Effects of Process Variables on the Physicochemical, Textural, and Structural Properties of an Isolated Pea Protein-Based High-Moisture Meat Analog
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
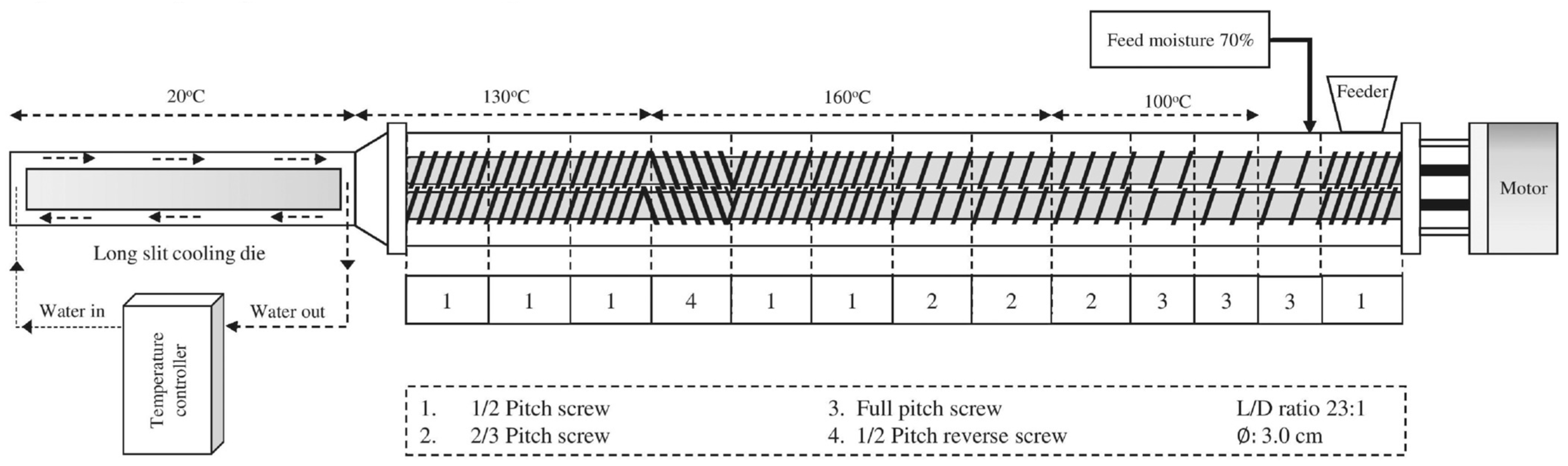
2.2. Extrusion Process
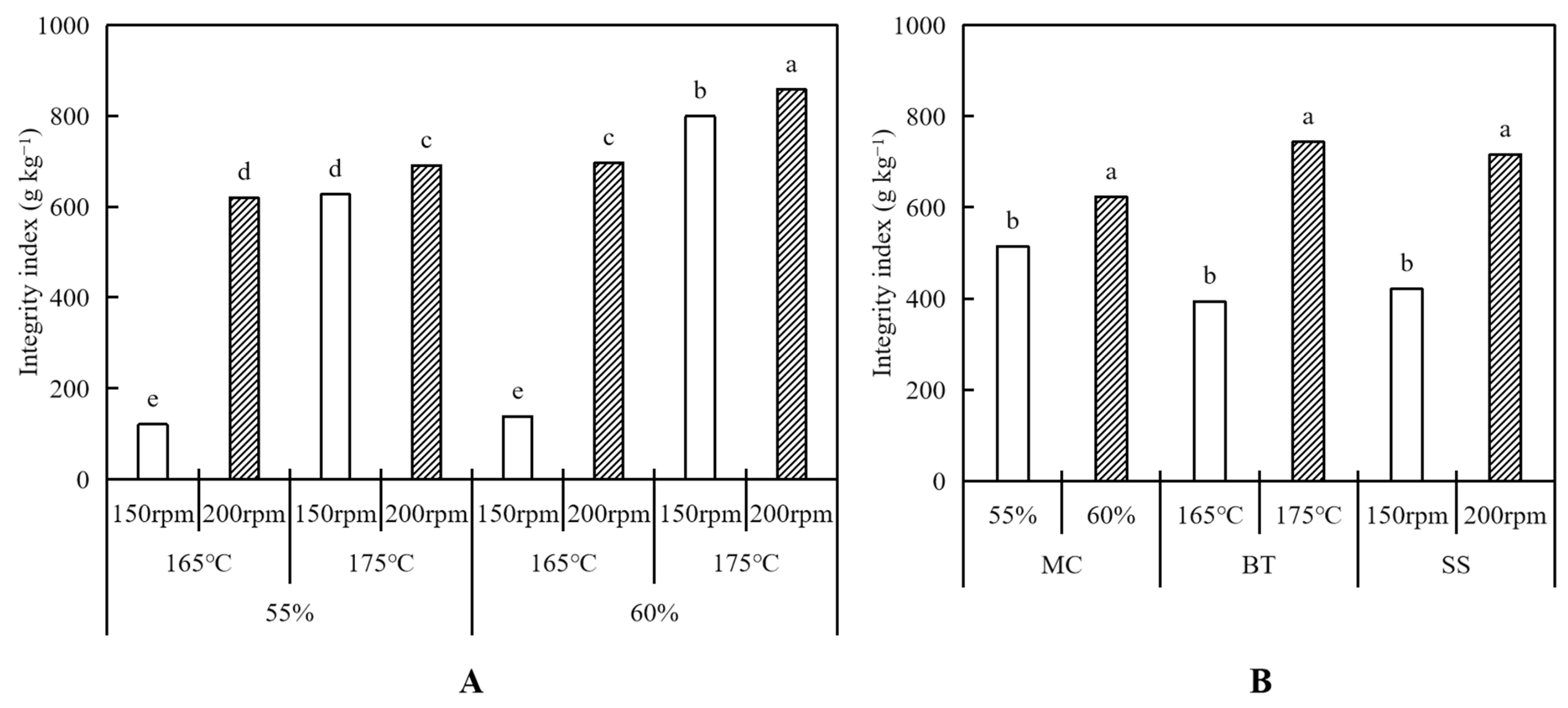
2.3. Integrity Index
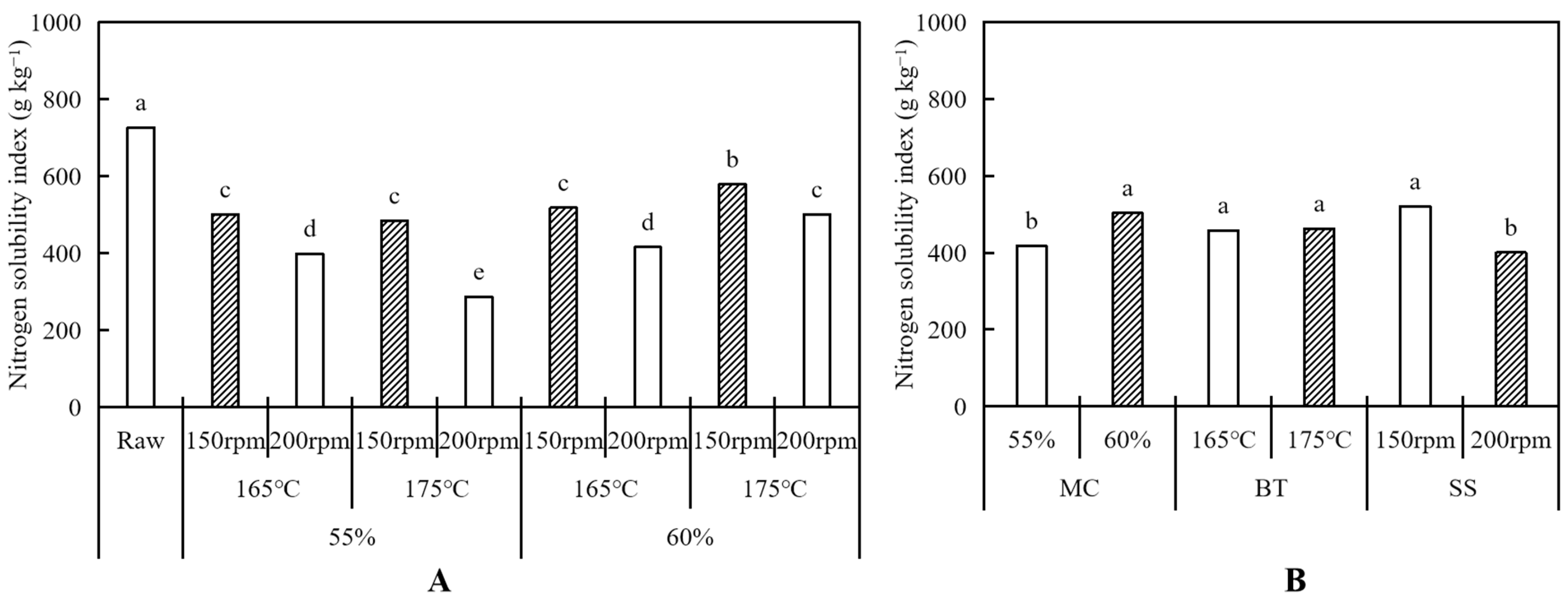
2.4. Nitrogen Solubility Index (NSI)
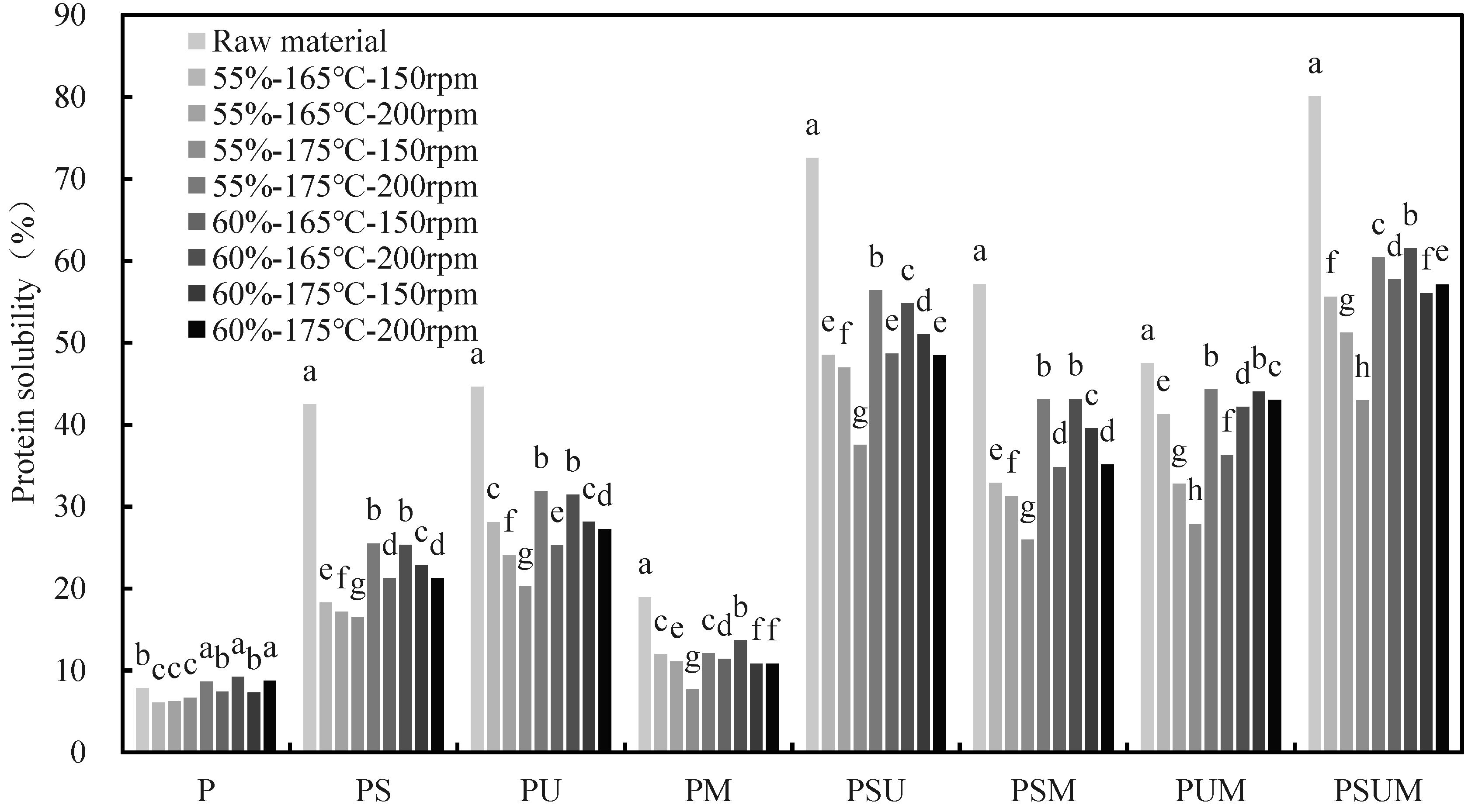
2.5. Protein Solubility
2.6. Texture Profile Analysis (TPA), Cutting Strength, and Texturization Degree
2.7. Scanning Electron Microscopy (SEM)
2.8. Fourier Transform Infrared Spectroscopy (FT-IR)
2.9. Experimental Design
3. Results and Discussion
3.1. Physicochemical Properties
3.1.1. Integrity Index
3.1.2. Nitrogen Solubility Index
3.1.3. Protein Solubility
3.2. Textural Profile Analysis, Cutting Strength, and Texturization Degree
3.3. Structural Properties
3.3.1. Macro- and Microstructure
3.3.2. Secondary Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismail, I.; Hwang, Y.; Joo, S. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111. [Google Scholar] [CrossRef]
- Malav, O.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat analog: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of ingredients and additives in plant-based meat analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.H. Physicochemical and functional characteristics of plant protein-based meat analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Park, J.