Anthocyanin-Rich Butterfly Pea Petal Extract Loaded Double Pickering Emulsion Containing Nanocrystalline Cellulose: Physicochemical Properties, Stability, and Rheology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Double Pickering Emulsion Preparation
2.3. Particle Size and ζ-Potential Measurement
2.4. Visual Creaming Assessment
2.5. Color
2.6. Rheological Properties
2.7. Confocal Laser Scanning Microscopy (CLSM)
2.8. Encapsulation Efficiency (EE)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Anthocyanin-Loaded Double Pickering Emulsions
3.1.1. Particle Size and Distribution
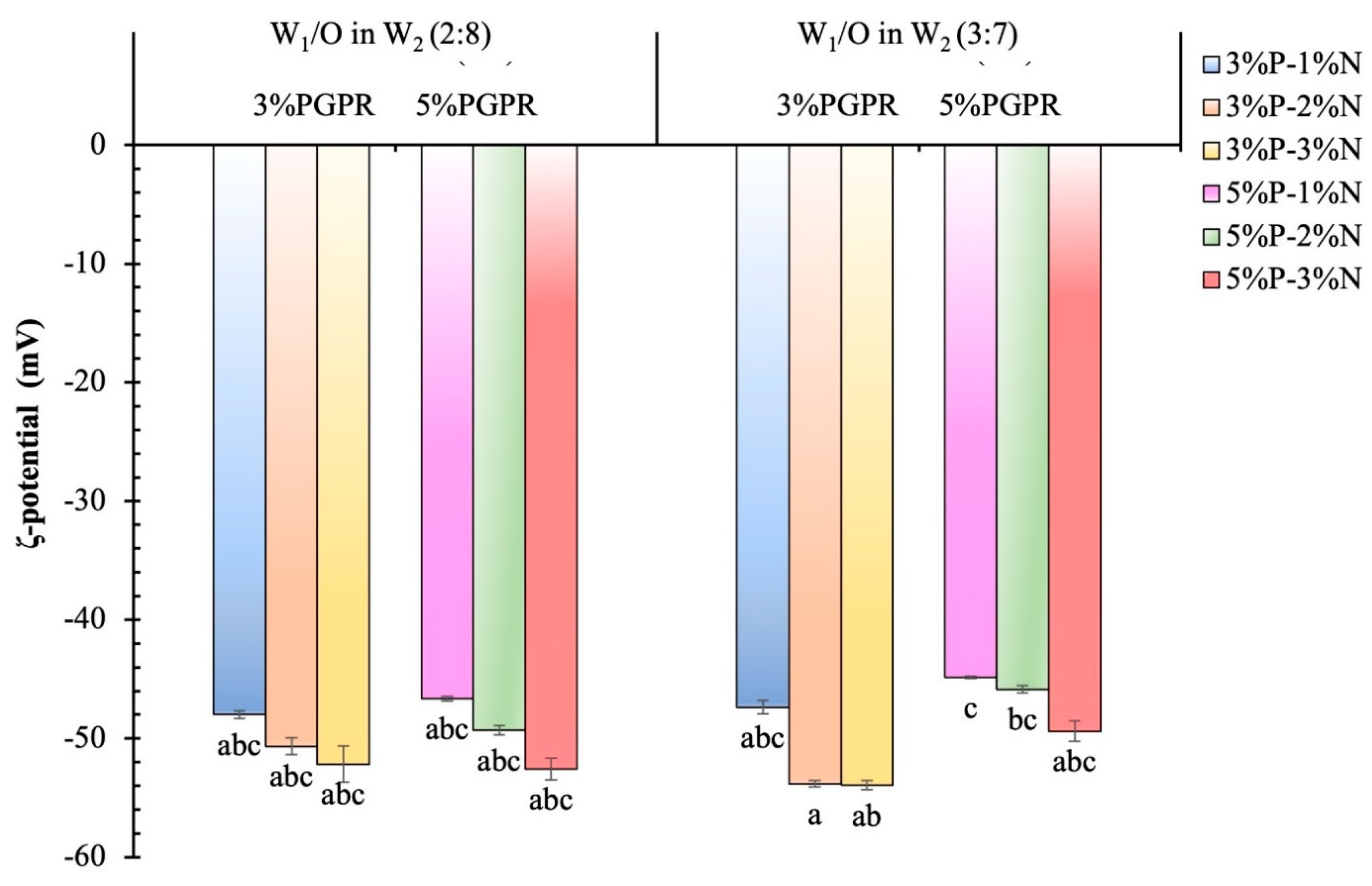
3.1.2. ζ-Potential
3.1.3. Visual Appearance
3.1.4. Visual Creaming
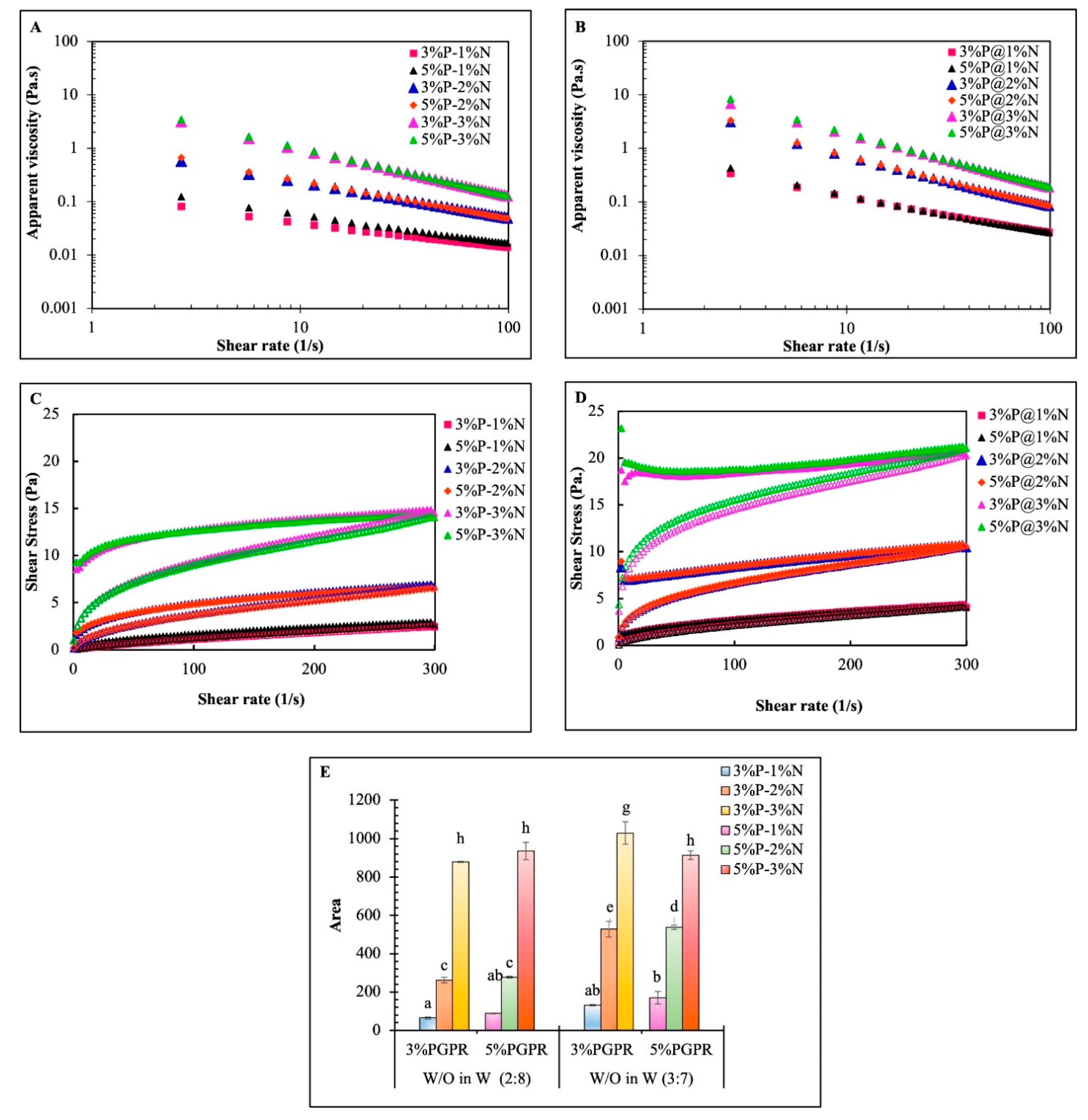
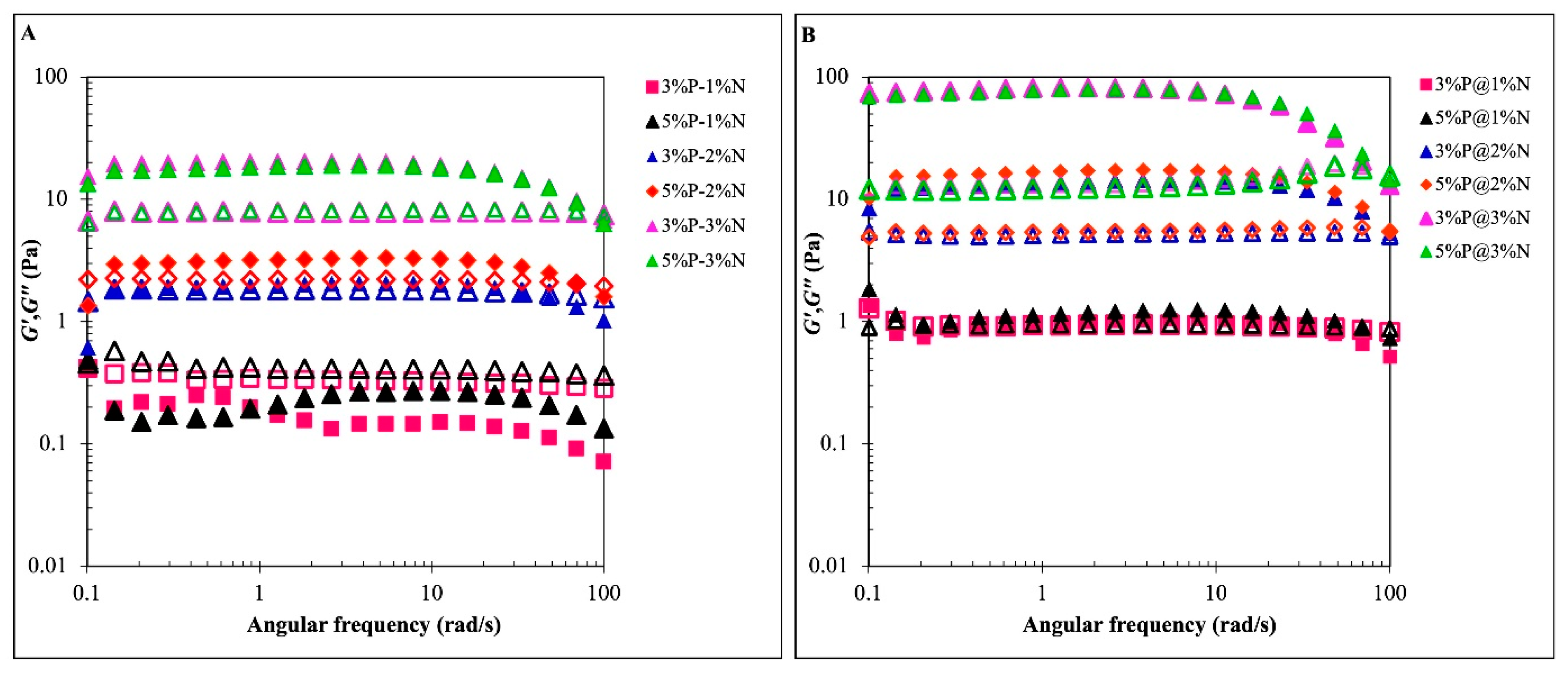
3.2. Rheological Properties
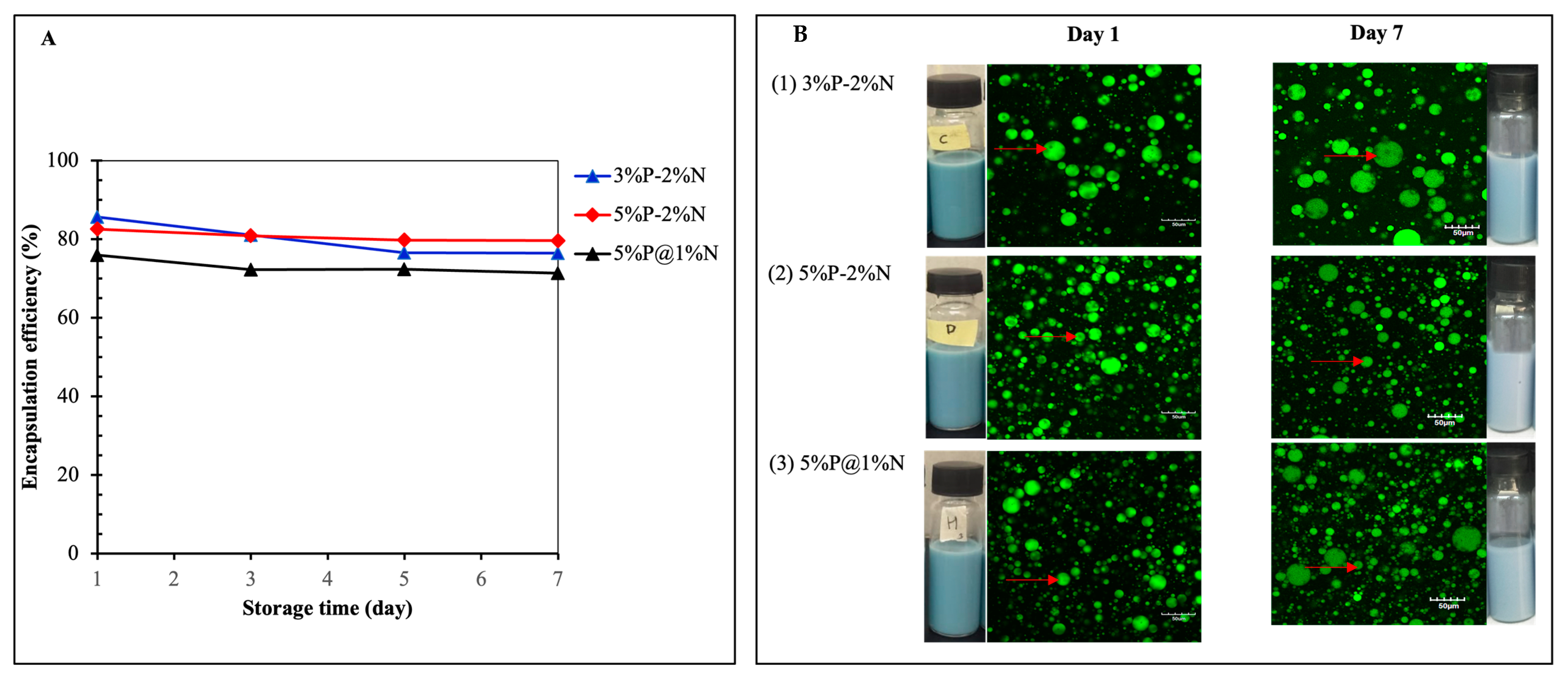
3.3. Encapsulation Efficiency and Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, T.; Xie, Y.; Li, N.; Liu, Y.; Wen, J.; Zhang, M.; Feng, W.; Huang, J.; Guo, Y. Clitoria ternatea blue petal extract protects against obesity, oxidative stress, and inflammation induced by a high-fat, high-fructose diet in C57BL/6 mice. Food Res. Int. 2022, 162, 112008. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Escher, G.B.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; da Silva, M.C.; Genovese, M.I.; Wen, M.; Zhang, L.; et al. Clitoria ternatea L. petal bioactive compounds display antioxidant, antihemolytic and antihypertensive effects, inhibit α-amylase and α-glucosidase activities and reduce human LDL cholesterol and DNA induced oxidation. Food Res. Int. 2020, 128, 108763. [Google Scholar] [CrossRef]
