Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours †
Abstract
1. Introduction
2. Materials and Methods
2.1. Grain Samples and Flour Preparation
2.2. Flour Characterization
2.3. Muffin Formulation and Preparation
2.4. Proximate Analysis
2.5. Evaluation of Glycemic Index
2.5.1. In Vivo Protocol
2.5.2. Calculation of Glycemic Index
2.6. Statistical Analysis
3. Results and Discussion
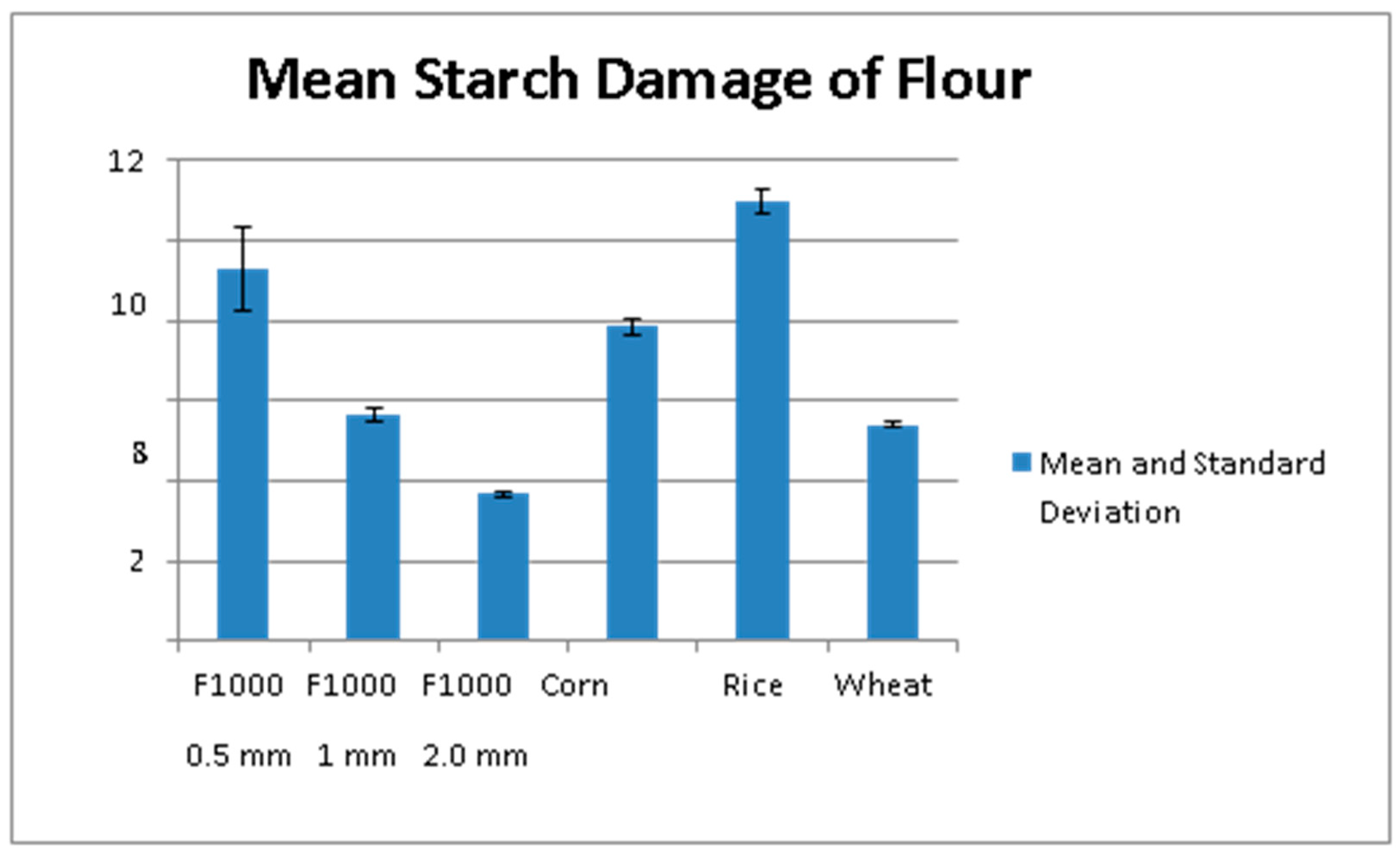
3.1. Flour Particle Size
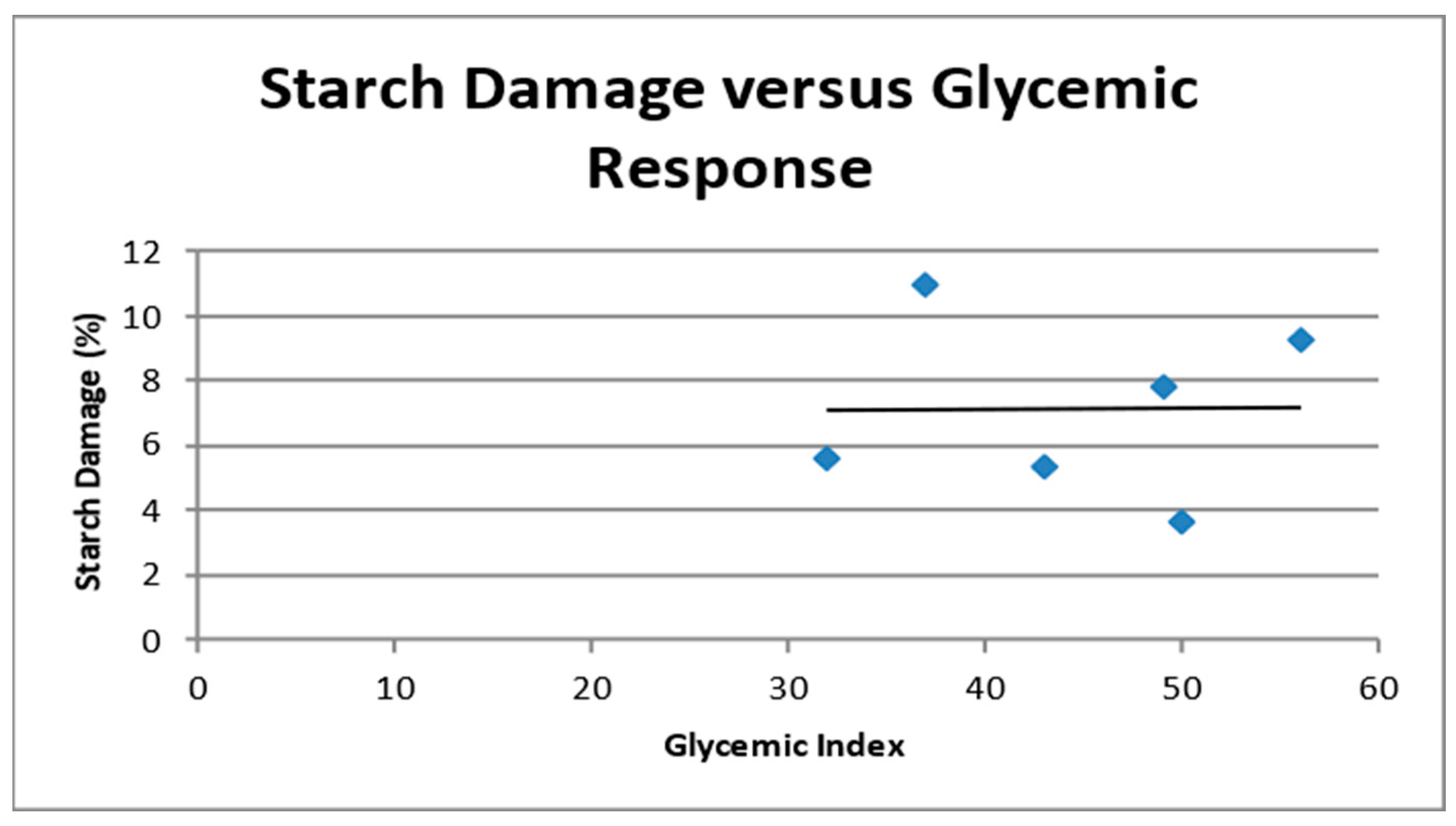
3.2. Flour Properties, Proximate Composition, and Relationships to GI
3.3. GI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J.M. Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; ACS Symposium Series; Awika, J.M., Piironen, V., Bean, S., Eds.; ACS Publications: Washington, DC, USA, 2011; Volume 1089, pp. 1–13. [Google Scholar]
- Friel, S.; Ford, L. Systems, food security and human health. Food Secur. 2015, 7, 437–451. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Borneo, R.; León, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110. [Google Scholar] [CrossRef]
- Milani, P.; Torres-Aquilar, P.; Hamaker, B.; Manary, M.; Abushamma, S.; Laar, A.; Steiner, R.; Ehsani, M.; de la Parra, J.; Skaven-Ruben, D.; et al. The whole grain manifesto: From green revolution to grain evolution. Glob. Food Secur. 2022, 34, 100649. [Google Scholar] [CrossRef]
- Venn, B.; Mann, J. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461. [Google Scholar] [CrossRef]
- Lutsey, P.; Jacobs, D.; Kori, S.; Mayer-Davis, E.; Shea, S.; Steffen, L.; Tracy, R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: The MESA Study. Br. J. Nutr. 2007, 98, 397–405. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: A meta-analysis of observational studies. Food Sci. Nutr. 2019, 7, 256–265. [Google Scholar] [CrossRef]
- Barrett, E.; Batterham, M.; Ray, S.; Beck, E. Whole grain, bran and cereal fibre consumption and CVD: A systematic review. Br. J. Nutr. 2019, 121, 914–937. [Google Scholar] [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef]
- Schacht, S.R.; Olsen, A.; Dragsted, L.O.; Overvad, K.; Tjønneland, A.; Kyrø, C. Whole-grain intake and pancreatic cancer risk—The Danish, diet, cancer and health cohort. J. Nutr. 2021, 151, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Koh-Banerjee, P.; Hu, F.B.; Franz, M.; Sampson, L.; Gronbaek, M.; Rimm, E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004, 80, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Hartvigsen, M.L.; Hedemann, M.S.; Hermansen, K. Mechanisms whereby whole grain cereals modulate the prevention of type 2 diabetes. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 87–103. [Google Scholar] [CrossRef]