-H.; Chatpaisarn, A.; Ryu, G.-H. Effects of gluten and moisture contents on texturization of extruded soy protein isolate. J. Korean Soc. Food Sci. Nutr. 2017, 46, 473–480. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ryu, G. Effects of green tea contents on the quality and antioxidant properties of textured vegetable protein by extrusion-cooking. Food Sci. Biotechnol. 2019, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Arêas, J.A. Extrusion of food proteins. Crit. Rev. Food Sci. Nutr. 1992, 32, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, A.S.; Singh, J.; Kaur, L.; Hardacre, A.; Singh, H. Meat analogs: Protein restructuring during thermomechanical processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1221–1249. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-moisture meat analogues produced from yellow pea and faba bean protein isolates/concentrate: Effect of raw material composition and extrusion parameters on texture properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Emin, M.A.; Boom, R.M.; van der Goot, A.J. The phase properties of soy protein and wheat gluten in a blend for fibrous structure formation. Food Hydrocoll. 2018, 79, 273–281. [Google Scholar] [CrossRef]
- Maung, T.-T.; Gu, B.-Y.; Ryu, G.-H. Influence of extrusion process parameters on specific mechanical energy and physical properties of high-moisture meat analog. Int. J. Food Eng. 2021, 17, 149–157. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, R.; Huo, J.; Wang, H.; Li, H.; Su, S.; Gao, Y.; Duan, Y. Rice bran meat analogs: Relationship between extrusion parameters, apparent properties and secondary structures. LWT 2022, 163, 113535. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.; Hsieh, F. Extrusion process parameters, sensory characteristics, and structural properties of a high moisture soy protein meat analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Emin, M.; Teumer, T.; Schmitt, W.; Rädle, M.; Schuchmann, H. Measurement of the true melt temperature in a twin-screw extrusion processing of starch based matrices via infrared sensor. J. Food Eng. 2016, 170, 119–124. [Google Scholar] [CrossRef]
- Thiébaud, M.; Dumay, E.; Cheftel, J.C. Influence of process variables on the characteristics of a high moisture fish soy protein mix texturized by extrusion cooking. LWT-Food Sci. Technol. 1996, 29, 526–535. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Cho, S.Y.; Ryu, G.H. Effects of mealworm larva composition and selected process parameters on the physicochemical properties of extruded meat analog. Food Sci. Nutr. 2021, 9, 4408–4419. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein–protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryu, G.H. Effects of pea protein content and extrusion types on physicochemical properties and texture characteristic of meat analogs. JSFA Rep. 2023, 3, 30–40. [Google Scholar] [CrossRef]