- Escher, G.B.; Wen, M.; Zhang, L.; Rosso, N.D.; Granato, D. Phenolic composition by UHPLC-Q-TOF-MS/MS and stability of anthocyanins from Clitoria ternatea L. (butterfly pea) blue petals. Food Chem. 2020, 331, 127341. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Osvaldt Rosales, T.K.; Pessoa da Silva, M.; Lourenço, F.R.; Aymoto Hassimotto, N.M.; Fabi, J.P. Nanoencapsulation of anthocyanins from blackberry (Rubus spp.) through pectin and lysozyme self-assembling. Food Hydrocoll. 2021, 114, 106563. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A. Double Emulsions. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 303–337. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Yin, J.; Chang, F.; Wang, C.; He, X.; Huang, Q.; Zhang, B. Anthocyanin-loaded double Pickering emulsion stabilized by octenylsuccinate quinoa starch: Preparation, stability and in vitro gastrointestinal digestion. Int. J. Biol. Macromol. 2020, 152, 1233–1241. [Google Scholar] [CrossRef]
- Nimaming, N.; Sadeghpour, A.; Murray, B.S.; Sarkar, A. Hybrid particles for stabilization of food-grade Pickering emulsions: Fabrication principles and interfacial properties. Trends Food Sci Technol. 2023, 138, 671–684. [Google Scholar] [CrossRef]
- Rescignano, N.; Fortunati, E.; Armentano, I.; Hernandez, R.; Mijangos, C.; Pasquino, R.; Kenny, J.M. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. J. Colloid Interface Sci. 2015, 445, 31–39. [Google Scholar] [CrossRef]
- Nurhadi, B.; Sulaeman, M.Y.; Mahani. Antioxidant stability of vitamin C in double Pickering emulsion W/O/W with microcrystalline cellulose. Int. J. Food Prop. 2023, 26, 567–580. [Google Scholar] [CrossRef]
