Succinate Enhances Lipolysis and Decreases Adipocytes Size in Both Subcutaneous and Visceral Adipose Tissue from High-Fat-Diet-Fed Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
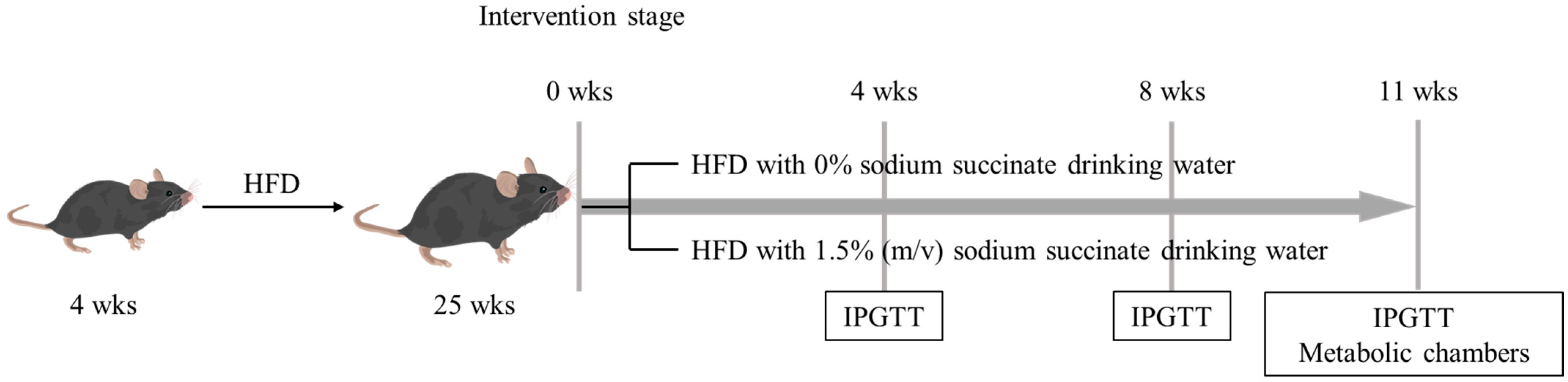
2.1. Animals and Experimental Design
2.2. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.3. Metabolic Phenotyping
2.4. Body Temperature and Cold Exposure
2.5. Histologic Analysis
2.6. Oil Red O Staining
2.7. Quantitative Real-Time qPCR
2.8. Biochemical Analysis for Liver, Adipose Tissue and Serum Samples
2.9. Statistical
3. Results
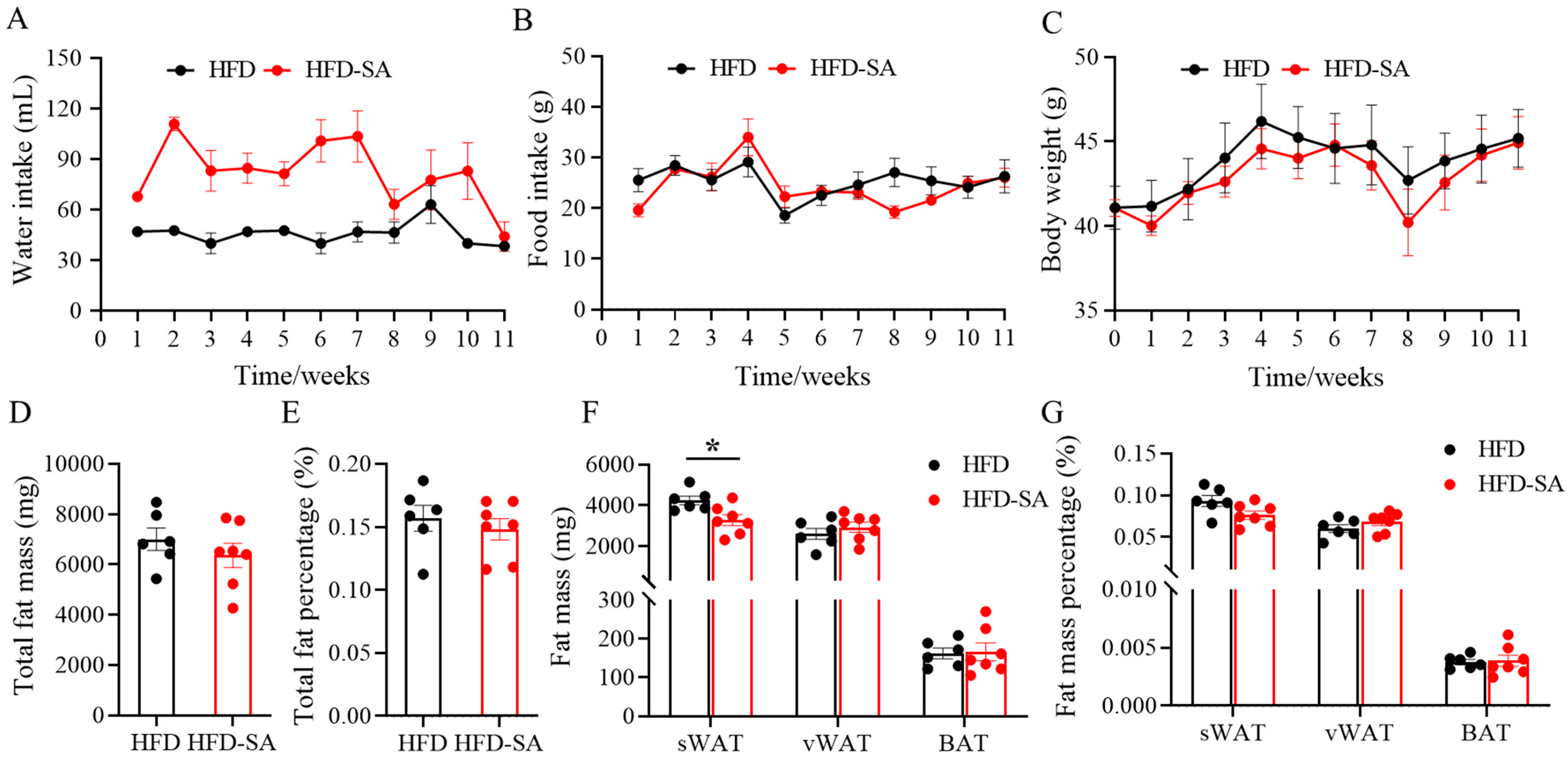
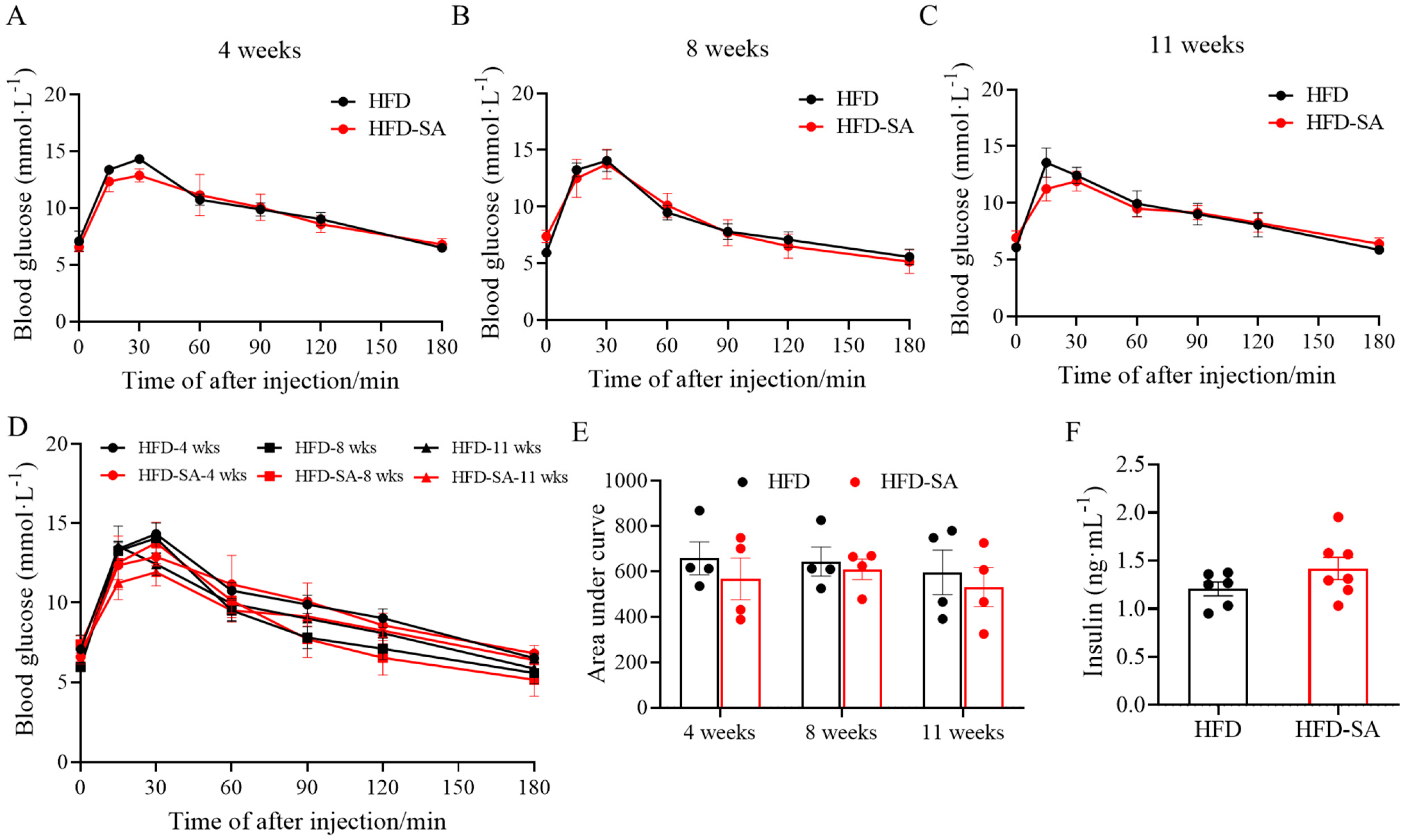
3.1. Succinate Improved Glucose Intolerance, Adipose Deposition and Energy Expenditure in Obese Mice
3.2. Succinate Decreases Triglyceride Content in Adipose Tissue and Adipocyte Size
3.3. Succinate Enhances Lipolysis in Both Adipose Tissue and Liver
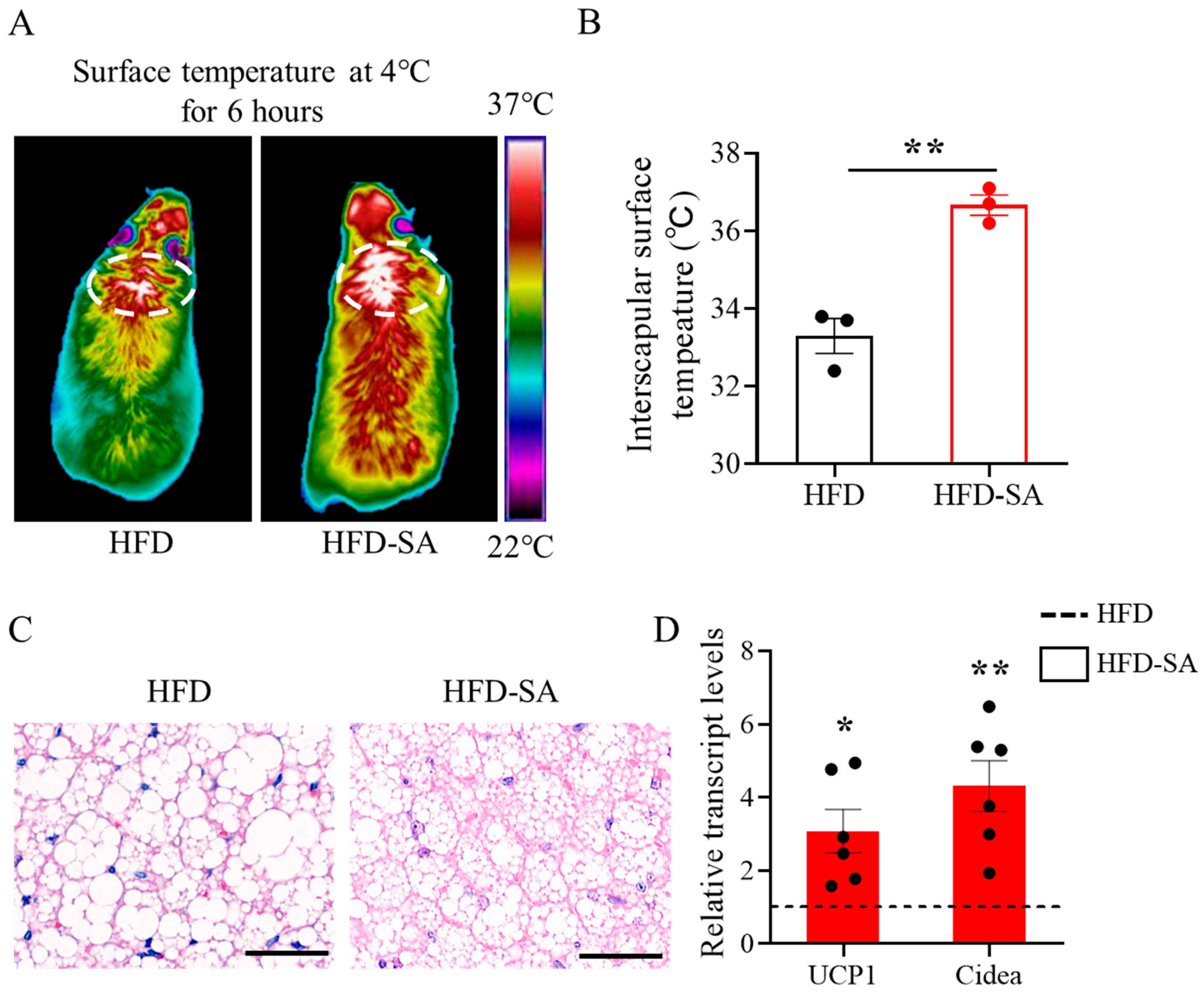
3.4. Succinate Enhances the Activation of BAT Thermogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, G.A.; Fruhbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Shi, Q.Y.; Wang, Y.; Hao, Q.K.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.S.; Shen, Y.J.; Ge, L.; et al. Pharmacotherapy for adults with overweight and obesity: A systematic review and network meta-analysis of randomised controlled trials. Lancet 2022, 399, 259–269. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; Le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.M.; Han, X.K.; Fu, S.L.; Zhu, C.; Chen, Q. Efficacy of resveratrol supplementation on glucose and lipid metabolism: A meta-analysis and systematic review. Front. Physiol. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Ceperuelo-Mallafre, V.; Llaurado, G.; Keiran, N.; Benaiges, E.; Astiarraga, B.; Martinez, L.; Pellitero, S.; Gonzalez-Clemente, J.M.; Rodriguez, A.; Fernandez-Real, J.M.; et al. Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery. Diabetes. Care 2019, 42, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Xia, Y.Y.; Chen, S.Y.; Wu, G.Y.; Bazer, F.W.; Zhou, B.Y.; Tan, B.; Zhu, G.Q.; Deng, J.P.; Yin, Y.L. Glutamine metabolism in macrophages: A novel target for obesity/type 2 diabetes. Adv. Nutr. 2019, 10, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Astiarraga, B.; Martinez, L.; Ceperuelo-Mallafre, V.; Llaurado, G.; Terron-Puig, M.; Rodriguez, M.M.; Casajoana, A.; Pellitero, S.; Megia, A.; Vilarrasa, N.; et al. Impaired succinate response to a mixed meal in obesity and type 2 diabetes is normalized after metabolic surgery. Diabetes Care 2020, 43, 2581–2587. [Google Scholar] [CrossRef]
- Fernandez-Veledo, S.; Ceperuelo-Mallafre, V.; Vendrell, J. Rethinking succinate: An unexpected hormone-like metabolite in energy homeostasis. Trends Endocrinol. Metab. 2021, 32, 680–692. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.F.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.Y.; Wang, Y.J.; Liu, C.; Wang, W.Z.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Liu, K.L.; Lin, L.Y.; Li, Q.; Xue, Y.Q.; Zheng, F.J.; Wang, G.; Zheng, C.X.; Du, L.M.; Hu, M.Y.; Huang, Y.; et al. Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl. Acad. Sci. USA 2020, 117, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; Delafiori, J.; Zuccoli, G.; Carregari, V.C.; Prado, T.P.; Morari, J.; Sidarta-Oliveira, D.; Solon, C.S.; Catharino, R.R.; Araujo, E.P.; et al. Exogenous succinate impacts mouse brown adipose tissue mitochondrial proteome and potentiates body mass reduction induced by liraglutide. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E226–E240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Chen, Y.T.; Zhao, L.; Tian, Q.Y.; deAvila, J.M.; Zhu, M.J.; Du, M. Dietary succinate supplementation to maternal mice improves fetal brown adipose tissue development and thermogenesis of female offspring. J. Nutr. Biochem. 2022, 100, 8. [Google Scholar] [CrossRef]
