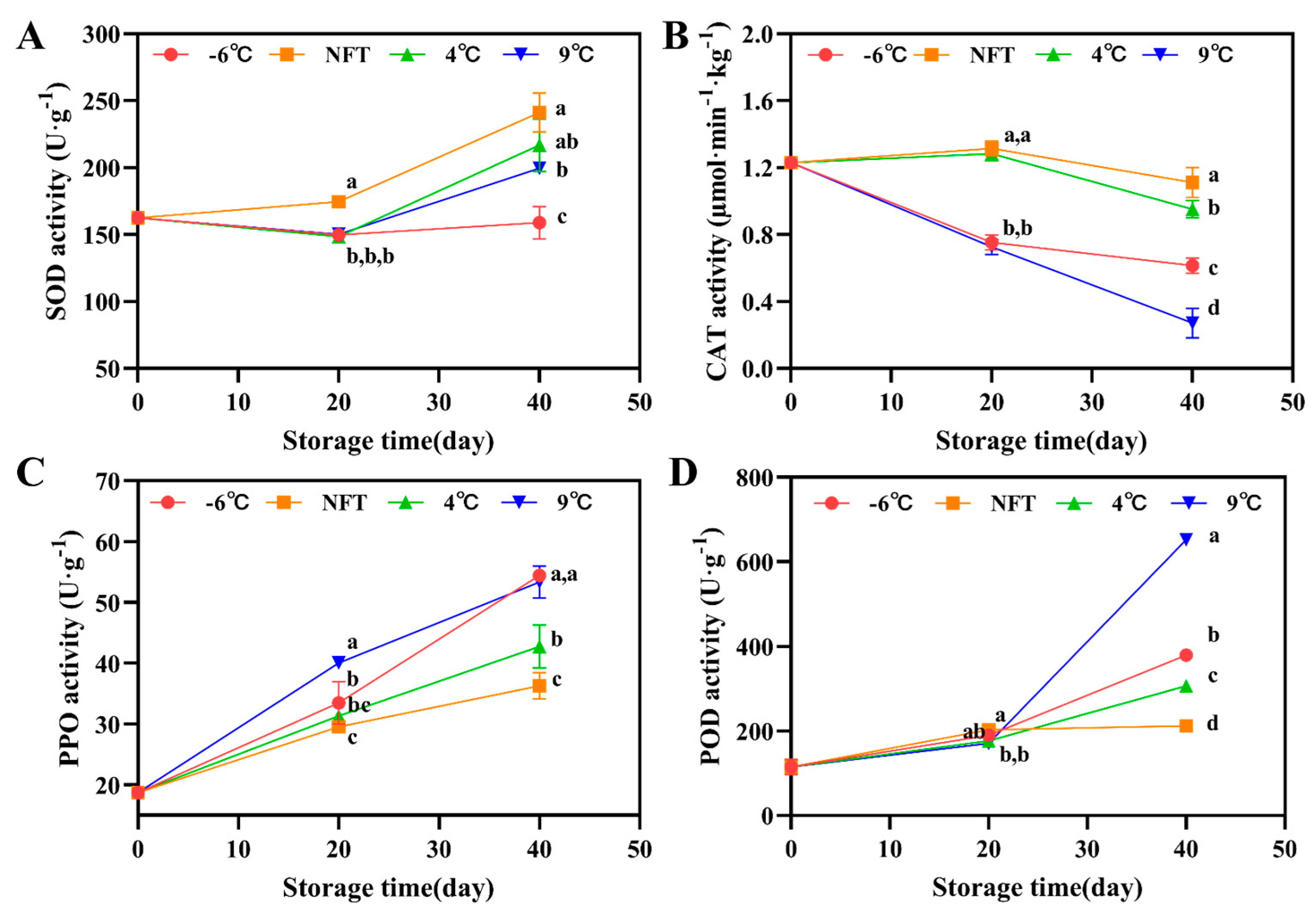
3.2.3. The Activity of SOD, CAT, PPO and POD
3.2.4. The Activity of PG, PME, Cx and β-Glu
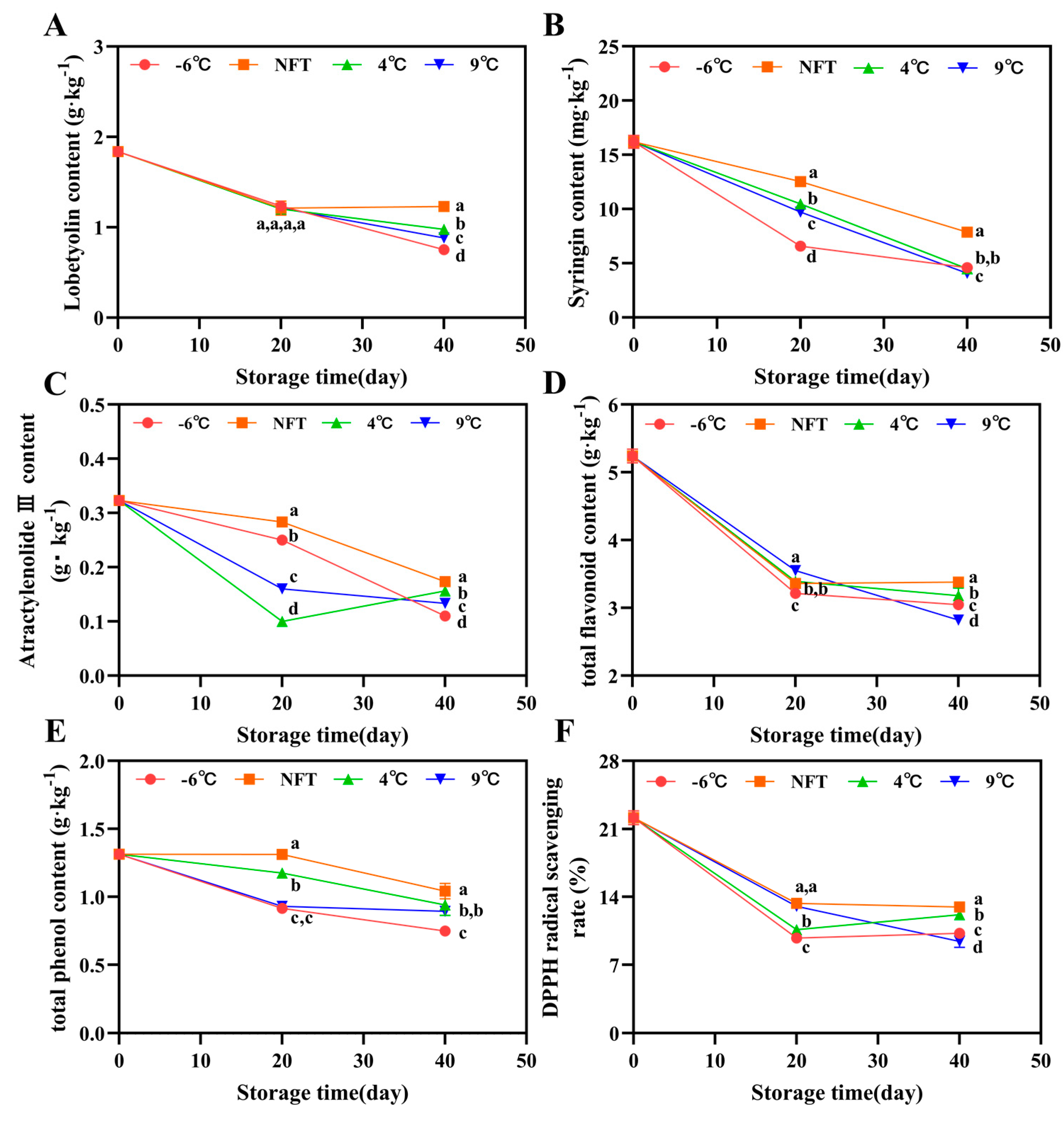
3.2.5. Changes in Lobetyolin Content, Syringin Content and Atractylenolide III Content
3.2.6. Changes in Total Flavonoid Content, Total Phenol Content, and DPPH Radical Scavenging Rate
3.3. Multivariate Statistical Analysis
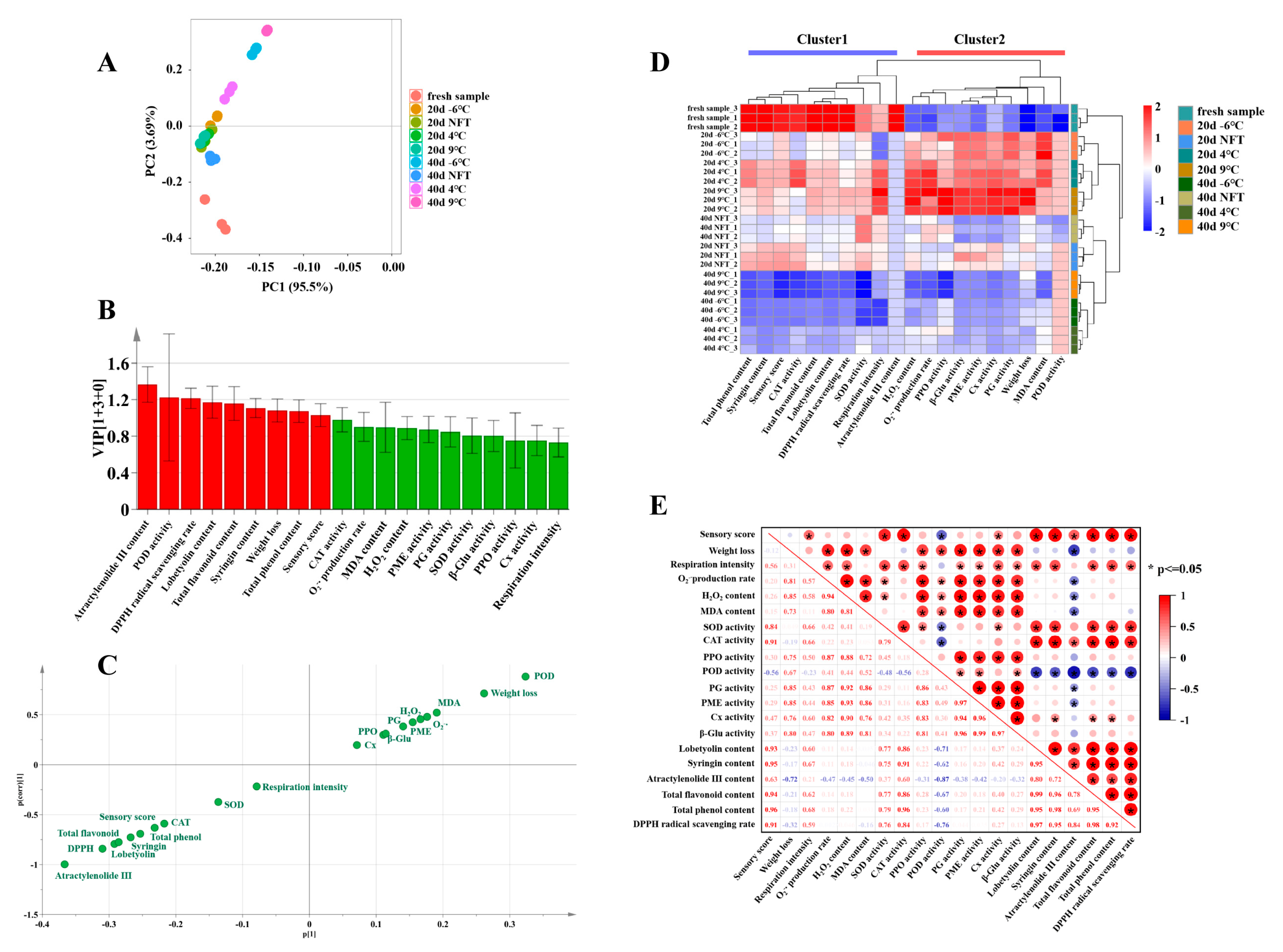
3.3.1. Effects of Near-Freezing Storage on Postharvest Quality of C. pilosula Roots Based on PCA
3.3.2. Effects of Near-Freezing Storage on Postharvest Quality of C. pilosula Roots Based on OPLS-DA
3.3.3. Effects of Near-Freezing Storage on Postharvest Quality of C. pilosula Roots Based on Cluster and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NFT | Near-freezing temperature |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| PPO | Polyphenol oxidase |
| POD | Peroxidase |
| PME | Pectin methylesterase |
| PG | Polygalacturonase |
| Cx | Cellulase |
| β-Glu | β-Glucosidase |
| ROS | Reactive oxygen species |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| HPLC | High-performance liquid chromatography |
| DAD | Diode array detector |
References
- Bai, R.; Wang, Y.; Zhang, Y.; Wang, Y.; Han, J.; Wang, Z.; Zhou, J.; Hu, F. Analysis and Health Risk Assessment of Potentially Toxic Elements in Three Codonopsis Radix Varieties in China. Biol. Trace Elem. Res. 2022, 200, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Zhu, S.; Goda, Y.; Cai, S.Q.; Komatsu, K. Quality evaluation of medicinally-used Codonopsis species and Codonopsis Radix based on the contents of pyrrolidine alkaloids, phenylpropanoid and polyacetylenes. J. Nat. Med. 2014, 68, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, J.; Leng, F.; Li, S.; Ma, X.; Wang, X.; Wang, Y. Effects of time-space conversion on microflora structure, secondary metabolites composition and antioxidant capacity of Codonopsis pilosula root. Plant Physiol. Biochem. PPB 2023, 198, 107659. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Ji, Y.; Peng, L.; Liu, Q.; Cao, H.; Yang, Y.; He, X.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Sisi, Q. Study on the Processing and Concocting Integration Technologies in Producing Areas based on Fresh Wendang. Master’s Thesis, Lanzhou University, Lanzhou, China, 2016. [Google Scholar]
