Insights into the Mechanisms of Reuterin against Staphylococcus aureus Based on Membrane Damage and Untargeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Production and Quantitation of Reuterin Stock
2.3. Determination of Minimum Inhibitory Concentration (MIC) Value
2.4. Growth Inhibition Curves
2.5. Extracellular Electrical Conductivity Assay
2.6. Membrane Potential Assay
2.7. Intracellular pH Assay
2.8. Membrane Permeability Assay
2.9. Intracellular Adenosine Triphosphate (ATP) Level Assay
2.10. Leakage of Intracellular DNA
2.11. Bacterial Morphology Assay
2.12. LC–MS-Based Untargeted Metabolomic Analysis
2.13. Statistical Analysis
3. Results and Discussion
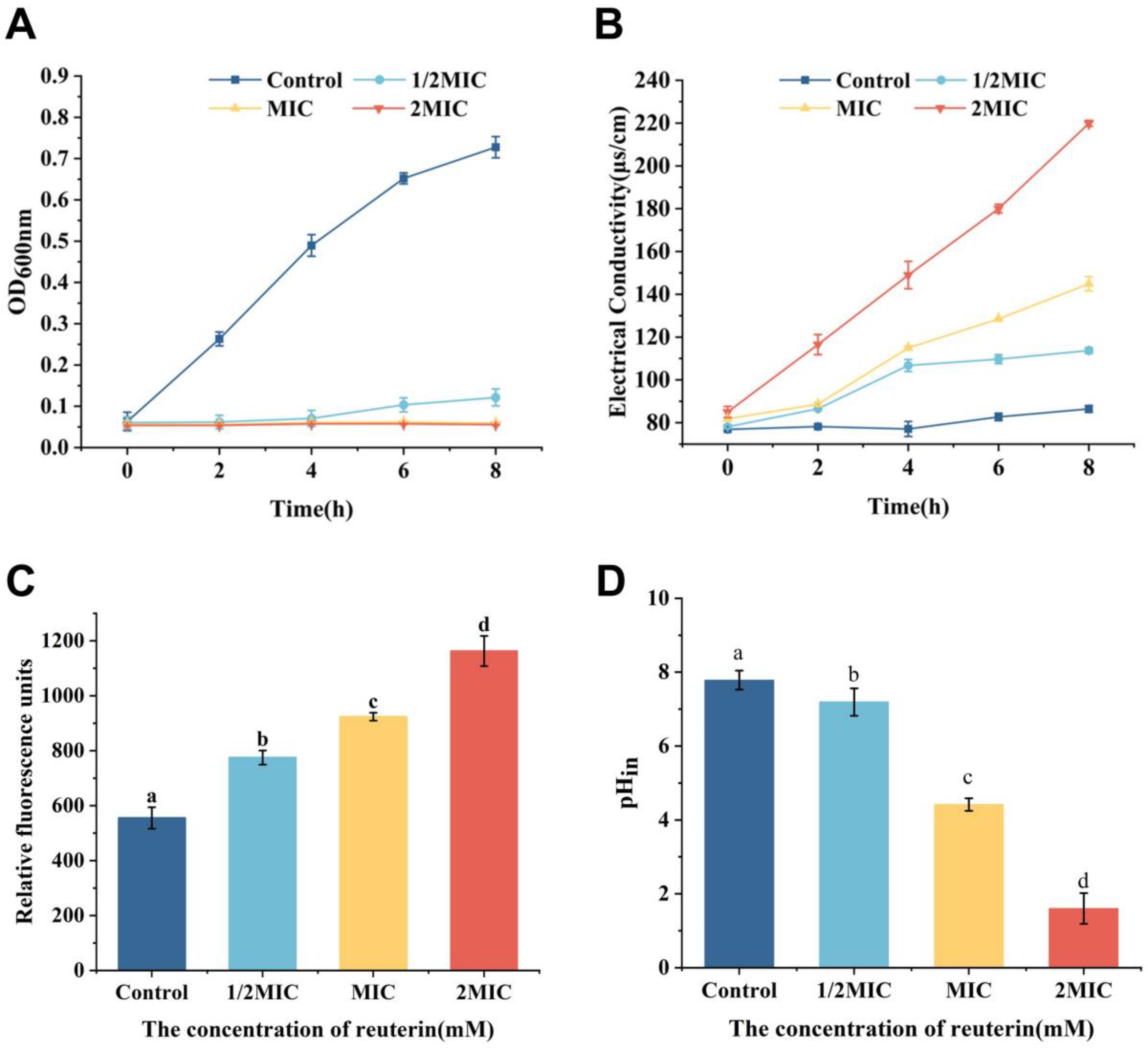
3.1. Antibacterial Activity
3.2. Effect of Reuterin on the Cell Membrane of S. aureus
3.2.1. Electrical Conductivity
3.2.2. Membrane Potential
3.2.3. Intracellular pH of S. aureus
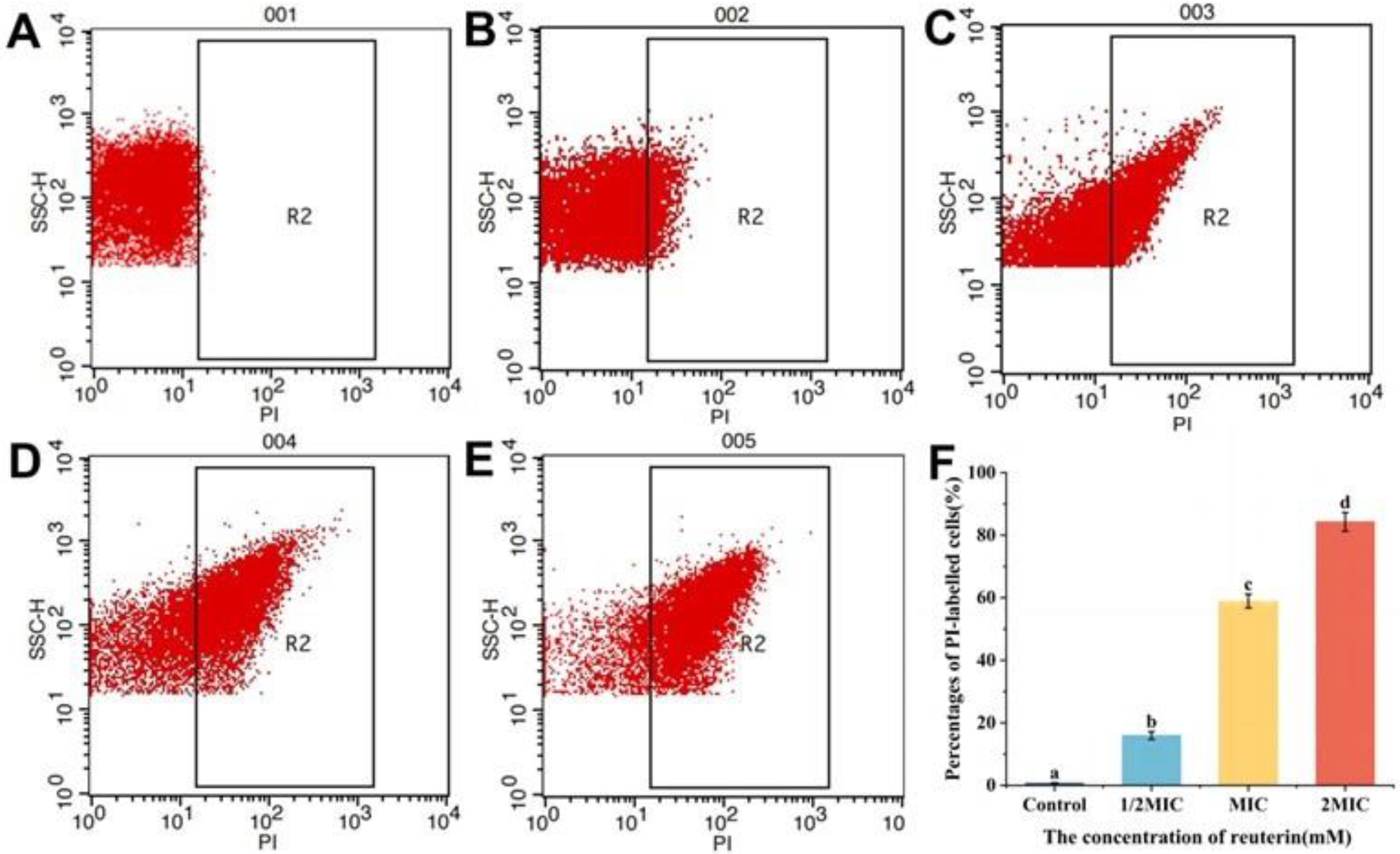
3.2.4. PI Staining of S. aureus
3.2.5. Intracellular ATP Concentration
3.2.6. Leakage of the Genomic DNA in S. aureus
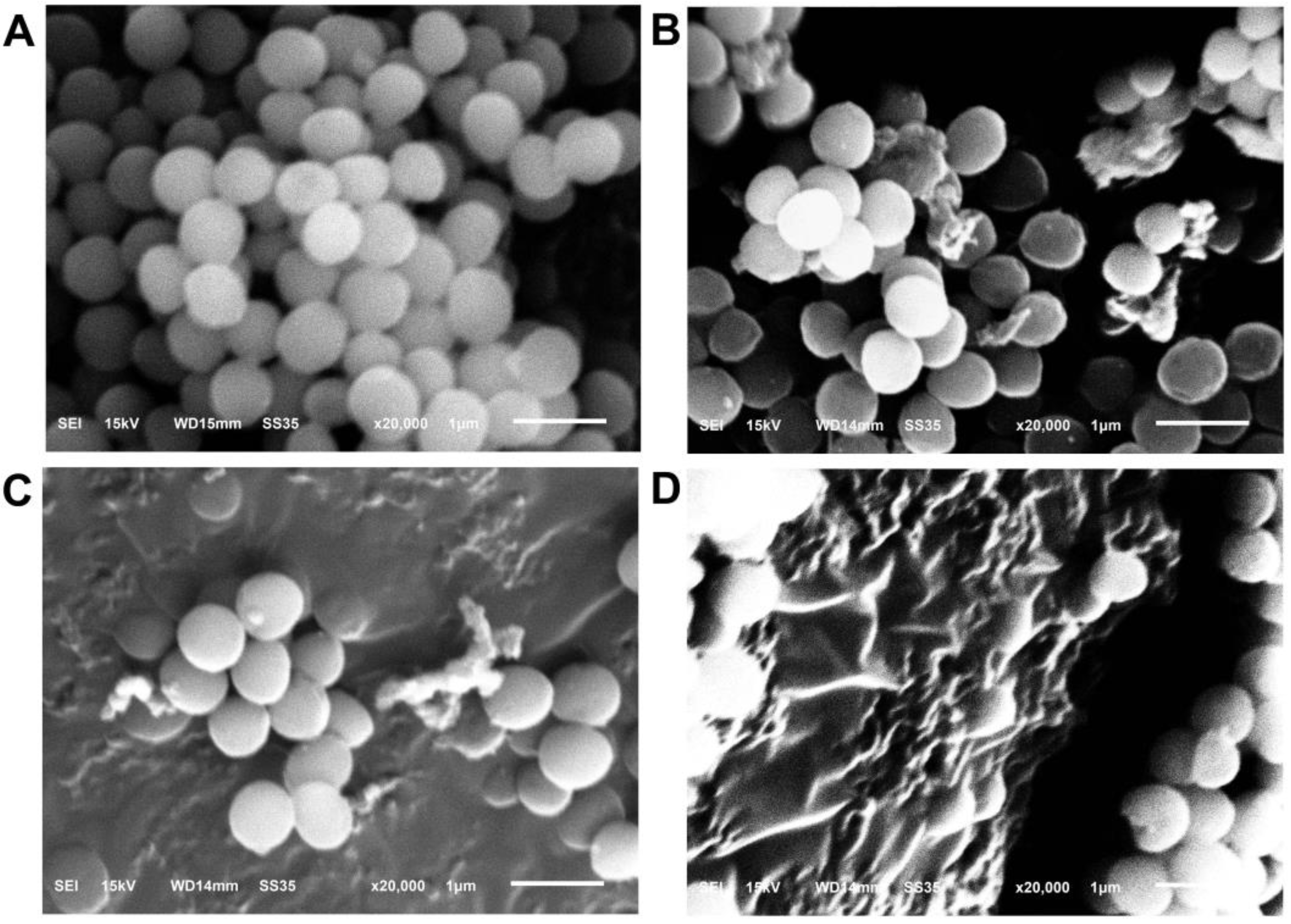
3.2.7. SEM Analysis
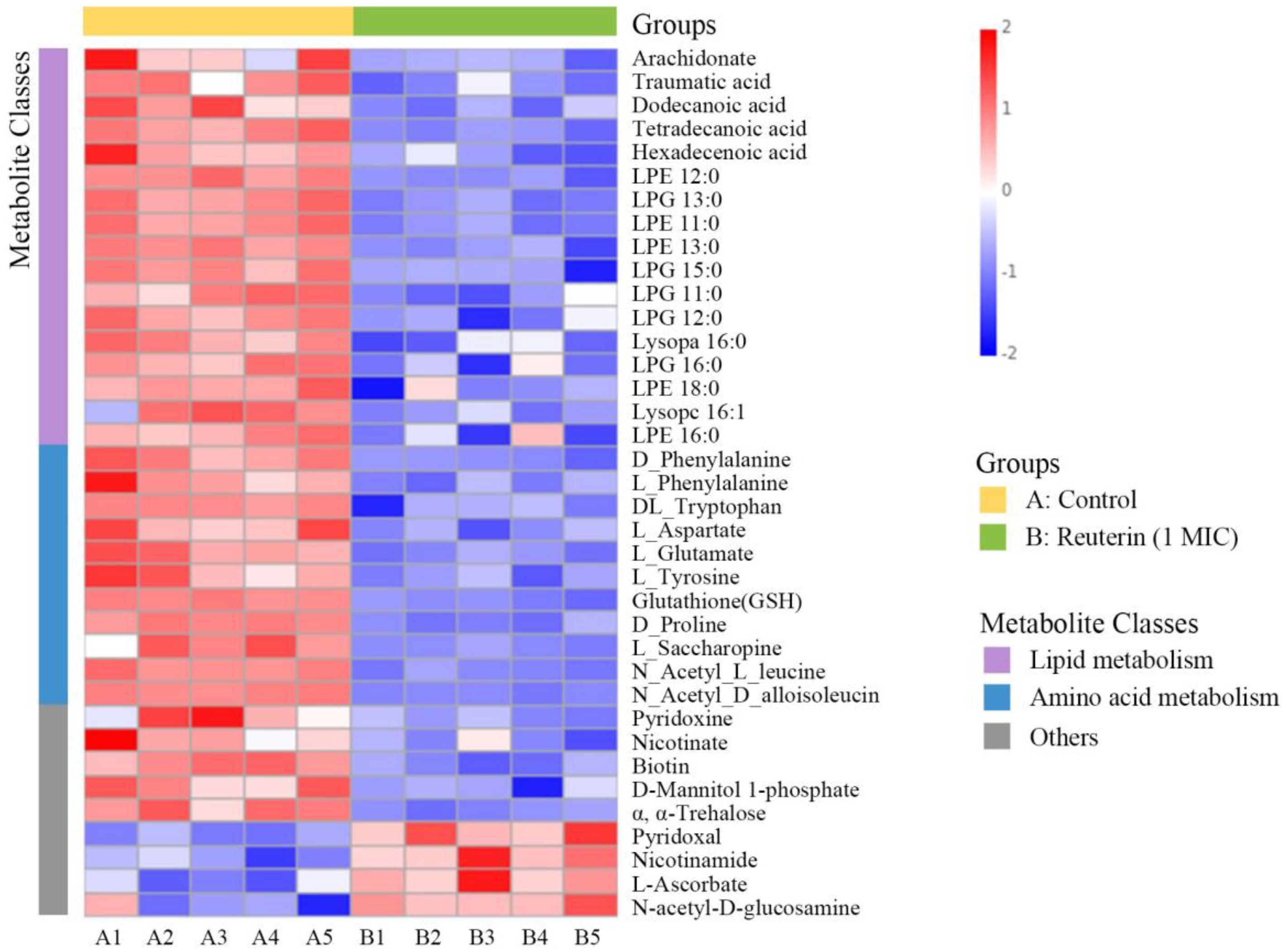
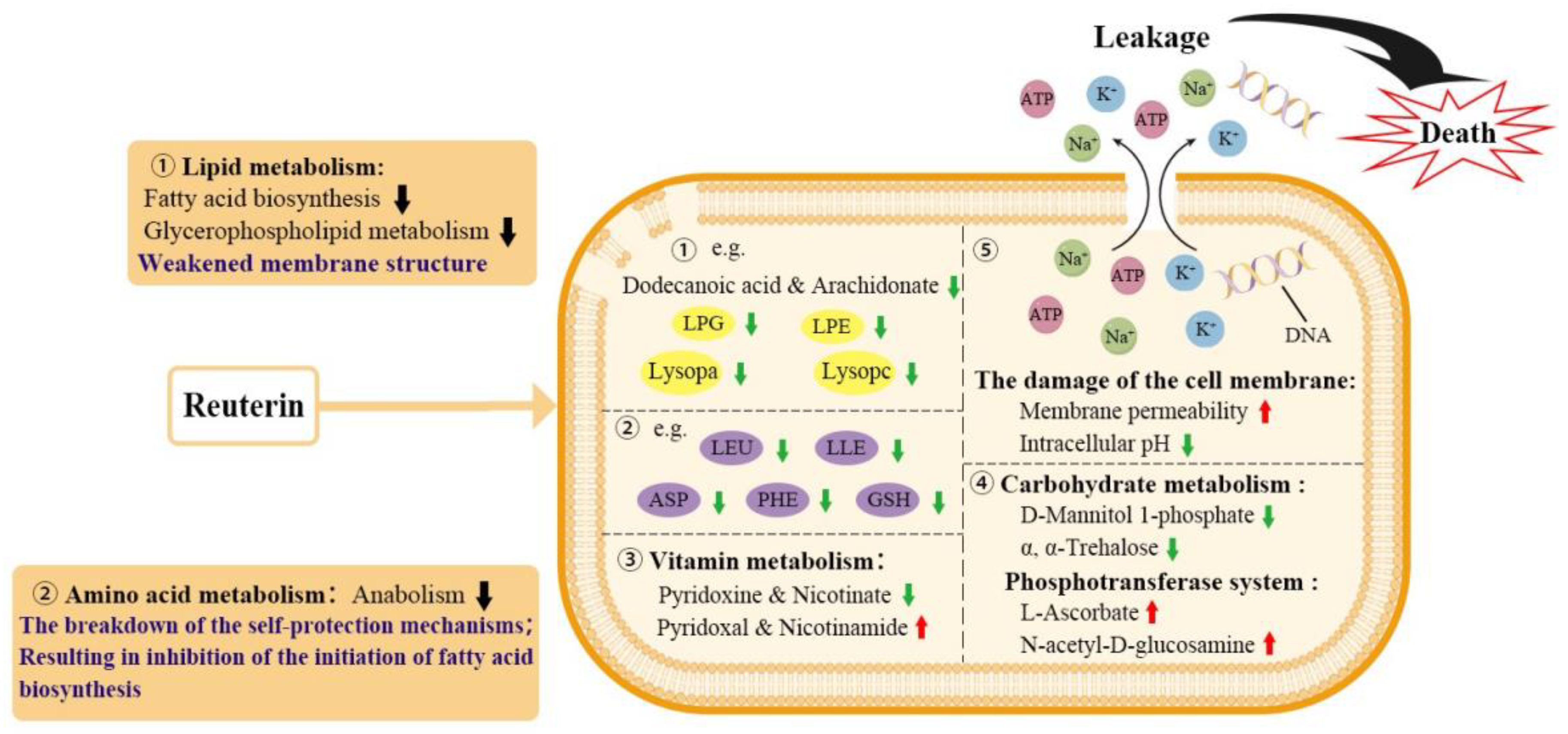
3.3. Untargeted Metabolomic Analysis of S. aureus Treated with Reuterin
3.3.1. Lipid Metabolism
3.3.2. Amino Acid Metabolism