- Fitri, I.A.; Mitbumrung, W.; Akanitkul, P.; Rungraung, N.; Kemsawasd, V.; Jain, S.; Winuprasith, T. Encapsulation of β-Carotene in Oil-in-Water Emulsions Containing Nanocellulose: Impact on Emulsion Properties, In Vitro Digestion, and Bioaccessibility. Polymers 2022, 14, 1414. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Niknam, R.; Mousavi, M.; Kiani, H. Effect of ultrasonication on rheological aspects and storage stability of O/W emulsions containing Gleditsia caspica galactomannan–Trigonella foenum–graceum galactomannan mixtures. Appl. Food Res. 2022, 2, 100109. [Google Scholar] [CrossRef]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Adun, P. Comprehensive study of intrinsic viscosity, steady and oscillatory shear rheology of Barhang seed hydrocolloid in aqueous dispersions. J. Food Process Eng. 2019, 42, e13047. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Du Le, H.; Loveday, S.M.; Singh, H.; Sarkar, A. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Panagopoulou, E.; Evageliou, V.; Kopsahelis, N.; Ladakis, D.; Koutinas, A.; Mandala, I. Stability of double emulsions with PGPR, bacterial cellulose and whey protein isolate. Colloids Surf. A Physicochem. Eng. 2017, 522, 445–452. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef]
- Saidane, D.; Perrin, E.; Cherhal, F.; Guellec, F.; Capron, I. Some modification of cellulose nanocrystals for functional Pickering emulsions. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150139. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Lu, S.; Fujii, Y.; Jin, J.; Xia, Q. Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum. Foods 2023, 12, 151. [Google Scholar] [CrossRef]
