Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Essential Oil
2.3. Sample of Beef Meat Preparation
2.4. Cultivation of the Samples
2.5. Identification of Bacteria with Mass Spectrometry
2.6. MALDI-TOF Matrix Solution Preparation
2.7. Identification of Microorganisms
2.8. Statistical Evaluations
3. Results
3.1. Number of Bacteria in log CFU/g
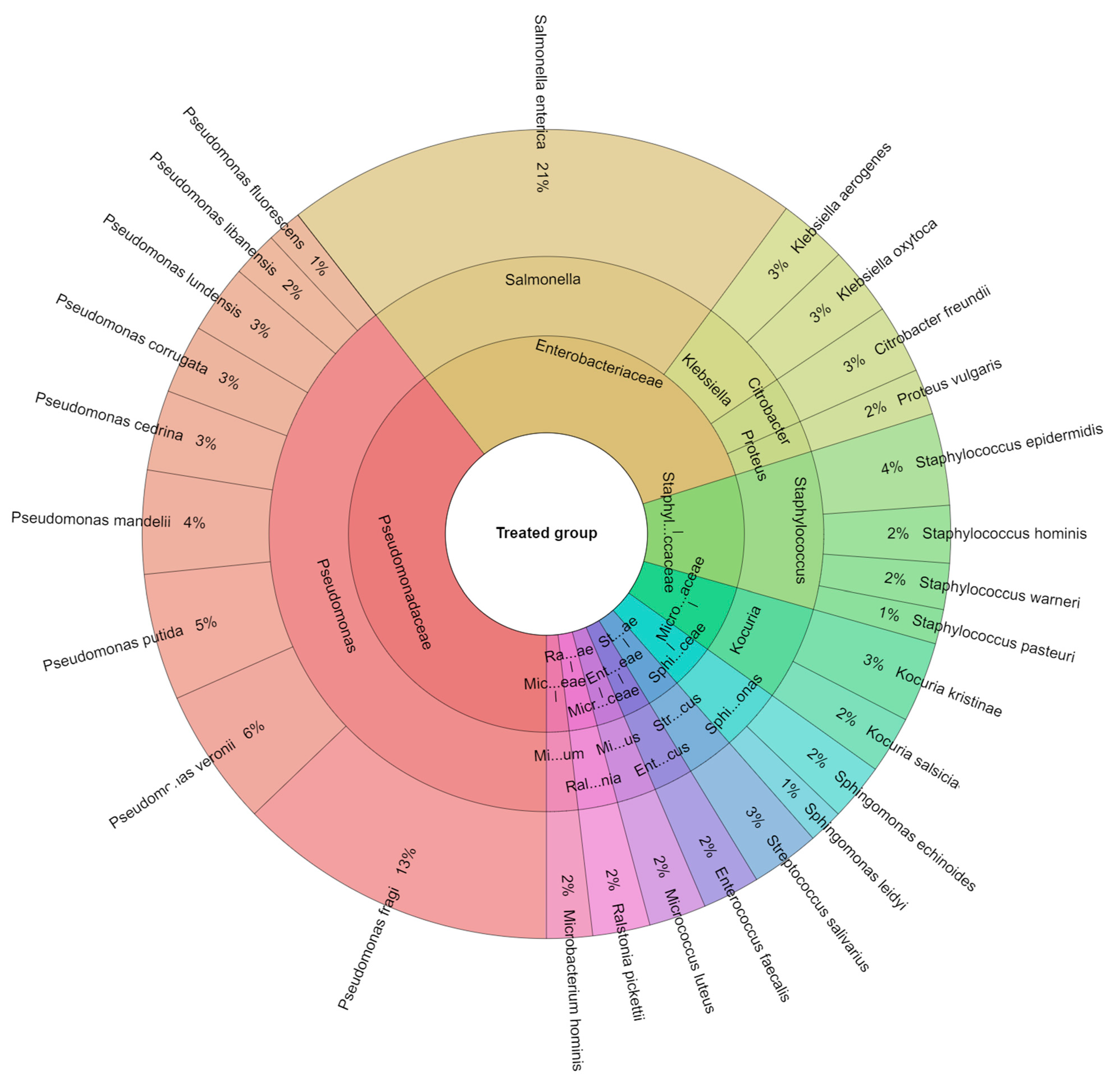
3.2. Isolated Bacteria from Beef Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Hunt, H.B.; Watson, S.C.; Chaves, B.D.; Sullivan, G.A. Inactivation of Salmonella in Nonintact Beef during Low-Temperature Sous Vide Cooking. J. Food Prot. 2023, 86, 100010. [Google Scholar] [CrossRef]
- Rhoades, J.R.; Duffy, G.; Koutsoumanis, K. Prevalence and Concentration of Verocytotoxigenic Escherichia coli, Salmonella enterica and Listeria monocytogenes in the Beef Production Chain: A Review. Food Microbiol. 2009, 26, 357–376. [Google Scholar] [CrossRef]
- Kargiotou, C.; Katsanidis, E.; Rhoades, J.; Kontominas, M.; Koutsoumanis, K. Efficacies of Soy Sauce and Wine Base Marinades for Controlling Spoilage of Raw Beef. Food Microbiol. 2011, 28, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Labuza, T.P.; Diez-Gonzalez, F. Comparison of Primary Predictive Models to Study the Growth of Listeria monocytogenes at Low Temperatures in Liquid Cultures and Selection of Fastest Growing Ribotypes in Meat and Turkey Product Slurries. Food Microbiol. 2008, 25, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Berends, B.R.; Van Knapen, F.; Mossel, D.A.A.; Burt, S.A.; Snijders, J.M.A. Impact on Human Health of Salmonella spp. on Pork in The Netherlands and the Anticipated Effects of Some Currently Proposed Control Strategies. Int. J. Food Microbiol. 1998, 44, 219–229. [Google Scholar] [CrossRef]
