Optimizing Processing Techniques of Oolong Tea Balancing between High Retention of Catechins and Sensory Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Experimental Instruments
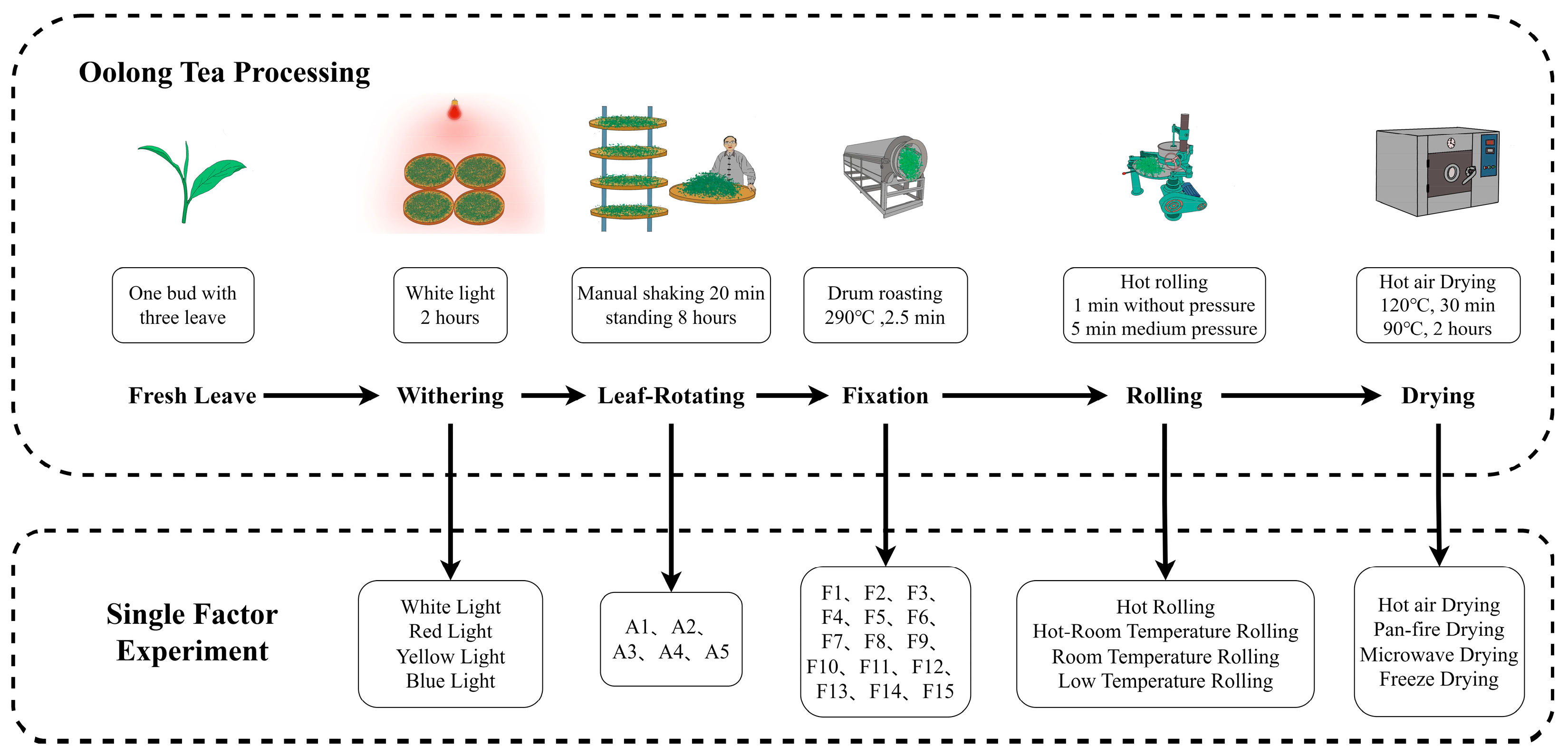
2.2. Experimental Materials and Oolong Tea Processing
2.3. Single-Factor Experiment
2.4. Effects of Different Comprehensive Processing Techniques on the Retention of Catechins
2.5. Detection of Catechin Content
2.6. Statistical Analysis
2.7. Sensory Evaluation
3. Results
3.1. Changes of Catechins in Different Withering Methods
3.2. Changes of Catechins in Different Leaf Rotating Methods
3.3. Changes of Catechins in Different Fixing Methods
3.4. Changes of Catechins under Different Rolling Methods
3.5. Changes of Catechins under Different Drying Methods
3.6. Changes of Catechins under the Combination of Comprehensive Processing Methods
3.7. Sensory Evaluation of Oolong Tea under the Combination of Comprehensive Processing Methods
4. Discussion
4.1. Degradation of EGCG Led to the Change in Catechin Components in the Enzymatic Oxidation Stage
4.2. Thermalization Affects Catechin Components in the Non-Enzymatic Stage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.L.; Duan, J.; Yang, S.Y.; Yang, E.; Jiang, Y.M. Effect of Girdling on Levels of Catechins in Fresh Leaf in Relation to Quality of ‘Huang Zhi Xiang’ Oolong Tea. Plant Foods Hum. Nutr. 2009, 64, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Lin, J.; Sun, Y.; Wei, S.; Wu, L. The Impact of Different Withering Approaches on the Metabolism of Flavor Compounds in Oolong Tea Leaves. Foods 2022, 11, 3601. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.T.; Fu, H.F.; Zhou, C.Z.; Chen, L.; Li, X.Z.; Lin, Y.L.; Lai, Z.X.; Guo, Y.Q. Transcriptome and Phytochemical Analyses Provide New Insights Into Long Non-Coding RNAs Modulating Characteristic Secondary Metabolites of Oolong Tea (Camellia sinensis) in Solar-Withering. Front. Plant Sci. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Shi, J.; Yang, G.Z.; You, Q.S.; Sun, S.L.; Chen, R.H.; Lin, Z.; Simal-Gandara, J.; Lv, H.P. Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001–2021). Crit. Rev. Food Sci. Nutr. 2023, 63, 4757–4784. [Google Scholar] [CrossRef]
- Mo, X.L.; Cheng, C.; Zeng, W.; Huang, Y.H. Changes in the Major Chemical Constituents of Oolong Tea under Continuous Low-speed Green-making Process. Sci. Technol. Food Ind. 2022, 43, 67–77. [Google Scholar] [CrossRef]
- Zhang, N.; Jing, T.T.; Zhao, M.Y.; Jin, J.Y.; Xu, M.J.; Chen, Y.X.; Zhang, S.R.; Wan, X.C.; Schwab, W.; Song, C.K. Untargeted metabolomics coupled with chemometrics analysis reveals potential non-volatile markers during oolong tea shaking. Food Res. Int. 2019, 123, 125–134. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lo, L.C.; Chen, I.Z.; Chen, P.A. Effect of shaking process on correlations between catechins and volatiles in oolong tea. J. Food Drug Anal. 2016, 24, 500–507. [Google Scholar] [CrossRef]