- Saechio, S.; Akanitkul, P.; Thiyajai, P.; Jain, S.; Tangsuphoom, N.; Suphantharika, M.; Winuprasith, T. Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility. Polymers 2023, 15, 901. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Zhou, H.; Muriel Mundo, J.L.; McClements, D.J. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J. Food Eng. 2019, 254, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Abbaspourrad, A. Phycocyanin-rich water-in-oil-in-water (W1/O/W2) double emulsion with nanosized particles: Improved color stability against light exposure. Colloids Surf. B 2022, 220, 112930. [Google Scholar] [CrossRef]
- McClements, D.J. Enhancing nutraceutical bioavailability through food matrix design. Curr. Opin. Food Sci. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and tribology of nanocellulose-based biodegradable greases: Wear and friction protection mechanisms of cellulose microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Saffarionpour, S. Nanocellulose for Stabilization of Pickering Emulsions and Delivery of Nutraceuticals and Its Interfacial Adsorption Mechanism. Food Bioproc. Technol. 2020, 13, 1292–1328. [Google Scholar] [CrossRef]
- Øye, G.; Simon, S.; Rustad, T.; Paso, K. Trends in food emulsion technology: Pickering, nano-, and double emulsions. Curr. Opin. Food Sci. 2023, 50, 101003. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-grade Pickering emulsions: Preparation, stabilization and applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, L.; Lv, C.; Guo, S.; Lu, X.; Tao, L.; Duan, Q.; Yang, Q.; Luo, Z. Preparation and characterization of pH-responsive Pickering emulsion stabilized by grafted carboxymethyl starch nanoparticles. Int. J. Biol. Macromol. 2020, 143, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, W. Chapter 12—Coacervation Processes. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 125–137. [Google Scholar] [CrossRef]