- Wong, T.L.; Nicol, C.; Cook, R.; Macdiarmid, S. Salmonella in Uncooked Retail Meats in New Zealand. J. Food Prot. 2007, 70, 1360–1365. [Google Scholar] [CrossRef]
- Botteldoorn, N.; Heyndrickx, M.; Rijpens, N.; Grijspeerdt, K.; Herman, L. Salmonella on Pig Carcasses: Positive Pigs and Cross Contamination in the Slaughterhouse. J. Appl. Microbiol. 2003, 95, 891–903. [Google Scholar] [CrossRef][Green Version]
- Meyer, C.; Thiel, S.; Ullrich And, U.; Stolle, A. Salmonella in Raw Meat and By-Products from Pork and Beef. J. Food Prot. 2010, 73, 1780–1784. [Google Scholar] [CrossRef]
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 765–801. ISBN 978-1-68367-058-2. [Google Scholar]
- Himanshu; Lanjouw, P.; Stern, N. How Lives Change; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-880650-9. [Google Scholar]
- Skandamis, P.; Tsigarida, E.; Nychas, G.-J.E. The Effect of Oregano Essential Oil on Survival/Death of Salmonella typhimurium in Meat Stored at 5 °C under Aerobic, VP/MAP Conditions. Food Microbiol. 2002, 19, 97–103. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical Composition and Biological Activity of Salvia officinalis Essential Oil. Acta Hortic. Regiotect. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Gál, R.; Čmiková, N.; Prokopová, A.; Kačániová, M. Antilisterial and Antimicrobial Effect of Salvia officinalis Essential Oil in Beef Sous-Vide Meat during Storage. Foods 2023, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in Cancer Invasion and Metastasis: Phytotherapeutics in Cancer Invasion and Metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef]
- Chaachouay, N. Ethnobotanical and Ethnopharmacological Study of Medicinal and Aromatic Plants Used in the Treatment of Neurological Disorders in the Moroccan Rif. J. Integr. Med. 2020, 8, e02191. [Google Scholar] [CrossRef]