- Tosh, S.M.; Bordenave, N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic index response and gut microbiota. Nutr. Rev. 2020, 78, 13–20. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2016, 57, 237–253. [Google Scholar] [CrossRef]
- Monro, J.; Mishra, S. In vitro digestive analysis of digestible and resistant starch fractions, with concurrent glycemic index determination, in whole grain wheat products minimally processed for reduced glycaemic impact. Foods 2020, 11, 1904. [Google Scholar] [CrossRef]
- Ciacci, C.; Maiuri, L.; Caporaso, N.; Bucci, C.; Guidice, L.D.; Massardo, D.R.; Pontieri, P.; Fonza, N.D.; Bean, S.R.; Ioerger, B.; et al. Celiac disease: In vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin. Nutr. 2007, 26, 799–805. [Google Scholar] [CrossRef]
- Pontieri, P.; Mamone, G.; de Caro, S.; Tuintra, M.R.; Roemer, E.; Okot, J.; de Vita, P.; Ficco, D.B.M.; Alifanio, P.; Pignone, D.; et al. Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analysis. J. Agric. Food Chem. 2013, 61, 2565–2571. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Khoddami, A.; Messina, V.; Venkata, K.V.; Farahnaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2023, 63, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.; Garner, P.L.; Mayer, E.P.; Hargrove, J.L.; Hartle, D.K.; Greenspan, P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J. Med. Food 2010, 13, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-induced RAW 264.7 macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic compounds in whole grain sorghum and their health benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, H.-S.; Lee, J.; Amarakoon, D.; Lou, Z.; Noronha, L.E.; Herald, T.J.; Perumal, R.; Smolensky, D. Polyphenol containing sorghum brans exhibit an anti-cancer effect in Apc Min/+ Mice treated with dextran sodium sulfate. Int. J. Mol. Sci. 2021, 22, 8286. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, E.H.; Yeo, M.A.; Kim, S.J.; Seo, M.C.; Moon, H.I. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res. Int. 2011, 44, 127–132. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Emmambux, M.N. Developments in our understanding of sorghum polysaccharides and their health benefits. Cereal Chem. 2010, 87, 263–271. [Google Scholar] [CrossRef]