3.3.3. Other Metabolic Pathways
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef]
- Liu, Y. Heat and Pressure Resistance of Escherichia coli and Its Inactivation in the Presence of Antimicrobial Compounds. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Engels, C.; Schwab, C.; Zhang, J.; Stevens, M.J.; Bieri, C.; Ebert, M.O.; Mcneill, K.; Sturla, S.J.; Lacroix, C. Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci. Rep. 2016, 6, 36246. [Google Scholar] [CrossRef]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156 Pt 6, 1589–1599. [Google Scholar] [CrossRef]
- Sun, M.C.; Hu, Z.Y.; Li, D.D.; Chen, Y.X.; Xi, J.H.; Zhao, C.H. Application of the Reuterin System as Food Preservative or Health-Promoting Agent: A Critical Review. Foods 2022, 11, 4000. [Google Scholar] [CrossRef]
- Vollenweider, S.; Grassi, G.; Iwo König, A.; Puhan, Z. Purification and Structural Characterization of 3-Hydroxypropionaldehyde and Its Derivatives. J. Agric. Food Chem. 2003, 51, 3287–3293. [Google Scholar] [CrossRef]
- Vollenweider, S.; Evers, S.; Zurbriggen, K.; Lacroix, C. Unraveling the hydroxypropionaldehyde (HPA) system: An active antimicrobial agent against human pathogens. J. Agric. Food Chem. 2010, 58, 10315–10322. [Google Scholar] [CrossRef]