- Salman, S.; Oz, G.; Felek, R.; Haznedar, A.; Turna, T.; Ozdemir, F. Effects of fermentation time on phenolic composition, antioxidant and antimicrobial activities of green, oolong, and black teas. Food Biosci. 2022, 49, 101884. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Liu, P.P.; Shi, J.; Gao, Y.; Wang, Q.S.; Yin, J.F. Quality development and main chemical components of Tieguanyin oolong teas processed from different parts of fresh shoots. Food Chem. 2018, 249, 176–183. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Q.Q.; Chen, Y.H.; Granato, D.; Wang, J.Q.; Yin, J.F.; Zhang, X.B.; Wang, F.; Chen, J.X.; Xu, Y.Q. Effects of the Baking Process on the Chemical Composition, Sensory Quality, and Bioactivity of Tieguanyin Oolong Tea. Front. Nutr. 2022, 9, 881865. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Yue, W.J.; Shen, Q.Y.; Wang, W.H.; Meng, H.Y.; Guo, Y.; Xu, W.J.; Guo, Y.L. Widely Targeted Metabolomics Analysis Reveals Great Changes in Nonvolatile Metabolites of Oolong Teas during Long-Term Storage. Molecules 2021, 26, 7278. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, H.W.; Lee, S.H.; Kim, Y.J.; Asamenew, G.; Choi, J.; Lee, J.W.; Jung, H.A.; Yoo, S.M.; Kim, J.B. Characterization of catechins, theaflavins, and flavonols by leaf processing step in green and black teas (Camellia sinensis) using UPLC-DAD-QToF/MS. Eur. Food Res. Technol. 2019, 245, 997–1010. [Google Scholar] [CrossRef]
- Qu, F.F.; Zhu, X.J.; Ai, Z.Y.; Ai, Y.J.; Qiu, F.F.; Ni, D.J. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT-Food Sci. Technol. 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Ntezimana, B.; Li, Y.; He, C.; Yu, X.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea. Foods 2021, 10, 2627. [Google Scholar] [CrossRef]
- Wang, R.X.; Huang, X.X.; Li, Q.; Li, J.; Tan, J.; Zhu, M.Z.; Wang, K.B. Comparison on catechins, aroma components and sensory quality of different types of white tea. Sci. Technol. Food Ind. 2022, 43, 315–321. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, J.; Mu, B.; Chen, Z.; Dai, W.; Lin, Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chem. 2020, 332, 127412. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Zhu, C.; Li, X.Z.; Chen, L.; Xie, S.Y.; Chen, G.W.; Zhang, H.; Lai, Z.X.; Lin, Y.L.; Guo, Y.Q. Transcriptome and phytochemical analyses reveal the roles of characteristic metabolites in the taste formation of white tea during the withering process. J. Integr. Agric. 2022, 21, 862–877. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT-Food Sci. Technol. 2019, 116, 108567. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, F.; Qin, L.; Zhang, N.; Cao, Q.; Schwab, W.; Li, D.X.; Song, C.K. Dynamic change in amino acids, catechins, alkaloids, and gallic acid in six types of tea processed from the same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 2019, 77, 28–38. [Google Scholar] [CrossRef]