- Lv, B.; Yang, X.; Xue, H.; Nan, M.; Zhang, Y.; Liu, Z.; Bi, Y.; Shang, S. Isolation of Main Pathogens Causing Postharvest Disease in Fresh Codonopsis pilosula during Different Storage Stages and Ozone Control against Disease and Mycotoxin Accumulation. J. Fungi 2023, 9, 146. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhao, Y.T.; Zhang, Z.C.; He, H.; Shi, L.; Zhu, X.; Cui, K.B. Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022, 387, 10. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.M.; Zhang, H.N.; Tian, C.Q.; Xu, H.B. Low temperature preservation developed for special foods in East Asia: A review. J. Food Process. Preserv. 2022, 46, 11. [Google Scholar] [CrossRef]
- Zhao, H.D.; Jiao, W.X.; Cui, K.B.; Fan, X.G.; Shu, C.; Zhang, W.L.; Cao, J.K.; Jiang, W.B. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Fan, X.; Jiao, W.; Cao, J.; Jiang, W. Near freezing point temperature storage inhibits chilling injury and enhances the shelf life quality of apricots following long-time cold storage. J. Food Process. Preserv. 2019, 43, e13958. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, K.; Wang, P.; Zhang, L.; Li, Y. Comprehensive quality evaluation of processed Scrophulariae Radix from different regions of China using HPLC coupled with chemometrics methods. Phytochem. Anal. 2023, 34, 816–829. [Google Scholar] [CrossRef]
- An, Q.; Sun, H.; Wu, L.; Liu, L.; Chen, S. Quality evaluation of Codonopsis Radix through high performance liquid chromatography fingerprint combined with chemometrics and simultaneous determination of five characteristic ingredients. Acta Chromatogr. 2023, 35, 247–258. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Wang, X.; Luo, W.; Yang, F. Quality Assessment and Classification of Codonopsis Radix Based on Fingerprints and Chemometrics. Molecules 2023, 28, 5127. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Anticancer Properties of Lobetyolin, an Essential Component of Radix Codonopsis (Dangshen). Nat. Product. Bioprospect. 2021, 11, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.X.; Lyu, X.; Chen, J.; Chen, X.M.; Guo, S.X. Untargeted Metabolomics Reveals Multiple Phytometabolites in the Agricultural Waste Materials and Medicinal Materials of Codonopsis pilosula. Front. Plant Sci. 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Wei, G.F.; Ning, K.; Zhang, G.Z.; Liu, Y.P.; Dong, L.L.; Chen, S.L. Contents of lobetyolin, syringin, and atractylolide III in Codonopsis pilosula are related to dynamic changes of endophytes under drought stress. Chin. Med. 2021, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Fan, R.N.; Sun, G.L.; Sun, T.; Fan, Y.T.; Bai, S.L.; Guo, S.Y.; Huang, S.Q.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Yang, W.T.; Liu, Y.X.; Sang, Y.Y.; Ma, Y.Y.; Guo, M.R.; Bai, G.R.; Cheng, S.B.; Chen, G.G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 10. [Google Scholar] [CrossRef]
- Salas-Sanjuán, M.D.; Rebolloso, M.D.; del Moral, F.; Valenzuela, J.L. Use of Sub-Atmospheric Pressure Storage to Improve the Quality and Shelf-Life of Marmande Tomatoes cv. Rojito. Foods 2023, 12, 1197. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Y.; Lin, H.; Lin, M.; Ritenour, M.A.; Chen, Y.; Wang, H.; Hung, Y.-C.; Lin, Y. Comparison between ‘Fuyan’ and ‘Dongbi’ longans in aril breakdown and respiration metabolism. Postharvest Biol. Technol. 2019, 153, 176–182. [Google Scholar] [CrossRef]
- Wang, H.F.; Shao, X.F. Fruit and Vegetable Storage and Processing Experimental Guidance; Science Press: Beijing, China, 2012. [Google Scholar]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Postharvest Physiological and Biochemical Experimental Guidance for Fruits and Vegetables; China Light Industry Press: Beijing, China, 2013. [Google Scholar]
- Ge, Y.; Duan, B.; Li, C.; Wei, M.; Chen, Y.; Li, X.; Tang, Q. Application of sodium silicate retards apple softening by suppressing the activity of enzymes related to cell wall degradation. J. Sci. Food Agric. 2019, 99, 1828–1833. [Google Scholar] [CrossRef]