- Miao, C.; Mirvakili, M.-N.; Hamad, W.Y. A rheological investigation of oil-in-water Pickering emulsions stabilized by cellulose nanocrystals. J. Colloid Interface Sci. 2022, 608, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. Rheology of simple and multiple emulsions. Curr. Opin. Colloid Interface Sci. 2011, 16, 41–60. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Properties and stability of oil-in-water emulsions stabilized by microfibrillated cellulose from mangosteen rind. Food Hydrocoll. 2015, 43, 690–699. [Google Scholar] [CrossRef]
- Choi, S.B.; Park, J.Y.; Moon, J.Y.; Lee, J.S. Effect of interactions between multiple interfaces on the rheological characteristics of double emulsions. Phys. Rev. E 2018, 97, 062603. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Bannwart, A.C.; Loh, W. Phase segregation, shear thinning and rheological behavior of crude oil-in-water emulsions. Chem. Eng. Res. Des. 2014, 92, 1629–1636. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Y.; Shi, C.; Ye, Y.; Abid, M.; Jabbar, S.; Wang, P.; Zeng, X.; Wu, T. Rheological properties of an amorphous cellulose suspension. Food Hydrocoll. 2014, 39, 27–33. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Durand, S.; Berot, S.; Blassel, C.; Gaillard, C.; Garnier, C.; Doublier, J.L. Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr. Polym. 2010, 80, 677–686. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of resveratrol using food-grade concentrated double emulsions: Emulsion characterization and rheological behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.; Kim, H. Rheological analysis of physical states of cellulose nanocrystal suspension and synergetic effect of aligned gel state. Carbohydr. Polym. 2022, 284, 119170. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and rheology of heavy crude oil/water emulsions stabilized by microfibrillated cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]