- Snow Setzer, M.; Sharifi-Rad, J.; Setzer, W. The Search for Herbal Antibiotics: An In-Silico Investigation of Antibacterial Phytochemicals. Antibiotics 2016, 5, 30. [Google Scholar] [CrossRef]
- Bagheri, G.; Mirzaei, M.; Mehrabi, R.; Sharifi-Rad, J. Cytotoxic and Antioxidant Activities of Alstonia scholaris, Alstonia venenata and Moringa oleifera Plants from India. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e31129. [Google Scholar] [CrossRef]
- Nikoli, B.; Miti-ulafi, D.; Vukovi-Gai, B.; Kneevi-Vukevi, J. Molecular Mechanisms of Action of Antimutagens from Sage (Salvia officinalis) and Basil (Ocimum basilicum). In Mutagenesis; Mishra, R., Ed.; InTechOpen: London, UK, 2012; ISBN 978-953-51-0707-1. [Google Scholar]
- Marino, M.; Bersani, C.; Comi, G. Impedance Measurements to Study the Antimicrobial Activity of Essential Oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. Essential Oils: Their Chemical Compositions and Their Preservative Effects against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- O’Brien, T.F. Emergence, Spread, and Environmental Effect of Antimicrobial Resistance: How Use of an Antimicrobial Anywhere Can Increase Resistance to Any Antimicrobial Anywhere Else. Clin. Infect. Dis. 2002, 34, S78–S84. [Google Scholar] [CrossRef]
- Karamanos, A.J. Cultivation and Breeding. In The Cultivation of Sage, 14th ed.; Kintzios, S.E., Ed.; Taylor & Francis e-Library: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Sensoy, N.D. Obtaining of Natural Antioxidant from Sage Leaves (Salvia officinalis) with Supercritical Carbon Dioxide Extraction. Master’s Thesis, Institute of Science and Technology, Gazi University, Ankara, Turkey, 2007. [Google Scholar]