- Trinh, K.T.; Glasgow, S. On the texture profile analysis test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012; pp. 23–26. [Google Scholar]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolym. Orig. Res. Biomol. 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Bhirud, P.; Tyler, R. Extrusion texturization of air-classified pea protein. J. Food Sci. 1999, 64, 509–513. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and rheological changes of texturized mung bean protein induced by feed moisture during extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; van den Berg, F.W.; Zhang, W.; Czaja, T.P.; Zhang, L.; Jespersen, B.M.; Lametsch, R. Differences in physicochemical properties of high-moisture extrudates prepared from soy and pea protein isolates. Food Hydrocoll. 2022, 128, 107540. [Google Scholar] [CrossRef]
- Sengar, A.S.; Beyrer, M.; McDonagh, C.; Tiwari, U.; Pathania, S. Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives. Foods 2023, 12, 3830. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, D.; Kang, L.; Zhang, B. Effects of Process Parameters on Products Characteristics of Textural Soy Protein. Chin. J. Grain Oil 2009, 24, 20–25. [Google Scholar]
- Zhang, J.; Ying, D.; Wei, Y.; Zhang, B.; Su, X.; Li, S. Thermal transition and decomposition properties of pH-and phosphate-induced defatted soybean meals. J. Therm. Anal. Calorim. 2017, 128, 699–706. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.; Hsieh, F. Texture and chemical characteristics of soy protein meat analog extruded at high moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Li, M.; Lee, T.-C. Effect of cysteine on the functional properties and microstructures of wheat flour extrudates. J. Agric. Food Chem. 1996, 44, 1871–1880. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Qi, P.X.; Onwulata, C.I. Physical properties, molecular structures, and protein quality of texturized whey protein isolate: Effect of extrusion temperature. J. Agric. Food Chem. 2011, 59, 4668–4675. [Google Scholar] [CrossRef] [PubMed]
- Afizah, M.N.; Rizvi, S.S. Functional properties of whey protein concentrate texturized at acidic pH: Effect of extrusion temperature. LWT-Food Sci. Technol. 2014, 57, 290–298. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Ren, H.; Zhao, Y.; Wang, Z.; Wu, F.; Xiao, Z. Structural changes in rice bran protein upon different extrusion temperatures: A Raman spectroscopy study. J. Chem. 2016, 2016, 6898715. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Tian, Z.; Zeng, H.; Temelli, F.; Chen, L. Impact of pH on molecular structure and surface properties of lentil legumin-like protein and its application as foam stabilizer. Colloids Surf. B Biointerfaces 2015, 132, 45–53. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, K.-X.; Guo, X.-N.; Zhou, H.-M. Egg white protein addition induces protein aggregation and fibrous structure formation of textured wheat gluten. Food Chem. 2022, 371, 131102. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate’s expansion, solubility, molecular weight distribution and secondary structure as determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