- Xiao, N.; Yang, L.L.; Yang, Y.L.; Liu, L.W.; Li, J.; Liu, B.L.; Liu, K.; Qi, L.W.; Li, P. Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front. Pharmacol. 2017, 8, 16. [Google Scholar] [CrossRef]
- Monfort-Ferre, D.; Caro, A.; Menacho, M.; Marti, M.; Espina, B.; Boronat-Toscano, A.; Nunez-Roa, C.; Seco, J.; Bautista, M.; Espin, E.; et al. The gut microbiota metabolite succinate promotes adipose tissue browning in crohn’s disease. J. Crohns. Colitis. 2022, 16, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Ceperuelo-Mallafre, V.; Keiran, N.; Queipo-Ortuno, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Perez-Brocal, V.; Andres-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. Isme. J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef]
- Elliott, P.; Posma, J.M.; Chan, Q.; Garcia-Perez, I.; Wijeyesekera, A.; Bictash, M.; Ebbels, T.M.D.; Ueshima, H.; Zhao, L.C.; van Horn, L.; et al. Urinary metabolic signatures of human adiposity. Sci. Transl. Med. 2015, 7, 16. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrne, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, 15. [Google Scholar] [CrossRef]
- Cummins, T.D.; Holden, C.R.; Sansbury, B.E.; Gibb, A.A.; Shah, J.; Zafar, N.; Tang, Y.N.; Hellmann, J.; Rai, S.N.; Spite, M.; et al. Metabolic remodeling of white adipose tissue in obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E262–E277. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Minze, L.J.; Reyes, M.; Ren, Y.L.; Wang, X.K.; Brunner, G.; Ghosn, M.; Cordero-Reyes, A.M.; Ding, K.R.; Pratico, D.; et al. High-fat feeding-induced hyperinsulinemia increases cardiac glucose uptake and mitochondrial function despite peripheral insulin resistance. Endocrinology 2013, 154, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Sundekilde, U.K.; Yde, C.C.; Honore, A.H.; Rae, J.M.C.; Burns, F.R.; Mukerji, P.; Mawn, M.P.; Stenman, L.; Dragan, Y.; Glover, K.; et al. An integrated multi-omics analysis defines key pathway alterations in a diet-induced obesity mouse model. Metabolites 2020, 10, 80. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodriguez-Pena, M.M.; Ceperuelo-Mallafre, V.; Cedo, L.; Nunez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodriguez, M.; Balvay, A.; et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Sadagopan, N.; Li, W.L.; Roberds, S.L.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar]
- Calderon-Santiago, M.; Priego-Capote, F.; Galache-Osuna, J.G.; de Castro, M.D.L. Method based on GC-MS to study the influence of tricarboxylic acid cycle metabolites on cardiovascular risk factors. J. Pharm. Biomed. Anal. 2013, 74, 178–185. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. GTTs and ITTs in mice: Simple tests, complex answers. Nat. Metab. 2021, 3, 883–886. [Google Scholar] [CrossRef]
- Bargut, T.C.; Silva-e-Silva, A.C.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Tschop, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Bruning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V., Jr.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods. 2011, 9, 57–63. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic acid and gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018, 27, 378–392. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Zhao, J.X.; Yang, Q.Y.; Wang, B.; Deavila, J.M.; Zhu, M.J.; Du, M. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell 2020, 19, 10. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, M.; Lee, D.; Jang, J.; Shin, S.S.; Yoon, M. Fenofibrate regulates visceral obesity and nonalcoholic steatohepatitis in obese female ovariectomized C57BL/6J mice. Int. J. Mol. Sci. 2021, 22, 3475. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 12, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L. Lipid droplet biogenesis. Curr. Opin. Cell Biol. 2019, 59, 88–96. [Google Scholar] [CrossRef]
- Goossens, G.H. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes. Facts. 2017, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Slawik, M.; Vidal-Puig, A.J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing. Res. Rev. 2006, 5, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Perfield, J.W.; Obin, M.S.; Greenberg, A.S. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell Biochem. 2008, 105, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Bulankina, A.V.; Hsiao, H.H.; Urlaub, H.; Jackle, H.; Kuhnlein, R.P. PERILIPIN-dependent control of lipid droplet structure and fat storage in drosophila. Cell Metab. 2010, 12, 521–532. [Google Scholar] [CrossRef]
- Martinez-Fernandez, L.; Laiglesia, L.M.; Huerta, A.E.; Martinez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins. Other. Lipid. Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Li, G.H.; Xing, Z.; Wang, W.Y.; Luo, W.Y.; Ma, Z.Y.; Wu, Z.C.; Chen, H.; Li, Y.H.; Wang, C.X.; Zeng, F.Y.; et al. Adipose-specific knockout of Protein Kinase D1 suppresses de novo lipogenesis in mice via SREBP1c-dependent signaling. Exp. Cell Res. 2021, 401, 11. [Google Scholar] [CrossRef]