- Cleusix, V.; Lacroix, C.; Vollenweider, S.; Duboux, M.; Le Blay, G. Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol. 2007, 7, 101. [Google Scholar] [CrossRef]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: A phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe 2023, 4, e75–e83. [Google Scholar] [CrossRef]
- Pal, M.; Berhanu, G.; Megersa, L.; Kandi, V. Epidemiology, Pathogenicity, Animal Infections, Antibiotic Resistance, Public Health Significance, and Economic Impact of Staphylococcus aureus: A Comprehensive Review. Am. J. Public Health Res. 2020, 8, 14–21. [Google Scholar]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Yehia, H.M.; Alkhuriji, A.F.; Savvaidis, I.; Al-Masoud, A.H. Bactericidal effect of nisin and reuterin on methicillin-resistant Staphylococcus aureus (MRSA) and S. aureus ATCC 25937. Food Sci. Technol. 2022, 42, e105321. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Combined effect of reuterin and lactic acid bacteria bacteriocins on the inactivation of food-borne pathogens in milk. Food Control 2011, 22, 457–461. [Google Scholar] [CrossRef]
- Vimont, A.; Fernandez, B.; Ahmed, G.; Fortin, H.P.; Fliss, I. Quantitative antifungal activity of reuterin against food isolates of yeasts and moulds and its potential application in yogurt. Int. J. Food Microbiol. 2019, 289, 182–188. [Google Scholar] [CrossRef]