- Shi, Y.; Liao, S.; Liu, J.; Ren, D.; Zou, Y. Effect of Processing Technique on Quality and Flavor of Mulberry Leaf Oolong-tea. Acta Sericologica Sin. 2015, 41, 525–533. [Google Scholar]
- You, F.; Chen, S.; Zhou, Z.; Li, X.; Hao, Z.; Sun, Y. Effect of Flavor Components on Different Illumination Time During Withering Process in Tieguanyin Tea. Chin. J. Trop. Crops 2018, 39, 1856–1862. [Google Scholar]
- Lin, Y.; Liu, J.; Dai, Q.; Li, Z.; Lin, J. Withering Technology of Different Light Quality to Increase Catechin Content in Fresh Tea Leaves. Chin. J. Trop. Crop 2021, 42, 3605–3616. [Google Scholar]
- Yuan, H.; Deng, Y.; Chen, G.; Xu, Y.; Wang, F.; Liu, P.; Zhong, X.; Yin, J. Effect of Raw Material Processing by Different Fixation Technology on the Stability with Quality of Tea Beverage. J. Tea Sci. 2012, 32, 236–246. [Google Scholar]
- Niu, G.J.; Zhu, S.; Wei, Q.Z.; Zeng, J.W.; Wei, Y.C.; Liang, Y.C. Influence of Steam Killenzyme Torrefaction on Quality of Camellia nitidissima Based on Multi-index Evaluation. J. Chin. Med. Mater. 2015, 38, 948–951. [Google Scholar]
- YUAN, H.B.; YIN, J.F.; DENG, Y.L.; XU, Y.Q.; WANG, F.; CHEN, G.S. Effect of Raw Tea Processing with Different Rolling Technology on the Quality of Green Tea Beverage. J. Tea Sci. 2014, 34, 29–35. [Google Scholar] [CrossRef]
- Gui, A.H.; Gao, S.W.; Zheng, P.C.; Feng, Z.H.; Liu, P.P.; Ye, F.; Wang, S.P.; Xue, J.J.; Xiang, J.; Ni, D.J.; et al. Dynamic Changes in Non-Volatile Components during Steamed Green Tea Manufacturing Based on Widely Targeted Metabolomic Analysis. Foods 2023, 12, 1551. [Google Scholar] [CrossRef]
- Loh, Z.H.; Lim, Y.Y. Drying effects on antioxidant activity, enzyme activity, and phytochemicals of avocado (Persea americana) leaves. J. Food Process. Preserv. 2018, 42, 13667. [Google Scholar] [CrossRef]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT-Food Sci. Technol. 2022, 161, 113394. [Google Scholar] [CrossRef]
- Lin, J.K.; Wilson, I.W.; Ge, G.P.; Sun, G.L.; Xie, F.L.; Yang, Y.F.; Wu, L.Y.; Zhang, B.H.; Wu, J.Q.; Zhang, Y.; et al. Whole transcriptome analysis of three leaf stages in two cultivars and one of their F-1 hybrid of Camellia sinensis L. with differing EGCG content. Tree Genet. Genomes 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Shi, Z.P.; Liu, Z.H. Probe into Mathematical Model of Chemical Essence of Bitterness and Astringency in Summer Green Tea. J. Tea Sci. 1987, 2, 7–12. [Google Scholar]
- Shi, Y.T.; Chen, J.L.; Xiao, X.D.; Chen, C.M.; Ni, D.J.; Yu, Z. Effect of Processing Technology on the components of Catechin in Fragrant Tea. Food Sci. Technol. 2020, 45, 70–76. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standardization Administration of the People’s Republic of China: Beijing, China, 2018; pp. 1–20.