- Sisi, Q.; Xia, G.; Yuling, M.; Xiaoping, Z.; Fangdi, H.; Yingdong, L. Research on Integrated Technology of Origin Processing and Processing Based on Fresh Chinese Herbal Codonopsis pilosula. Chin. J. Inf. Tradit. Chin. Med. 2017, 24, 6. [Google Scholar]
- Wang, Y.; Wang, Z.; Zhang, J.; Yu, H.; Chen, Y.; Gao, Y.; Li, X.; Li, W.; Hu, F. Evaluation of the Quality of Codonopsis Radix in Different Growth Years by the AHP-CRITIC Method. Chem. Biodivers. 2023, 20, e202201108. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ying, X.; Zhang, Q.; Xu, Y.; Jiang, C.; Shang, J.; Zang, Z.; Wan, F.; Huang, X. Evaluation of the Effect of Ultrasonic Pretreatment on the Drying Kinetics and Quality Characteristics of Codonopsis pilosula Slices Based on the Grey Correlation Method. Molecules 2023, 28, 5596. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.L.I.; Tong, L.I.; Wei, L.I.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Min, T.; Lu, K.Y.; Chen, J.H.; Niu, L.F.; Lin, Q.; Yi, Y.; Hou, W.F.; Ai, Y.W.; Wang, H.X. Biochemical Mechanism of Fresh-Cut Lotus (Nelumbo nucifera Gaertn.) Root with Exogenous Melatonin Treatment by Multiomics Analysis. Foods 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Jin, P.C.; Fu, J.; Du, W.W.; Li, H.; Cui, G.F. Effects of 1-MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures. Horticulturae 2022, 8, 954. [Google Scholar] [CrossRef]
- Lv, J.Y.; Bai, L.; Han, X.Z.; Xu, D.L.; Ding, S.Y.; Li, C.Y.; Ge, Y.H.; Li, J.R. Effects of 1-MCP treatment on sprouting and preservation of ginger rhizomes during storage at room temperature. Food Chem. 2021, 349, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Tong, X.; Tan, Z. Syringin protects against cerebral ischemia/reperfusion injury via inhibiting neuroinflammation and TLR4 signaling. Perfusion 2021, 37, 562–569. [Google Scholar] [CrossRef]