- Circle, S.J.; Stone, L.; Boruff, C.S. Acrolein Determination by Means of Tryptophane. A Colorimetric Micromethod. Ind. Eng. Chem. 1945, 17, 259–262. [Google Scholar] [CrossRef]
- Ju, J.-H.; Jeon, S.-G.; Lee, K.M.; Heo, S.-Y.; Kim, M.-S.; Kim, C.-H.; Oh, B.-R. The biocatalytic production of 3-hydroxypropionaldehyde and evaluation of its stability. Catalysts 2021, 11, 1139. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Escamilla-Garcia, E.; de la Garza-Ramos, M.A.; Tamez-Guerra, P.; Gomez-Flores, R.; Urbina-Rios, C.S. In Vitro Antimicrobial Activity and Downregulation of Virulence Gene Expression on Helicobacter pylori by Reuterin. Probiotics Antimicrob. Proteins 2018, 10, 168–175. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.; Meng, R.; Liu, Z.; Zhang, G.; Guo, N. Synergistic antimicrobial effects of nisin and p-Anisaldehyde on Staphylococcus aureus in pasteurized milk. LWT 2017, 84, 222–230. [Google Scholar] [CrossRef]
- Tao, Y.; Qian, L.-H.; Xie, J. Effect of chitosan on membrane permeability and cell morphology of Pseudomonas aeruginosa and Staphyloccocus aureus. Carbohydr. Polym. 2011, 86, 969–974. [Google Scholar] [CrossRef]
- Sanchez, E.; Garcia, S.; Heredia, N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Env. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef]