| Sample Code | Treatments | Phase Volume (W1:O) in W2 | PGPR (%) | NCC (%) |
|---|---|---|---|---|
| A | 3%P-1%N | 2:8 | 3 | 1 |
| B | 5%P-1%N | 2:8 | 5 | 2 |
| C | 3%P-2%N | 2:8 | 3 | 3 |
| D | 5%P-2%N | 2:8 | 5 | 1 |
| E | 3%P-3%N | 2:8 | 3 | 2 |
| F | 5%P-3%N | 2:8 | 5 | 3 |
| G | 3%P@1%N | 3:7 | 3 | 1 |
| H | 5%P@1%N | 3:7 | 5 | 2 |
| I | 3%P@2%N | 3:7 | 3 | 3 |
| J | 5%P@2%N | 3:7 | 5 | 1 |
| K | 3%P@3%N | 3:7 | 3 | 2 |
| L | 5%P@3%N | 3:7 | 5 | 3 |
| Sample | L* | a* | b* | D [3,2] (μm) |
|---|---|---|---|---|
| 3%P-1%N | 49.0 ± 1.2 ab | −10.8 ± 0.2 a | −10.1 ± 0.8 a | 12.3 ± 0.8 bc |
| 5%P-1%N | 49.7 ± 2.1 abcd | −10.5 ± 0.2 bcd | −10.3 ± 0.8 a | 11.0 ± 0.9 abc |
| 3%P-2%N | 48.8 ± 0.2 a | −10.6 ± 0.1 abc | −10.4 ± 0.5 a | 12.4 ± 0.7 c |
| 5%P-2%N | 50.5 ± 0.6 abcd | −10.2 ± −0.1 de | −10.1 ± 0.6 a | 9.9 ± 1.1 a |
| 3%P-3%N | 50.4 ± 1.1 abcd | −10.4 ± 0.1 cde | −9.4 ± 1.0 a | 12.4 ± 0.6 c |
| 5%P-3%N | 51.8 ± 1.5 d | −10.2 ± 0.1 e | −9.4 ± 1.0 a | 9.9 ± 0.9 a |
| 3%P@1%N | 50.3 ± 0.6 abcd | −10.8 ± 0.1 a | −10.0 ± 0.3 a | 10.5 ± 0.5 ab |
| 5%P@1%N | 50.3 ± 2.2 abcd | −10.4 ± 0.2 cd | −10.3 ± 0.6 a | 10.7 ± 1.2 abc |
| 3%P@2%N | 49.1 ± 0.6 abc | −10.8 ± 0.2 ab | −9.9 ± 0.6 a | 12.6 ± 0.9 bc |
| 5%P@2%N | 50.1 ± 0.9 abcd | −10.6 ± 0.1 abc | −10.2 ± 0.5 a | 10.4 ± 0.2 abc |
| 3%P@3%N | 51.3 ± 0.1 cd | −10.4 ± 0.2 cd | −9.2 ± 0.3 a | 11.3 ± 0.8 abc |
| 5%P@3%N | 51.6 ± 1.0 bcd | −10.2 ± 0.1 de | −9.3 ± 0.5 a | 10.4 ± 1.1 a |
| W1/O in W2 (w/v) (2:8) | W1/O in W2 (w/v) (3:7) | ||||||