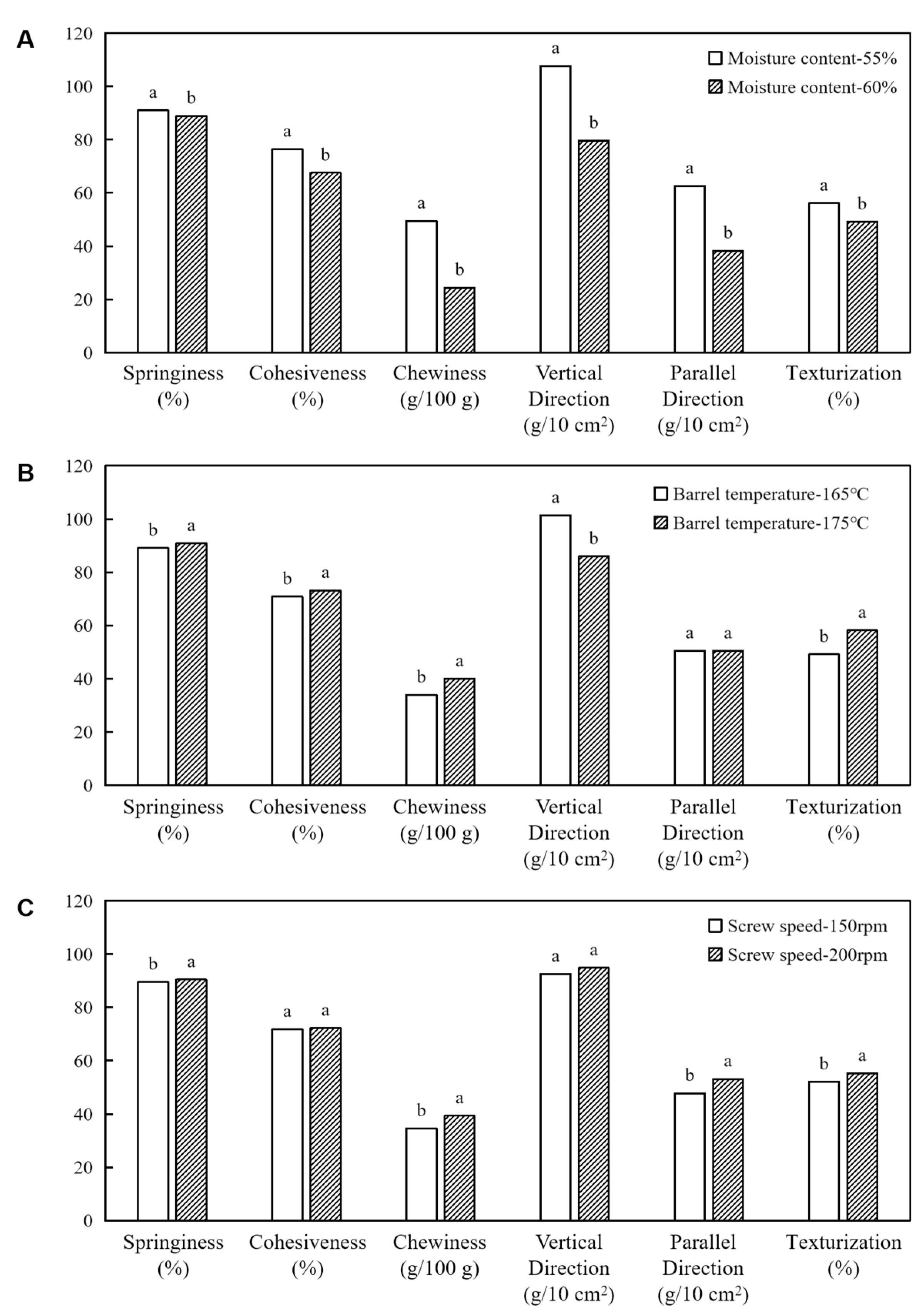
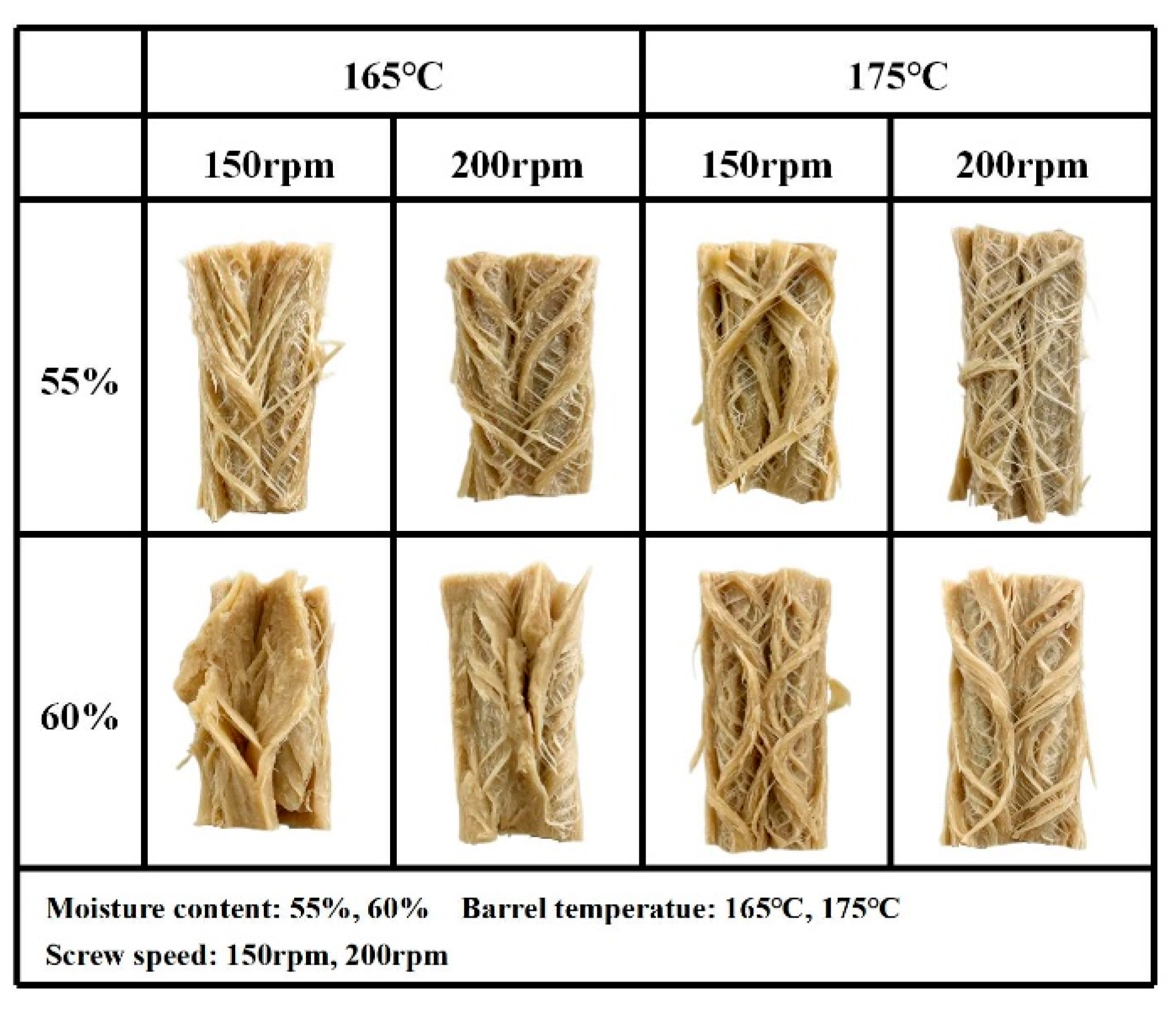
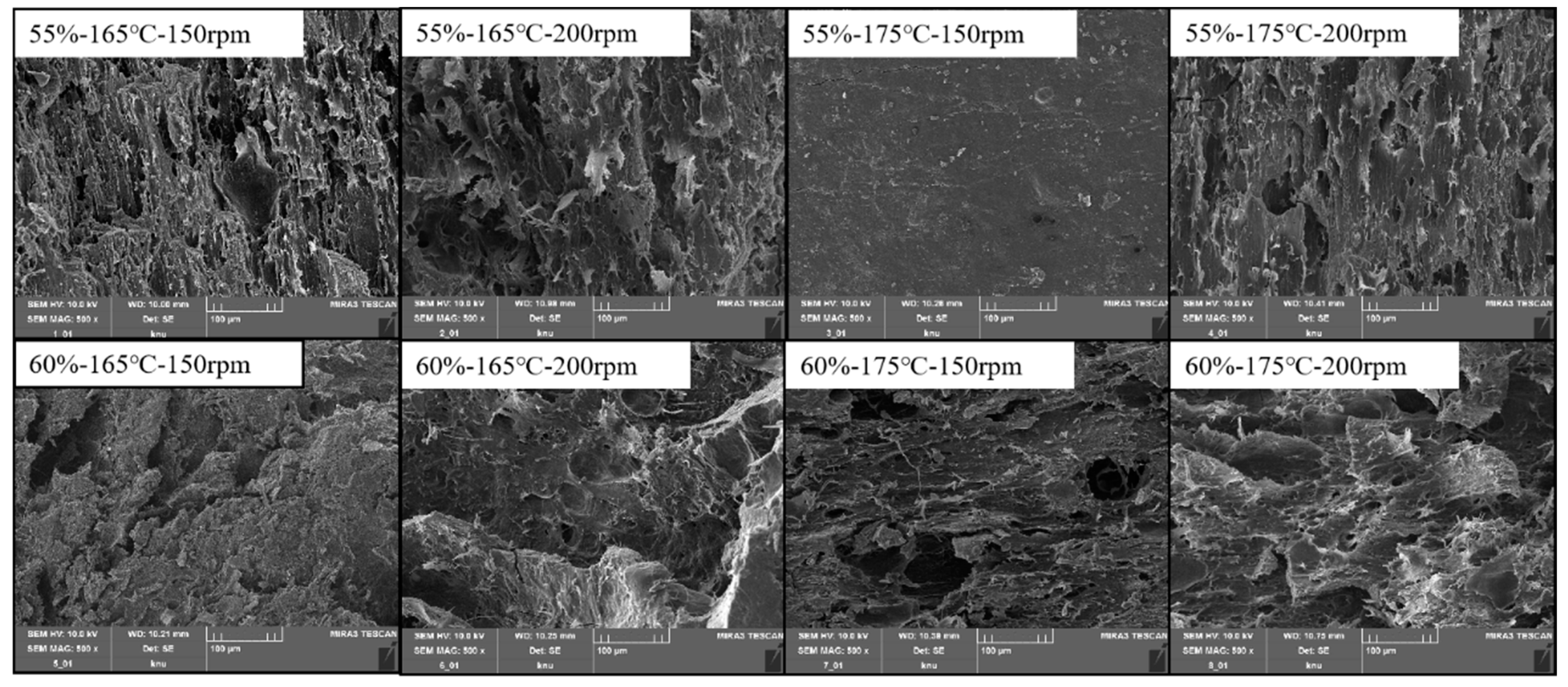
| Integrity Index (g kg−1) | Nitrogen Solubility Index (g kg−1) | Texture Profile Analysis | Cutting Strength (g cm−2) | ||||
|---|---|---|---|---|---|---|---|
| Springiness (%) | Cohesiveness (%) | Chewiness (kg) | Vertical Direction | Parallel Direction | |||
| Moisture content (A) | 83.55 *** | 34.515 *** | 3.18 × 10 *** | 8.90 × 10 *** | 6.76 × 102 *** | 9.91 × 10 *** | 3.53 × 102 *** |
| Barrel temperature (B) | 871.893 *** | 0.093 | 2.30 × 10 *** | 6.27 * | 3.92 × 10 *** | 3.00 × 10 *** | 0.00 |
| Screw speed (C) | 620.353 *** | 67.659 *** | 5.81 * | 3.23 × 10−1 | 2.38 × 10 *** | 6.60 × 10−1 | 1.71 × 10 *** |
| A × B | 26.867 *** | 21.954 *** | 6.29 ** | 1.59 × 10 *** | 2.42 × 10 *** | 9.70 × 10−2 | 7.40 ** |
| A × C | 1.281 | 4.087 | 8.33 × 10−1 | 2.13 | 5.66 × 10 *** | 1.45 × 10 *** | 1.81 × 10 *** |
| B × C | 389.471 *** | 1.495 | 4.17 × 10−1 | 5.16 * | 9.59 ** | 1.32 × 10 ** | 9.79 ** |
| Process Variables | TPA | Cutting Strength (g cm −2) | ||||||
|---|---|---|---|---|---|---|---|---|
| MC (%) | BT (°C) | SS (rpm) | Springiness (%) | Cohesiveness (%) | Chewiness (g) | Vertical Direction | Parallel Direction | Texturization (%) |