- Mohammad, S.M. A Study on Sage (Salvia officinalis). J. Appl. Sci Res. 2011, 7, 1261–1262. [Google Scholar]
- Karyotis, D.; Skandamis, P.N.; Juneja, V.K. Thermal Inactivation of Listeria monocytogenes and Salmonella spp. in Sous-Vide Processed Marinated Chicken Breast. Food Res. Int. 2017, 100, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Bari, M.L.; Inatsu, Y.; Kawamoto, S.; Friedman, M. Thermal Destruction of Escherichia Coli O157:H7 in Sous-Vide Cooked Ground Beef as Affected by Tea Leaf and Apple Skin Powders. J. Food Prot. 2009, 72, 860–865. [Google Scholar] [CrossRef]
- Juneja, V.K.; Osoria, M.; Tiwari, U.; Xu, X.; Golden, C.E.; Mukhopadhyay, S.; Mishra, A. The Effect of Lauric Arginate on the Thermal Inactivation of Starved Listeria monocytogenes in Sous-Vide Cooked Ground Beef. Food Res. Int. 2020, 134, 109280. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.; Boulaaba, A.; Lis, K.; Abdulmawjood, A.; Plötz, M.; Becker, A. Inactivation of Listeria monocytogenes in Game Meat Applying Sous Vide Cooking Conditions. Meat Sci. 2020, 167, 108164. [Google Scholar] [CrossRef]
- Smith, C.J.; Olszewska, M.A.; Diez-Gonzalez, F. Selection and Application of Natural Antimicrobials to Control Clostridium perfringens in Sous-Vide Chicken Breasts. Int. J. Food Microbiol. 2021, 347, 109193. [Google Scholar] [CrossRef]
- Nissen, H.; Rosnes, J.T.; Brendehaug, J.; Kleiberg, G.H. Safety Evaluation of Sous Vide-Processed Ready Meals. Lett. Appl. Microbiol. 2002, 35, 433–438. [Google Scholar] [CrossRef]