- Simnadis, T.G.; Tapsell, L.C.; Beck, E.J. Effect of sorghum consumption on health outcomes: A systematic review. Nutr. Rev. 2016, 74, 690–707. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Xiong, Y.X.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Comp. Rev. Food Sci. Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Chaloulos, P.; Papalexi, A.; Mandala, I. Effects of bran size and carob seed flour of optimized bread formulas on glycemic responses in humans: A randomized clinical trial. J. Funct. Foods 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Kathirvel, P.; Yamazaki, Y.; Zhu, W.; Luhovyy, B. Glucose release from lentil flour digested in vitro: The role of particle size. Cereal Chem. 2019, 96, 1126–1136. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Mann, J.; Elbalshy, M.; Mete, E.; Robinson, C.; Oey, I.; Silcock, P.; Downes, N.; Perry, T.; Morenga, L.E. Whole grain particle size influences postprandial glycemia in type 2 diabetes: A randomized crossover study comparing four wholegrain breads. Diabetes Care 2020, 43, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.B.J. Criteria of flour quality. In Wheat Chemistry and Technology; Pomeranz, Y., Ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1978; p. 212. [Google Scholar]
- Campbell, G.M.; Muhamad, C.F. On predicting roller milling performance VI: Effect of kernel hardness and shape on the particle size distribution from first break milling of wheat. Food BioProd. Proc. 2007, 85, 7–23. [Google Scholar] [CrossRef]
- Chen, J.; D’Appolonia, B.L. Effect of starch damage and oxidizing agents on alveogram properties. In Fundamentals of Dough Rheology; Faridi, H., Faubion, J.M., Eds.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1986; pp. 117–129. [Google Scholar]
- Evers, A.D.; Stevens, D.J. Starch damaged. In Advances in Cereal Science and Technology; Pomeranz, Y., Ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1985; Volume VII, pp. 321–349. [Google Scholar]
- Diabetes Council. 20 Healthy Flours from Lowest to Highest Carbohydrates. Available online: https://www.thediabetescouncil.com/20-healthy-flours/ (accessed on 8 November 2023).
- The University of Sydney Glycemic Index Research Service—Glycemic Index Search. Available online: https://glycemicindex.com/gi-search/ (accessed on 8 November 2023).