| 55 | 165 | 150 | 90.44 ± 0.85 ab | 77.25 ± 2.41 a | 4.516.37 ± 545.34 c | 1122.13 ± 134.75 a | 629.69 ± 47.62 b | 56.16 ± 3.38 bc |
| 200 | 90.74 ± 1.12 ab | 77.11 ± 2.99 a | 5251.18 ± 482.39 b | 1177.48 ± 95.38 a | 657.70 ± 48.35 ab | 55.86 ± 1.51 bc | ||
| 175 | 150 | 91.03 ± 0.53 ab | 73.78 ± 1.79 ab | 4184.50 ± 462.09 c | 901.79 ± 147.07 b | 513.80 ± 56.14 c | 56.98 ± 3.73 b | |
| 200 | 91.82 ± 0.80 a | 77.76 ± 1.58 a | 5841.75 ± 525.09 a | 1106.54 ± 156.73 a | 703.00 ± 97.47 a | 63.53 ± 2.45 a | ||
| 60 | 165 | 150 | 87.12 ± 2.04 d | 66.02 ± 5.24 c | 2097.40 ± 171.94 e | 985.28 ± 123.42 b | 366.42 ± 22.40 d | 37.19 ± 3.07 e |
| 200 | 88.11 ± 1.59 cd | 62.94 ± 3.12 c | 1705.61 ± 116.58 f | 770.91 ± 75.63 c | 364.60 ± 23.42 d | 47.29 ± 2.20 d | ||
| 175 | 150 | 89.56 ± 2.37 c | 69.93 ± 4.38 b | 3039.21 ± 235.91 d | 692.00 ± 62.68 c | 401.15 ± 40.12 d | 57.97 ± 2.10 b | |
| 200 | 91.01 ± 1.37 ab | 71.32 ± 6.14 b | 2920.11 ± 270.18 d | 737.83 ± 57.06 c | 400.50 ± 38.92 d | 54.22 ± 2.27 c | ||
| Process Variables | β-Sheet (%) | Random Coil (%) | α-Helix (%) | β-Turn (%) | ||
|---|---|---|---|---|---|---|
| MC (%) | BT (°C) | SS (rpm) | ||||
| Raw material | 49.64 ± 0.10 a | 19.16 ± 0.11 b | 14.77 ± 0.20 d | 15.77 ± 0.17 bc | ||
| 55 | 165 | 150 | 45.67 ± 0.32b cd | 22.53 ± 0.20 a | 17.69 ± 0.11 ab | 14.32 ± 0.15 d |
| 200 | 47.57 ± 0.22 b | 21.44 ± 0.08 a | 16.24 ± 0.09 c | 16.66 ± 0.12 b | ||
| 175 | 150 | 45.10 ± 0.33 cd | 22.85 ± 0.21 a | 17.90 ± 0.15 ab | 14.35 ± 0.15 d | |