- Hyytiä-Trees, E.; Skyttä, E.; Mokkila, M.; Kinnunen, A.; Lindström, M.; Lähteenmäki, L.; Ahvenainen, R.; Korkeala, H. Safety Evaluation of Sous Vide-Processed Products with Respect to Nonproteolytic Clostridium botulinum by Use of Challenge Studies and Predictive Microbiological Models. Appl. Environ. Microbiol. 2000, 66, 223–229. [Google Scholar] [CrossRef]
- Cosansu, S.; Juneja, V.K. Growth of Clostridium perfringens in Sous Vide Cooked Ground Beef with Added Grape Seed Extract. Meat Sci. 2018, 143, 252–256. [Google Scholar] [CrossRef]
- Lindström, M.; Mokkila, M.; Skyttä, E.; Hyytiä-Trees, E.; Lähteenmäki, L.; Hielm, S.; Ahvenainen, R.; Korkeala, H. Inhibition of Growth of Nonproteolytic Clostridium botulinum Type B in Sous Vide Cooked Meat Products Is Achieved by Using Thermal Processing but Not Nisin. J. Food Prot. 2001, 64, 838–844. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The Effect of Essential Oil and Extract from Sage (Salvia officinalis L.) Herbal Dust (Food Industry by-Product) on the Oxidative and Microbiological Stability of Fresh Pork Sausages. LWT 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Bor, T.; Aljaloud, S.O.; Gyawali, R.; Ibrahim, S.A. Antimicrobials from Herbs, Spices, and Plants. In Fruits, Vegetables, and Herbs; Elsevier: Amsterdam, The Netherlands, 2016; pp. 551–578. ISBN 978-0-12-802972-5. [Google Scholar]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plant Extracts as Antimicrobials in Food Products. In Handbook of Natural Antimicrobials for Food Safety and Quality; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–47. ISBN 978-1-78242-034-7. [Google Scholar]
- Raeisi, S.; Ojagh, S.M.; Sharifi-Rad, M.; Sharifi-Rad, J.; Quek, S.Y. Evaluation of Allium paradoxum (M.B.) G. Don. and Eryngium caucasicum Trauve. Extracts on the Shelf-Life and Quality of Silver Carp (Hypophthalmichthys molitrix) Fillets during Refrigerated Storage: RAEISI et al. J. Food Saf. 2017, 37, e12321. [Google Scholar] [CrossRef]