- Xu, K.; Tian, C.Y.; Zhou, C.Z.; Zhu, C.; Weng, J.J.; Sun, Y.; Lin, Y.L.; Lai, Z.X.; Guo, Y.Q. Non-Targeted Metabolomics Analysis Revealed the Characteristic Non-Volatile and Volatile Metabolites in the Rougui Wuyi Rock Tea (Camellia sinensis) from Different Culturing Regions. Foods 2022, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Yanase, E.; Sawaki, K.; Nakatsuka, S. The isolation of a bicyclo 3.2.1 intermediate during formation of benzotropolones, a common nucleus found in black tea pigments: Theaflavins. Synlett 2005, 2005, 2661–2663. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Ikai, H.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Photo-Irradiation of Proanthocyanidin as a New Disinfection Technique via Reactive Oxygen Species Formation. PLoS ONE 2013, 8, e60053. [Google Scholar] [CrossRef]
- Neilson, A.P.; Ferruzzi, M.G. Influence of Formulation and Processing on Absorption and Metabolism of Flavan-3-Ols from Tea and Cocoa. Annu. Rev. Food Sci. Technol. 2011, 2, 125–151. [Google Scholar] [CrossRef]
- Liu, Z.B.; Chen, F.C.; Sun, J.Y.; Ni, L. Dynamic changes of volatile and phenolic components during the whole manufacturing process of Wuyi Rock tea (Rougui). Food Chem. 2022, 367, 130624. [Google Scholar] [CrossRef]
- Li, Y.C.; He, C.; Yu, X.L.; Zhou, J.T.; Ran, W.; Chen, Y.Q.; Ni, D.J. Effects of red-light withering on the taste of black tea as revealed by non-targeted metabolomics and transcriptomics analysis. LWT-Food Sci. Technol. 2021, 147, 111620. [Google Scholar] [CrossRef]
- Kong, X.H.; Xu, W.Q.; Zhang, K.X.; Chen, G.J.; Zeng, X.X. Effects of reaction temperature, pH and duration on conversion of tea catechins and formation of theaflavins and theasinensins. Food Biosci. 2023, 54, 102911. [Google Scholar] [CrossRef]
- Dai, X.L.; Liu, Y.J.; Zhuang, J.H.; Yao, S.B.; Liu, L.; Jiang, X.L.; Zhou, K.; Wang, Y.S.; Xie, D.Y.; Bennetzen, J.L.; et al. Discovery and characterization of tannase genes in plants: Roles in hydrolysis of tannins. New Phytol. 2020, 226, 1104–1116. [Google Scholar] [CrossRef]
- Liu, P.-P.; Yin, J.-F.; Chen, G.-S.; Wang, F.; Xu, Y.-Q. Flavor characteristics and chemical compositions of oolong tea processed using different semi-fermentation times. J. Food Sci. Technol.-Mysore 2018, 55, 1185–1195. [Google Scholar] [CrossRef]
- Hua, J.J.; Wang, H.J.; Yuan, H.B.; Yin, P.; Wang, J.J.; Guo, G.Y.; Jiang, Y.W. New insights into the effect of fermentation temperature and duration on catechins conversion and formation of tea pigments and theasinensins in black tea. J. Sci. Food Agric. 2022, 102, 2750–2760. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Zeng, Y.T.; Zhao, X. Stability and Chemical Changes of Catechins during Oolong Tea Processing. Food Sci. 2019, 40, 69–74. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Dong, J.J.; Jin, J.; Liu, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Roasting process shaping the chemical profile of roasted green tea and the association with aroma features. Food Chem. 2021, 353, 129428. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.-H.; Liang, Y.