- Wang, S.-S.; Zhang, T.; Wang, L.; Dong, S.; Wang, D.-H.; Li, B.; Cao, X.-Y. The Dynamic Changes in the Main Substances in Codonopsis pilosula Root Provide Insights into the Carbon Flux between Primary and Secondary Metabolism during Different Growth Stages. Metabolites 2023, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Sun, Y.; Cao, L.; Chen, L.; Chen, J.; Cheng, X.; Wang, C. Antifatigue and antihypoxia activities of oligosaccharides and polysaccharides from Codonopsis pilosula in mice. Food Funct. 2020, 11, 6352–6362. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Xie, J.; Zheng, M.L.; Yi, Y.; Hou, W.F.; Wang, L.M.; Ai, Y.W.; Wang, H.X. The effect of different temperatures on browning incidence and phenol compound metabolism in fresh-cut lotus (Nelumbo nucifera G.) root. Postharvest Biol. Technol. 2017, 123, 69–76. [Google Scholar] [CrossRef]
- Sathya, R.; Rasane, P.; Singh, J.; Kaur, S.; Bakshi, M.; Gunjal, M.; Kaur, J.; Sharma, K.; Sachan, S.; Singh, A.; et al. Strategic Advances in the Management of Browning in Fruits and Vegetables. Food Bioprocess Technol. 2023, 26. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.Y.; Wang, X.Q.; Liu, X.X.; Zhu, X.L.; Zhang, J. Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Effect of postharvest oxalic acid application on enzymatic browning and quality of lotus (Nelumbo nucifera Gaertn.) root slices. Food Chem. 2020, 312, 126051. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Chen, X.F.; Malterud, K.E.; Rise, F.; Barsett, H.; Inngjerdingen, K.T.; Michaelsen, T.E.; Paulsen, B.S. Structural features and complement fixing activity of polysaccharides from Codonopsis pilosula Nannf var. modesta L.T.Shen roots. Carbohydr. Polym. 2014, 113, 420–429. [Google Scholar] [CrossRef]
- Shi, Y.N.; Li, B.J.; Grierson, D.; Chen, K.S. Insights into cell wall changes during fruit softening from transgenic and naturally occurring mutants. Plant Physiol. 2023, 192, 1671–1683. [Google Scholar] [CrossRef]
- Li, X.W.; Cao, S.F.; Zheng, Y.H.; Sun, A.P. 1-MCP suppresses ethylene biosynthesis and delays softening of ‘Hami’ melon during storage at ambient temperature. J. Sci. Food Agric. 2011, 91, 2684–2688. [Google Scholar] [CrossRef]