- Shobana, S.; Geetha, G.; Bai, M.R.; Vijayalakshmi, P.; Gayathri, R.; Lakshmipriya, N.; Unnikrishnan, R.; Anjana, R.M.; Malleshi, N.G.; Krishnaswamy, K.; et al. Carbohydrate profiling & glycaemic indices of selected traditional Indian foods. Indian J. Med. Res. 2022, 155, 55–56. [Google Scholar] [CrossRef]
- Moraes, E.M.; da Silva Marineli, R.; Lenquiste, S.A.; Steel, C.J.; Menezes, C.B.; Queiroz, V.A.V.; Marostica, M.R., Jr. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef]
- Prasad, M.P.R.; Rao, B.D.; Kalpana, K.; Rao, M.V.; Patil, J.V. Glycaemic index and glycaemic load of sorghum products. J. Sci. Food Agric. 2014, 95, 1626–1630. [Google Scholar] [CrossRef]
- Holt, S.H.; Miller, J.B. Particle size, satiety and the glycaemic response. Eur. J. Clin. Nutr. 1994, 48, 496–502. [Google Scholar]
- O’Dea, K.; Nestel, P.J.; Antonoff, L. Physical factors influencing postprandial glucose and insulin responses to starch. Am. J. Clin. Nutr. 1980, 33, 760–765. [Google Scholar] [CrossRef]
- O’Donnell, L.J.D.; Emmett, P.M.; Heaton, K.W. Size of flour particles and its relation to glycaemia, insulinaemia, and colonic disease. Brit. Med. J. 1989, 298, 1616–1617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. The Effect of Particle Size of Whole-Grain Flour on Plasma Glucose, Insulin, Glucagon and Thyroid-Stimulating Hormone in Humans. J. Am. Coll. Nutr. 1999, 18, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Flavel, M.; Jois, M.; Kitchen, B. Potential contributions of the methodology to the variability of glycaemic index of foods. World J. Diabetes 2021, 12, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Pyler, E.J.; Gorton, L.A. Baking Science & Technology. Volume I: Fundamentals and Ingredients, 4th ed.; Sosland Publishing Co.: Kansas City, MO, USA, 2009; p. 127. [Google Scholar]
- Khan, K.; Shewry, P.R. Wheat: Chemistry and Technology, 4th ed.; AACC International: St. Paul, MN, USA, 2009; p. 141. [Google Scholar]
- Berdanier, C.D. Carbohydrate Metabolism: Regulation and Physiological Role; Hemisphere Publishing Corporation: Washington, DC, USA, 1976; Volume 1. [Google Scholar]
| Flour Type | Water Amount (g) |
|---|---|
| Sorghum (Fine) | 415 g |
| Sorghum (Intermediate) | 350 g |
| Sorghum (Coarse) | 315 g |
| Corn | 400 g |
| Rice | 385 g |
| Whole Wheat | 385 g |
| All-Purpose | 375 g |
| Flour Type | Serving Size (g) |
|---|---|
| Sorghum (Fine) | 53.3 |
| Sorghum (Intermediate) | 47.8 |
| Sorghum (Coarse) | 55.0 |
| Corn | 49.1 |
| Rice | 44.3 |