- Chang, Y.; Xing, M.; Hu, X.; Feng, H.; Wang, Y.; Guo, B.; Sun, M.; Ma, L.; Fei, P. Antibacterial Activity of Chrysanthemum buds Crude Extract against Cronobacter sakazakii and Its Application as a Natural Disinfectant. Front. Microbiol. 2020, 11, 632177. [Google Scholar] [CrossRef]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Liu, Y.; Cao, H.; Han, Q.; Xie, B.; Fan, L.; Li, X.; Hu, J.; Yang, G. Effect of fermented corn-soybean meal on serum immunity, the expression of genes related to gut immunity, gut microbiota, and bacterial metabolites in grower-finisher pigs. Front. Microbiol. 2019, 10, 2620. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sanchez-Vega, R.; Gutierrez-Mendez, N.; Leon-Felix, J.; Acosta-Muniz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef]
- Bennett, S.; Ben Said, L.; Lacasse, P.; Malouin, F.; Fliss, I. Susceptibility to nisin, bactofencin, pediocin and reuterin of multidrug resistant Staphylococcus aureus, Streptococcus dysgalactiae and Streptococcus uberis causing bovine mastitis. Antibiotics 2021, 10, 1418. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Clementi, E.A.; Marks, L.R.; Roche-Håkansson, H.; Håkansson, A.P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Vis. Exp. 2014, 84, e51008. [Google Scholar] [CrossRef]