- Chen, Z.S.Z.; He, M.Y.; Zhou, Y.J.; Chen, X.; Zhu, H.; Yang, B.; Jiang, Y.M.; Qu, H.X. Degradation of water-soluble polysaccharides in pulp of litchi during storage. Food Chem. 2023, 402, 10. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zahid, N.; Nawaz, A.; Naz, S.; Ejaz, S.; Ullah, S.; Siddiq, B. Tragacanth gum coating suppresses the disassembly of cell wall polysaccharides and delays softening of harvested mango (Mangifera indica L.) fruit. Int. J. Biol. Macromol. 2022, 222, 521–532. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Wang, Y.; Song, P.; Wang, Z.; Tang, Z.; Guo, Y.; Yu, H.; Hu, F. Exploration of Postharvest Conditions for Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen Roots Based on Sensory Quality, Active Components, Antioxidant Capacity and Physiological Changes at Different Storage Temperatures. Foods 2023, 12, 4418. https://doi.org/10.3390/foods12244418
Wen L, Wang Y, Song P, Wang Z, Tang Z, Guo Y, Yu H, Hu F. Exploration of Postharvest Conditions for Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen Roots Based on Sensory Quality, Active Components, Antioxidant Capacity and Physiological Changes at Different Storage Temperatures. Foods. 2023; 12(24):4418. https://doi.org/10.3390/foods12244418
Chicago/Turabian StyleWen, Longxia, Yanping Wang, Pingping Song, Zixia Wang, Zhuoshi Tang, Yina Guo, Huaqiao Yu, and Fangdi Hu. 2023. "Exploration of Postharvest Conditions for Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen Roots Based on Sensory Quality, Active Components, Antioxidant Capacity and Physiological Changes at Different Storage Temperatures" Foods 12, no. 24: 4418. https://doi.org/10.3390/foods12244418
APA StyleWen, L., Wang, Y., Song, P., Wang, Z., Tang, Z., Guo, Y., Yu, H., & Hu, F. (2023). Exploration of Postharvest Conditions for Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen Roots Based on Sensory Quality, Active Components, Antioxidant Capacity and Physiological Changes at Different Storage Temperatures. Foods, 12(24), 4418. https://doi.org/10.3390/foods12244418