|---|---|---|---|---|---|---|---|
| Sample | K (Pa·sn) | n (-) | R2 | Sample | K (Pa·sn) | n (-) | R2 |
| 3%P-1%N | 0.033 | 0.351 | 0.988 | 3%P@1%N | 0.183 | 0.341 | 0.976 |
| 5%P-1%N | 0.063 | 0.333 | 0.981 | 5%P@1%N | 0.348 | 0.327 | 0.958 |
| 3%P-2%N | 0.217 | 0.533 | 0.958 | 3%P@2%N | 2.968 | 0.427 | 0.846 |
| 5%P-2%N | 0.339 | 0.547 | 0.957 | 5%P@2%N | 3.069 | 0.353 | 0.829 |
| 3%P-3%N | 2.041 | 0.685 | 0.837 | 3%P@3%N a | - | - | - |
| 5%P-3%N a | - | - | - | 5%P@3%N a | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, P.; Sriprablom, J.; Winuprasith, T. Anthocyanin-Rich Butterfly Pea Petal Extract Loaded Double Pickering Emulsion Containing Nanocrystalline Cellulose: Physicochemical Properties, Stability, and Rheology. Foods 2023, 12, 4173. https://doi.org/10.3390/foods12224173
Koirala P, Sriprablom J, Winuprasith T. Anthocyanin-Rich Butterfly Pea Petal Extract Loaded Double Pickering Emulsion Containing Nanocrystalline Cellulose: Physicochemical Properties, Stability, and Rheology. Foods. 2023; 12(22):4173. https://doi.org/10.3390/foods12224173
Chicago/Turabian StyleKoirala, Pankaj, Jiratthitikan Sriprablom, and Thunnalin Winuprasith. 2023. "Anthocyanin-Rich Butterfly Pea Petal Extract Loaded Double Pickering Emulsion Containing Nanocrystalline Cellulose: Physicochemical Properties, Stability, and Rheology" Foods 12, no. 22: 4173. https://doi.org/10.3390/foods12224173
APA StyleKoirala, P., Sriprablom, J., & Winuprasith, T. (2023). Anthocyanin-Rich Butterfly Pea Petal Extract Loaded Double Pickering Emulsion Containing Nanocrystalline Cellulose: Physicochemical Properties, Stability, and Rheology. Foods, 12(22), 4173. https://doi.org/10.3390/foods12224173