- Morici, P.; Rizzato, C.; Ghelardi, E.; Rossolini, G.M.; Lupetti, A. Sensitization of KPC and NDM Klebsiella pneumoniae To Rifampicin by the Human Lactoferrin-Derived Peptide hLF1-11. Microbiol. Spectr. 2023, 11, e0276722. [Google Scholar] [CrossRef]
- Kumari, J.; Rathore, M.S. Na+/K+-ATPase a Primary Membrane Transporter: An Overview and Recent Advances with Special Reference to Algae. J. Membr. Biol. 2020, 253, 191–204. [Google Scholar] [CrossRef]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef]
- Suh, S.-J.; Shuman, J.; Carroll, L.P.; Silo-Suh, L. BEEP: An assay to detect bio-energetic and envelope permeability alterations in Pseudomonas aeruginosa. J. Microbiol. Methods 2016, 125, 81–86. [Google Scholar] [CrossRef]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Koko, M.Y.F.; Zhang, S.; Li, Y. Encapsulation of cinnamaldehyde: An insight on delivery systems and food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2521–2543. [Google Scholar] [CrossRef]
- Šajbidor, J. Effect of Some Environmental Factors on the Content and Composition of Microbial Membrane Lipids. Crit. Rev. Biotechnol. 1997, 17, 87–103. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Yao, J.; Rock, C.O. Bacterial fatty acid metabolism in modern antibiotic discovery. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1300–1309. [Google Scholar] [CrossRef]