| 200 | 45.04 ± 0.32 cd | 22.84 ± 0.20 a | 17.82 ± 0.10 ab | 14.51 ± 0.21 d | ||
| 60 | 165 | 150 | 37.24 ± 0.08 e | 21.12 ± 0.18 a | 18.63 ± 0.21 a | 23.54 ± 0.76 a |
| 200 | 46.44 ± 2.27 bc | 21.45 ± 1.82 a | 17.08 ± 1047 bc | 15.22 ± 0.85 cd | ||
| 175 | 150 | 46.56 ± 2.26 bc | 21.41 ± 1.85 a | 17.42 ± 0.88 abc | 15.15 ± 0.87 cd | |
| 200 | 44.23 ± 1.21 d | 22.47 ± 1.53 a | 17.76 ± 1.31 ab | 12.74 ± 1.17 bc | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ryu, G.H. Effects of Process Variables on the Physicochemical, Textural, and Structural Properties of an Isolated Pea Protein-Based High-Moisture Meat Analog. Foods 2023, 12, 4413. https://doi.org/10.3390/foods12244413
Zhang Y, Ryu GH. Effects of Process Variables on the Physicochemical, Textural, and Structural Properties of an Isolated Pea Protein-Based High-Moisture Meat Analog. Foods. 2023; 12(24):4413. https://doi.org/10.3390/foods12244413
Chicago/Turabian StyleZhang, Yu, and Gi Hyung Ryu. 2023. "Effects of Process Variables on the Physicochemical, Textural, and Structural Properties of an Isolated Pea Protein-Based High-Moisture Meat Analog" Foods 12, no. 24: 4413. https://doi.org/10.3390/foods12244413
APA StyleZhang, Y., & Ryu, G. H. (2023). Effects of Process Variables on the Physicochemical, Textural, and Structural Properties of an Isolated Pea Protein-Based High-Moisture Meat Analog. Foods, 12(24), 4413. https://doi.org/10.3390/foods12244413