-R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Wang, J.Q.; Chen, J.X.; Wang, F.; Yin, J.F.; Zeng, L.; Shi, J.; Xu, Y.Q. Effect of baking on the flavor stability of green tea beverages. Food Chem. 2020, 331, 127258. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Meng, Q.L.; Wang, Y.J.; Long, P.P.; Ho, C.T.; Cui, C.J.; Cao, L.T.; Li, D.X.; Wan, X.C. Roasting improves the hypoglycemic effects of a large-leaf yellow tea infusion by enhancing the levels of epimerized catechins that inhibit alpha-glucosidase. Food Funct. 2018, 9, 5162–5168. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.B.; Jiang, X.H. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.B.; Wen, R.A.H. Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J. Agric. Food Chem. 2006, 54, 5924–5932. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, H.; Lu, F.; Ma, C.; Zhu, S.; Li, G.; Huang, S.; Zhang, Y.; Lv, C.; Xiao, R. Widely targeted metabolomics and HPLC analysis elaborated the quality formation of Yunnan pickled tea during the whole process at an industrial scale. Food Chem. 2023, 422, 135716. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Ye, Y.; Yin, J.F.; Jin, J.; Liang, Y.R.; Liu, R.Y.; Tang, P.; Xu, Y.Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Turkmen, N.; Velioglu, Y.S. Determination of alkaloids and phenolic compounds in black tea processed by two different methods in different plucking seasons. J. Sci. Food Agric. 2007, 87, 1408–1416. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, X.Y.; Liu, W.; Lin, H.Z.; Huang, Y.P.; Zhou, Y.F.; Wang, J. Physicochemical and Aroma Quality Changes of Oolong Tea in Modeling Process under Different Forces Models. Sci. Technol. Food Ind. 2018, 39, 1–10+17. [Google Scholar] [CrossRef]
- Hao, Z.L.; Cai, Y.B.; Jin, X.Y.; Chen, X.Y.; Lin, Y.P. Effect of Modeling Methods on the Quality of Southern Fujian Oolong Tea. Food Sci. 2012, 33, 105–109. [Google Scholar]
- Sun, Y.; Shen, Y.; Liu, D.; Ye, X. Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT-Food Sci. Technol. 2015, 60, 1269–1275. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, X.Y.; Jiao, Y.F.; Hao, J.; Ni, D.J.; Yu, Z.; Chen, Y.Q. Effects of different processing technology for raw tea on the quality of Qingzhuan tea. J. Food Saf. Qual. 2022, 13, 1919–1926. [Google Scholar] [CrossRef]
- Li, S.; Liu, F.W.; Wu, M.L.; Li, Y.H.; Song, X.X.; Yin, J.Y. Effects of Drying Treatments on Nutritional Compositions, Volatile Flavor Compounds, and Bioactive Substances of Broad Beans. Foods 2023, 12, 2160. [Google Scholar] [CrossRef]
- Li, F.N.; Boateng, I.D.; Yang, X.M.; Li, Y.Y.; Liu, W.M. Effects of processing methods on quality, antioxidant capacity, and cytotoxicity of Ginkgo biloba leaf tea product. J. Sci. Food Agric. 2023, 103, 4993–5003. [Google Scholar] [CrossRef]





| No. | Shaking | Standing |
|---|---|---|
| A1 | Manual shaking, 30 r/min, 10 min for total shaking time: the first shaking for 2 min; the second shaking for 3 min; the third shaking for 5 min. | 8 h for total standing time: the first standing for 1.5 h; the second standing for 2.5 h; the third standing for 4 h. |