- Kamischke, C.; Fan, J.; Bergeron, J.; Kulasekara, H.D.; Dalebroux, Z.D.; Burrell, A.; Kollman, J.M.; Miller, S.I. The Acinetobacter baumannii Mla system and glycerophospholipid transport to the outer membrane. eLife 2019, 8, e40171. [Google Scholar] [CrossRef]
- Frank, M.W.; Whaley, S.G.; Rock, C.O. Branched-chain amino acid metabolism controls membrane phospholipid structure in Staphylococcus aureus. J. Biol. Chem. 2021, 297, 101255. [Google Scholar] [CrossRef]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernández, S.H.; Wei, X.; Fan, M. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef]
- Hussein, M.; Karas, J.A.; Schneider-Futschik, E.K.; Chen, F.; Swarbrick, J.; Paulin, O.K.A.; Hoyer, D.; Baker, M.; Zhu, Y.; Li, J.; et al. The Killing Mechanism of Teixobactin against Methicillin-Resistant Staphylococcus aureus: An Untargeted Metabolomics Study. mSystems 2020, 5, 10-1288. [Google Scholar] [CrossRef]
- Yang, S.; Tian, L.; Wang, X.; Wu, M.; Liao, S.; Fu, J.; Xiong, W.; Gong, G. Metabolomics analysis and membrane damage measurement reveal the antibacterial mechanism of lipoic acid against Yersinia enterocolitica. Food Funct. 2022, 13, 11476–11488. [Google Scholar] [CrossRef]