| Whole Wheat | 55.0 |
| All-Purpose | 45.0 |
| Flour Type | Mean d90 Flour Particle Size Distribution (μm) | Mean Particle Size Distribution (μm) |
|---|---|---|
| Sorghum (fine) | 190.9 ± 0.6 | 82.2 ± 1.1 |
| Sorghum (intermediate) | 394.4 ± 11.4 | 166.9 ± 4.0 |
| Sorghum (coarse) | 731.7 ± 42.6 | 303.3 ± 15.5 |
| Corn (fine | 246.6 ± 47.1 | 92.6 ± 16.1 |
| Rice (fine) | 154.2 ± 2.6 | 67.9 ± 0.4 |
| Wheat (fine) | 307.4 ± 2.2 | 325.1 ± 4.5 |
| Muffin Type | %Moisture | % Crude Protein | % Crude Fat | % Crude Fiber | % Ash | % Total Carbohydrate |
|---|---|---|---|---|---|---|
| Sorghum (fine) | 55.62 ± 0.20 a | 4.38 ± 0.20 e | 0.79 ± 0.01 c | 0.38 ± 0.01 c | 1.33 ± 0.01 cd | 37.50 ± 0.20 e |
| Sorghum (intermediate) | 50.25 ± 0.04 c | 4.87 ± 0.05 d | 0.97 ± 0.02 b | 0.52 ± 0.03 b | 1.54 ± 0.01 ab | 41.80 ± 0.14 c |
| Sorghum (coarse) | 55.47 ± 0.01 a | 5.05 ± 0.02 c | 1.02 ± 0.01 b | 0.70 ± 0.02 a | 1.34 ± 0.02 cd | 36.41 ± 0.00 f |
| Corn (fine) | 52.73 ± 0.21 b | 3.74 ± 0.01 g | 1.17 ± 0.00 a | 0.28 ± 0.02 d | 1.38 ± 0.02 c | 40.72 ± 0.18 d |
| Rice (fine) | 48.81 ± 0.07 d | 4.15 ± 0.03 f | 0.29 ± 0.01 d | 0.06 ± 0.0 e | 1.50 ± 0.02 b | 45.20 ± 0.07 a |
| Wheat (fine) | 52.96 ± 0.05 b | 8.05 ± 0.05 a | 0.31 ± 0.00 d | 0.65 ± 0.02 a | 1.58 ± 0.01 a | 36.40 ± 0.03 f |
| All-purpose | 47.71 ± 0.10 d | 6.80 ± 0.02 b | 0.14 ± 0.00 e | 0.02 ± 0.00 e | 1.31 ± 0.01 d | 44.00 ± 0.07 b |
| Sorghum (Fine) | Sorghum (Intermediate) | Sorghum (Coarse) | Corn (Fine) | Rice (Fine) | Wheat (Fine) | All-Purpose Flour | |
|---|---|---|---|---|---|---|---|
| GI | 56 ± 33 b | 32 ± 17 a | 50 ± 26 ab | 49 ± 29 ab | 37 ± 18 a | 43 ± 23 ab | 44 ± 22 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruett, A.; Aramouni, F.M.; Bean, S.R.; Haub, M.D. Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours. Foods 2023, 12, 4188. https://doi.org/10.3390/foods12234188
Pruett A, Aramouni FM, Bean SR, Haub MD. Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours. Foods. 2023; 12(23):4188. https://doi.org/10.3390/foods12234188
Chicago/Turabian StylePruett, Ashley, Fadi M. Aramouni, Scott R. Bean, and Mark D. Haub. 2023. "Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours" Foods 12, no. 23: 4188. https://doi.org/10.3390/foods12234188
APA StylePruett, A., Aramouni, F. M., Bean, S. R., & Haub, M. D. (2023). Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours. Foods, 12(23), 4188. https://doi.org/10.3390/foods12234188