- Ji, L.L.; Zhao, Y.; He, L.J.; Zhao, J.; Gao, T.; Liu, F.Z.; Qi, B.C.; Kang, F.; Wang, G.; Zhao, Y.L.; et al. AKAP1 deficiency attenuates diet-induced obesity and insulin resistance by promoting fatty acid oxidation and thermogenesis in brown adipocytes. Adv. Sci. 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Kingwell, B.A. Brown adipose tissue in humans: Therapeutic potential to combat obesity. Pharmacol. Ther. 2013, 140, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Becerril, S.; Sainz, N.; Garrastachu, P.; Garcia-Velloso, M.J. BAT: A new target for human obesity? Trends. Pharmacol. Sci. 2009, 30, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Milbank, E.; Dragano, N.R.V.; Gonzalez-Garcia, I.; Garcia, M.R.; Rivas-Limeres, V.; Perdomo, L.; Hilairet, G.; Ruiz-Pino, F.; Mallegol, P.; Morgan, D.A.; et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPK alpha 1 corrects obesity through BAT activation. Nat. Metab. 2021, 3, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.X.; Wei, G.; You, Y.L.; Huang, Y.Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.L.; Zhang, C.H.; et al. Rutin ameliorates obesity through brown fat activation. Faseb. J. 2017, 31, 333–345. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Johnson, J.M.; Peterlin, A.D.; Balderas, E.; Sustarsic, E.G.; Maschek, J.A.; Lang, M.J.; Jara-Ramos, A.; Panic, V.; Morgan, J.T.; Villanueva, C.J.; et al. Mitochondrial phosphatidylethanolamine modulates UCP1 to promote brown adipose thermogenesis. Sci. Adv. 2023, 9, 15. [Google Scholar] [CrossRef]
- Jash, S.; Banerjee, S.; Lee, M.J.; Farmer, S.R.; Puri, V. CIDEA transcriptionally regulates ucp1 for britening and thermogenesis in human fat cells. iScience 2019, 20, 73–89. [Google Scholar] [CrossRef]
- Vasan, S.K.; Noordam, R.; Gowri, M.S.; Neville, M.J.; Karpe, F.; Christodoulides, C. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: Evidence from a large human cross-sectional study. Diabetologia 2019, 62, 2079–2087. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Xie, C.Z.; Li, X.Y.; Yang, J.; Yu, T.; Zhang, R.H.; Zhang, T.Q.; Saxena, D.; Snyder, M.; Wu, Y.J.; et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat. Commun. 2017, 8, 12. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Gao, Y.; Pang, Y.; Xie, C.; Jiang, C. An integrated lc-ms/ms strategy for quantifying the oxidative-redox metabolome in multiple biological samples. Anal. Chem. 2020, 92, 8810–8818. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Deja, S.; Kucejova, B.; Duarte, J.A.G.; McDonald, J.G.; Burgess, S.C. Targeted determination of tissue energy status by lc-ms/ms. Anal. Chem. 2019, 91, 5881–5887. [Google Scholar] [CrossRef] [PubMed]



| Diet | Catalog# | Cal Density (kcal/g) | Fat (%) | Protein (%) | Carbohydrate (%) |
|---|---|---|---|---|---|
| HFD | D12492 | 5.24 | 60 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, T.; Fang, B.; Zhang, M.; Liu, Y. Succinate Enhances Lipolysis and Decreases Adipocytes Size in Both Subcutaneous and Visceral Adipose Tissue from High-Fat-Diet-Fed Obese Mice. Foods 2023, 12, 4285. https://doi.org/10.3390/foods12234285
Ji T, Fang B, Zhang M, Liu Y. Succinate Enhances Lipolysis and Decreases Adipocytes Size in Both Subcutaneous and Visceral Adipose Tissue from High-Fat-Diet-Fed Obese Mice. Foods. 2023; 12(23):4285. https://doi.org/10.3390/foods12234285
Chicago/Turabian StyleJi, Tengteng, Bing Fang, Ming Zhang, and Yaqiong Liu. 2023. "Succinate Enhances Lipolysis and Decreases Adipocytes Size in Both Subcutaneous and Visceral Adipose Tissue from High-Fat-Diet-Fed Obese Mice" Foods 12, no. 23: 4285. https://doi.org/10.3390/foods12234285
APA StyleJi, T., Fang, B., Zhang, M., & Liu, Y. (2023). Succinate Enhances Lipolysis and Decreases Adipocytes Size in Both Subcutaneous and Visceral Adipose Tissue from High-Fat-Diet-Fed Obese Mice. Foods, 12(23), 4285. https://doi.org/10.3390/foods12234285