| A2 | Manual shaking, 30 r/min, 20 min for total shaking time: the first shaking for 4 min; the second shaking for 6 min; the third shaking for 10 min. | 8 h for total standing time: the first standing for 1.5 h; the second standing for 2.5 h; the third standing for 4 h. |
| A3 | Manual shaking, 30 r/min, 30 min for total shaking time: the first shaking for 6 min; the second shaking for 10 min; the third shaking for 14 min. | 8 h for total standing time: the first standing for 1.5 h; the second standing for 2.5 h; the third standing for 4 h. |
| A4 | Manual shaking, 30 r/min, 20 min for total shaking time: the first shaking for 4 min; the second shaking for 6 min; the third shaking for 10 min. | 6 h for total standing time: the first standing for 1 h; the second standing for 2 h; the third standing for 3 h. |
| A5 | Manual shaking, 30 r/min, 20 min for total shaking time: the first shaking for 4 min; the second shaking for 6 min; the third shaking for 10 min. | 10 h for total standing time: the first standing for 2 h; the second standing for 3 h; the third standing for 5 h. |
| No. | Fixings |
|---|---|
| F1 | Drum roasting at 290 °C for 2 min, microwave at 800 W for 30 s |
| F2 | Pan-firing roasting at 260 °C for 4 min |
| F3 | Drum roasting at 290 °C for 2.5 min |
| F4 | Microwave at 800 W for 10 s |
| F5 | Microwave at 800 W for 20 s |
| F6 | Microwave at 800 W for 30 s |
| F7 | Microwave at 800 W for 40 s |
| F8 | Microwave at 800 W for 50 s |
| F9 | Microwave at 800 W for 60 s |
| F10 | Steam at 100 °C for 10 s |
| F11 | Steam at 100 °C for 20 s |
| F12 | Steam at 100 °C for 30 s |
| F13 | Steam at 100 °C for 40 s |
| F14 | Steam at 100 °C for 50 s |
| F15 | Steam at 100 °C for 60 s |
| No. | Rolling |
|---|---|
| HR | Rolling while hot, no pressure rolling for 1 min, middle pressure rolling for 5 min |
| H-RTR | Rolling while hot, no pressure rolling for 1 min, spreading for 5 min, middle pressure rolling for 5 min |
| RTR | Spreading for 5 min, no pressure rolling for 1 min, middle pressure rolling for 5 min |
| LTR | Spreading at 4 °C for 5 min, no pressure rolling for 1 min, middle pressure rolling for 5 min |
| No. | Drying |
|---|---|
| HD | Hot-air baking at 120 °C for 30 min, 90 °C for 120 min |
| PD | Pan-firing for 90 min |
| MD | Microwave at 800 W for 8 min |
| FD | Freeze drying for 4 h |
| Compound | Treatment | ||||
|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | |
| Gallic acid | 12.15 ± 0.64 a | 10.96 ± 0.31 b | 10.98 ± 0.32 b | 10.35 ± 0.26 b | 10.62 ± 0.31 b |
| Epigallocatechin | 0.28 ± 0.07 b | 0.09 ± 0.00 c | 0.42 ± 0.03 a | 0.25 ± 0.06 b | 0.42 ± 0.01 a |
| Catechin | 12.30 ± 0.44 a | 9.95 ± 0.17 b | 9.94 ± 0.77 b | 10.64 ± 0.04 b | 10.30 ± 0.26 b |
| Epicatechin | 31.25 ± 1.01 a | 21.47 ± 1.24 c | 22.42 ± 1.23 bc | 21.26 ± 1.39 c | 24.26 ± 0.48 b |
| Epigallocatechin gallate | 147.83 ± 3.74 a | 102.95 ± 2.04 d | 109.46 ± 4.29 c | 118.63 ± 3.52 b | 123.38 ± 2.08 b |
| Gallocatechin-3-gallate | 20.75 ± 0.96 a | 19.06 ± 0.67 b | 19.79 ± 0.27 ab | 21.11 ± 1.14 a | 20.42 ± 0.25 ab |