- Tan, S.; Hua, X.; Xue, Z.; Ma, J. Cajanin Stilbene Acid Inhibited Vancomycin-Resistant Enterococcus by Inhibiting Phosphotransferase System. Front. Pharmacol. 2020, 11, 473. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.-C.; Li, D.-D.; Chen, Y.-X.; Fan, X.-J.; Gao, Y.; Ye, H.; Zhang, T.; Zhao, C. Insights into the Mechanisms of Reuterin against Staphylococcus aureus Based on Membrane Damage and Untargeted Metabolomics. Foods 2023, 12, 4208. https://doi.org/10.3390/foods12234208
Sun M-C, Li D-D, Chen Y-X, Fan X-J, Gao Y, Ye H, Zhang T, Zhao C. Insights into the Mechanisms of Reuterin against Staphylococcus aureus Based on Membrane Damage and Untargeted Metabolomics. Foods. 2023; 12(23):4208. https://doi.org/10.3390/foods12234208
Chicago/Turabian StyleSun, Mao-Cheng, Dian-Dian Li, Yu-Xin Chen, Xiu-Juan Fan, Yu Gao, Haiqing Ye, Tiehua Zhang, and Changhui Zhao. 2023. "Insights into the Mechanisms of Reuterin against Staphylococcus aureus Based on Membrane Damage and Untargeted Metabolomics" Foods 12, no. 23: 4208. https://doi.org/10.3390/foods12234208
APA StyleSun, M.-C., Li, D.-D., Chen, Y.-X., Fan, X.-J., Gao, Y., Ye, H., Zhang, T., & Zhao, C. (2023). Insights into the Mechanisms of Reuterin against Staphylococcus aureus Based on Membrane Damage and Untargeted Metabolomics. Foods, 12(23), 4208. https://doi.org/10.3390/foods12234208