| Epicatechin-3-gallate | 31.19 ± 1.46 b | 33.83 ± 0.52 a | 28.76 ± 2.55 c | 28.44 ± 0.87 c | 27.40 ± 0.36 c |
| Catechin gallate | 13.59 ± 0.48 ab | 12.77 ± 0.30 bc | 14.33 ± 0.59 a | 12.13 ± 0.64 cd | 11.63 ± 0.39 d |
| Total simple catechin | 43.83 ± 1.35 a | 31.51 ± 1.27 c | 32.77 ± 2.02 bc | 32.16 ± 1.34 c | 34.98 ± 0.43 b |
| Total ester-type catechin | 213.35 ± 6.60 a | 168.61 ± 2.19 d | 172.34 ± 7.67 cd | 180.32 ± 4.46 bc | 182.83 ± 2.30 b |
| TETC/TSC | 4.87 ± 0.03 c | 5.35 ± 0.15 b | 5.26 ± 0.09 b | 5.61 ± 0.15 a | 5.23 ± 0.08 b |
| Total catechin | 257.18 ± 7.92 a | 200.12 ± 3.46 d | 205.11 ± 9.68 cd | 212.48 ± 5.59 bc | 217.81 ± 2.42 b |
| Bitterness index | 4.43 ± 0.04 c | 4.77 ± 0.10 b | 4.73 ± 0.06 b | 5.00 ± 0.14 a | 4.71 ± 0.08 b |
| Compound | Treatment | |||
|---|---|---|---|---|
| HR | H-RTR | RTR | LTR | |
| Gallic acid | 10.23 ± 0.56 b | 10.39 ± 0.21 b | 11.61 ± 0.56 a | 10.63 ± 0.17 b |
| Epigallocatechin | 0.13 ± 0.01 b | 0.11 ± 0.01 b | 0.22 ± 0.02 a | 0.11 ± 0.01 b |
| Catechin | 11.07 ± 0.42 a | 9.91 ± 0.37 c | 10.11 ± 0.09 bc | 10.54 ± 0.17 ab |
| Epicatechin | 22.27 ± 0.87 b | 19.52 ± 0.53 c | 25.16 ± 0.51 a | 21.21 ± 0.64 b |
| Epigallocatechin gallate | 123.44 ± 4.97 a | 114.24 ± 2.25 b | 127.79 ± 1.85 a | 122.24 ± 3.33 a |
| Gallocatechin-3-gallate | 19.37 ± 0.45 b | 17.42 ± 0.90 c | 20.83 ± 0.82 a | 17.82 ± 0.59 c |
| Epicatechin-3-gallate | 34.53 ± 0.59 a | 31.56 ± 1.56 b | 35.13 ± 1.60 a | 33.11 ± 0.44 ab |
| Catechin gallate | 4.34 ± 0.28 b | 4.21 ± 0.21 b | 5.08 ± 0.19 a | 4.23 ± 0.06 b |
| Total simple catechin | 33.47 ± 1.16 b | 29.54 ± 0.85 c | 35.49 ± 0.43 a | 31.86 ± 0.81 d |
| Total ester-type catechin | 181.68 ± 6.24 ab | 167.42 ± 4.38 c | 188.84 ± 4.35 a | 177.41 ± 4.21 b |
| TETC/TSC | 5.43 ± 0.14 ab | 5.67 ± 0.19 a | 5.32 ± 0.06 b | 5.57 ± 0.08 ab |
| Total catechin | 215.15 ± 7.11 ab | 196.97 ± 4.66 c | 224.34 ± 4.77 a | 209.27 ± 4.90 b |
| Bitterness index | 4.87 ± 0.13 bc | 5.10 ± 0.17 a | 4.77 ± 0.05 c | 5.03 ± 0.08 ab |
| Compound | Treatment | |||
|---|---|---|---|---|
| HD | PD | MD | FD | |
| Gallic acid | 11.26 ± 0.16 a | 8.53 ± 0.34 b | 10.84 ± 0.11 a | 11.16 ± 0.27 a |
| Epigallocatechin | 0.18 ± 0.01 b | 0.16 ± 0.03 c | 0.22 ± 0.01 b | 0.35 ± 0.04 a |
| Catechin | 10.72 ± 0.13 b | 10.55 ± 0.22 b | 10.85 ± 0.17 b | 11.27 ± 0.24 a |
| Epicatechin | 22.84 ± 0.28 a | 19.22 ± 0.78 b | 19.33 ± 0.58 b | 22.33 ± 0.57 a |
| Epigallocatechin gallate | 115.08 ± 0.69 a | 109.03 ± 0.48 b | 115.36 ± 5.43 a | 101.64 ± 0.49 c |
| Gallocatechin-3-gallate | 19.54 ± 0.43 b | 17.18 ± 0.20 d | 26.17 ± 0.33 a | 18.52 ± 0.44 c |
| Epicatechin-3-gallate | 33.11 ± 1.28 a | 28.94 ± 0.51 b | 32.23 ± 0.51 a | 28.66 ± 1.36 b |
| Catechin gallate | 4.83 ± 0.61 ab | 3.84 ± 0.27 b | 5.43 ± 0.10 a | 4.71 ± 0.18 c |
| Total simple catechin | 33.74 ± 0.20 a | 29.93 ± 0.75 b | 30.40 ± 0.75 b | 33.94 ± 0.78 a |
| Total ester-type catechin | 172.55 ± 1.68 b | 158.99 ± 1.15 c | 179.20 ± 4.66 a | 153.52 ± 1.61 d |
| TETC/TSC | 5.11 ± 0.04 b | 5.31 ± 0.11 b | 5.90 ± 0.10 a | 4.53 ± 0.15 c |
| Total catechin | 206.29 ± 1.81 a | 188.91 ± 1.75 b | 209.60 ± 5.26 a | 187.46 ± 0.83 b |
| Bitterness index | 4.57 ± 0.04 c | 4.77 ± 0.10 b | 5.08 ± 0.11 a | 4.03 ± 0.13 d |
| Level | Factor | |||
|---|---|---|---|---|
| Withering (A) | Fixing (B) | Rolling (C) | Drying (D) | |
| 1 | RL8 | F5 | HR | HD |
| 2 | BL6 | F12 | RTR | PD |
| 3 | YL4 | F3 | LTR | MD |
| No. | Withering (A) | Fixing (B) | Rolling (C) | Drying (D) |
|---|---|---|---|---|
| OE1 | RL8 | F5 | HR | HD |
| OE2 | RL8 | F12 | RTR | PD |
| OE3 | RL8 | F3 | LTR | MD |
| OE4 | BL6 | F5 | RTR | MD |
| OE5 | BL6 | F12 | LTR | HD |
| OE6 | BL6 | F3 | HR | PD |
| OE7 | YL4 | F5 | LTR | PD |
| OE8 | YL4 | F12 | HR | MD |
| OE9 | YL4 | F3 | RTR | HD |
| NO. | Appearance | Liquor Color | Aroma | Taste | Infused Leaf | Score |
|---|---|---|---|---|---|---|
| CK | Even color and lightly dry | Orange | Pure and sweet | Mellow and lightly astringent | Even and lightly dull | 86.27 |
| OE1 | Lightly mixed and lightly dry | Lightly dull yellow | Sweet and lightly caramel | Mellow and lightly coarse | Even and light dull | 84.68 |
| OE2 | Lightly dull dry | Orange red | Sweet and lightly caramel | Mellow with grassy | Even and lightly dark | 84.70 |
| OE3 | Even color and smooth | Bright yellow | Fruity and pure | Mellow and lightly bitter | Even | 88.60 |
| OE4 | Smooth and lightly dry | Yellow | Clean and fresh, lasting | Mellow and brisk | Even | 89.25 |
| OE5 | Even color and clean | Light yellow | Pure and sweet | Mellow and lightly astringent | Even | 85.83 |
| OE6 | Even color and lightly dry | Yellow | Grassy | Coarse and astringent | Dull and lightly dark | 82.23 |
| OE7 | Even color | Light yellow | Less pure and grassy | Astringent and lightly bitter | Dull and lightly dark | 78.52 |
| OE8 | Smooth | Yellow | Sweet and lightly weak | Coarse and lightly bitter | Even | 82.98 |
| OE9 | Even color | Lightly dull yellow | Pure | Mellow and lightly astringent | Even and lightly blue leaf | 85.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Lin, Y.; Tuo, Y.; Liu, L.; Du, X.; Zhu, Q.; Hu, Y.; Shi, Y.; Wu, L.; Lin, J. Optimizing Processing Techniques of Oolong Tea Balancing between High Retention of Catechins and Sensory Quality. Foods 2023, 12, 4334. https://doi.org/10.3390/foods12234334
Lu X, Lin Y, Tuo Y, Liu L, Du X, Zhu Q, Hu Y, Shi Y, Wu L, Lin J. Optimizing Processing Techniques of Oolong Tea Balancing between High Retention of Catechins and Sensory Quality. Foods. 2023; 12(23):4334. https://doi.org/10.3390/foods12234334
Chicago/Turabian StyleLu, Xiaofeng, Yanyan Lin, Yanming Tuo, Lijia Liu, Xinxin Du, Qiufang Zhu, Yunfei Hu, Yutao Shi, Liangyu Wu, and Jinke Lin. 2023. "Optimizing Processing Techniques of Oolong Tea Balancing between High Retention of Catechins and Sensory Quality" Foods 12, no. 23: 4334. https://doi.org/10.3390/foods12234334
APA StyleLu, X., Lin, Y., Tuo, Y., Liu, L., Du, X., Zhu, Q., Hu, Y., Shi, Y., Wu, L., & Lin, J. (2023). Optimizing Processing Techniques of Oolong Tea Balancing between High Retention of Catechins and Sensory Quality. Foods, 12(23), 4334. https://doi.org/10.3390/foods12234334