- Raeisi, S.; Sharifi-Rad, M.; Quek, S.Y.; Shabanpour, B.; Sharifi-Rad, J. Evaluation of Antioxidant and Antimicrobial Effects of Shallot (Allium ascalonicum L.) Fruit and Ajwain (Trachyspermum ammi (L.) Sprague) Seed Extracts in Semi-Fried Coated Rainbow Trout (Oncorhynchus mykiss) Fillets for Shelf-Life Extension. LWT Food Sci. Technol. 2016, 65, 112–121. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Abdelkader, M.; Ahcen, B.; Rachid, D.; Hakim, H. Phytochemical Study and Biological Activity of Sage (Salvia officinalis L.). Int. J. Biol. Life Agric. Sci. 2015, 8, 1253–1257. [Google Scholar] [CrossRef]
- Miladinovic, D.; Lj, M. Antimicrobial Activity of Essential Oil of Sage from Serbia. Facta Univ. Ser. Phys. Chem. Technol. 2000, 2, 97–100. [Google Scholar]
- Youssef, M.K.; Gill, C.O.; Yang, X. Storage Life at 2 °C or −1.5 °C of Vacuum-Packaged Boneless and Bone-in Cuts from Decontaminated Beef Carcasses: Storage Life of Vacuum-Packaged Beef Primals from Decontaminated Carcasses. J. Sci. Food Agric. 2014, 94, 3118–3124. [Google Scholar] [CrossRef]
- Youssef, M.K.; Gill, C.O.; Tran, F.; Yang, X. Unusual Compositions of Microflora of Vacuum-Packaged Beef Primal Cuts of Very Long Storage Life. J. Food Prot. 2014, 77, 2161–2167. [Google Scholar] [CrossRef]
- Yang, X.; Tran, F.; Wolters, T. Microbial Ecology of Decontaminated and Not Decontaminated Beef Carcasses. J. Food Res. 2017, 6, 85. [Google Scholar] [CrossRef]
- Wang, H.; He, A.; Yang, X. Dynamics of Microflora on Conveyor Belts in a Beef Fabrication Facility during Sanitation. Food Control 2018, 85, 42–47. [Google Scholar] [CrossRef]
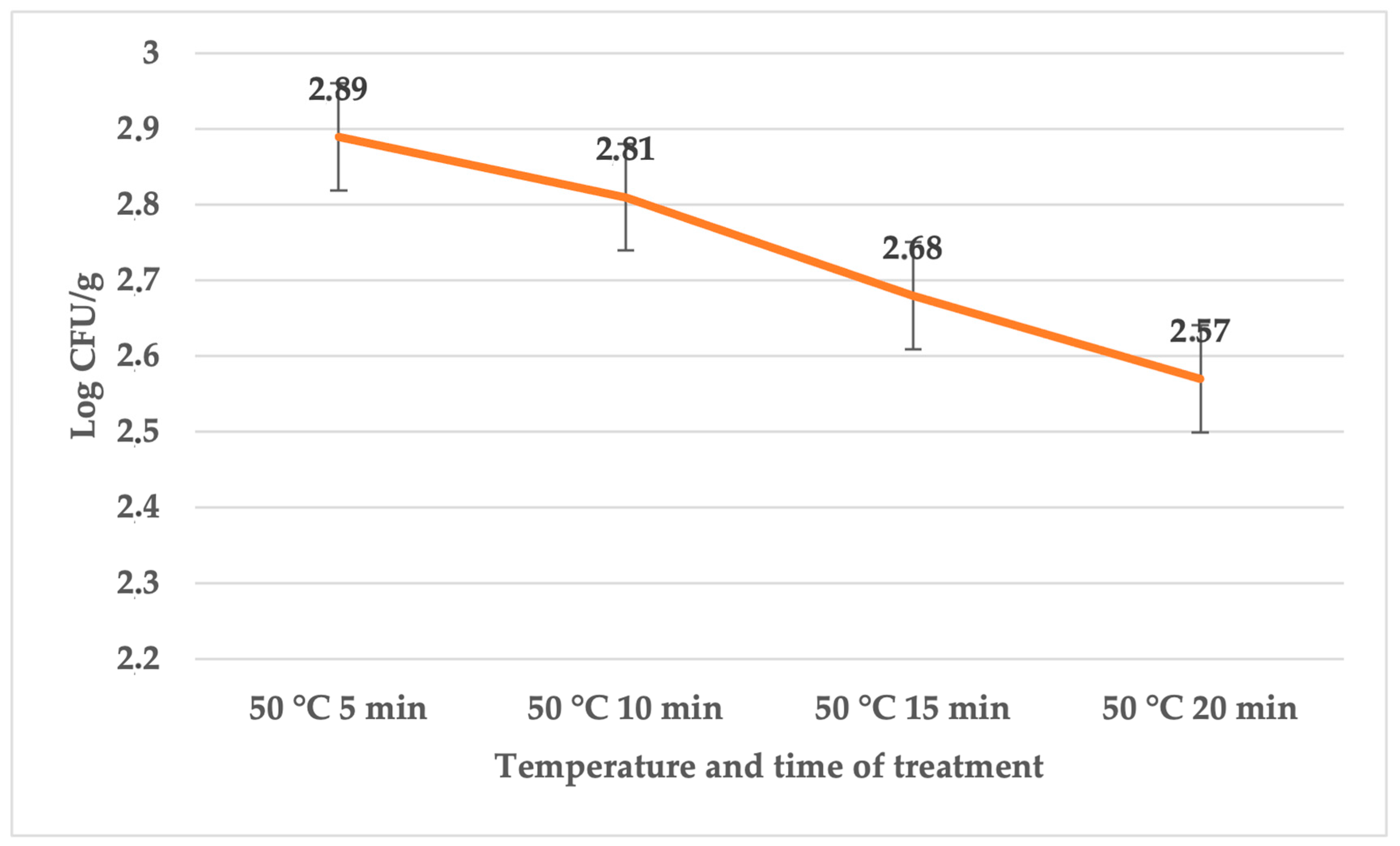
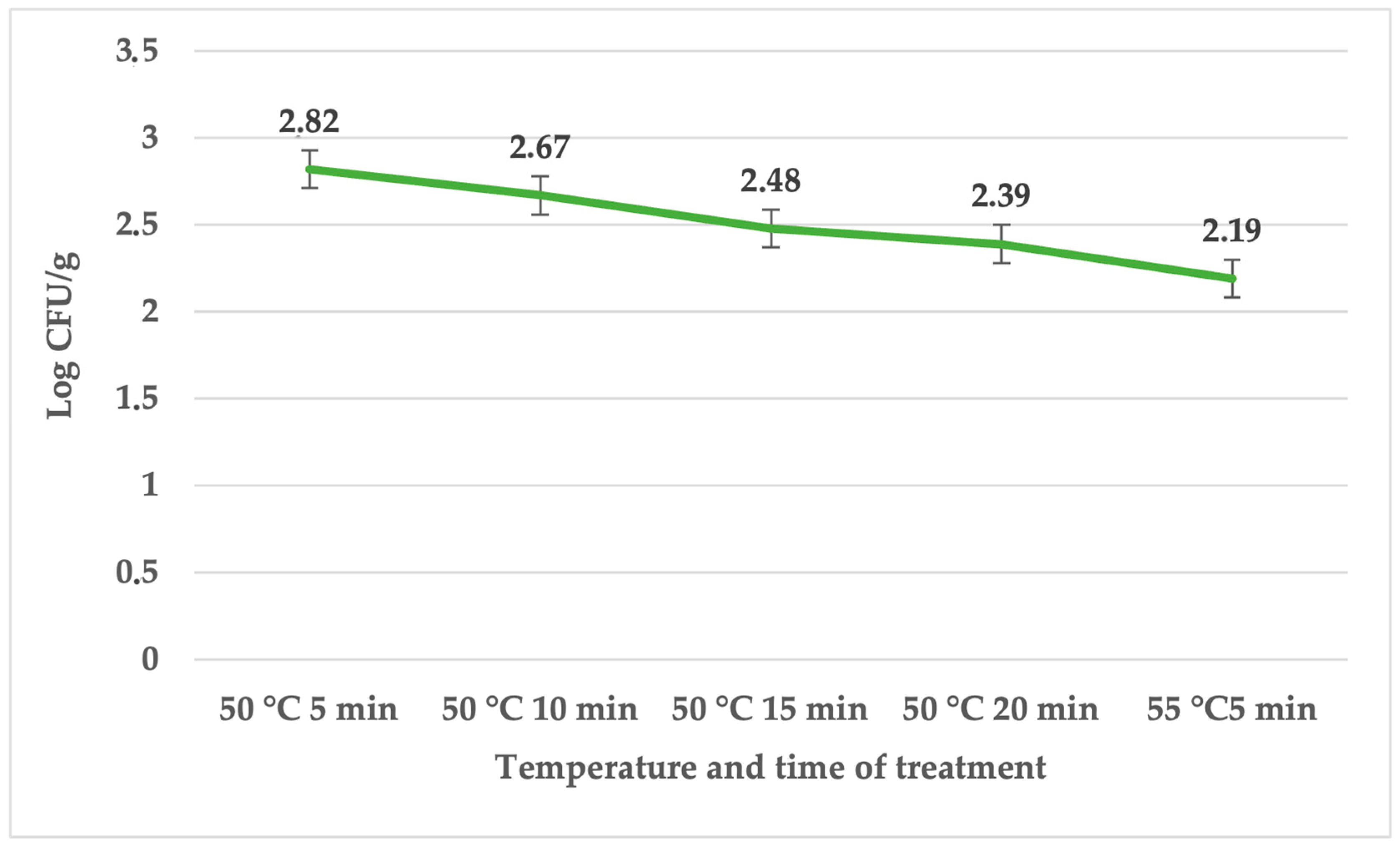
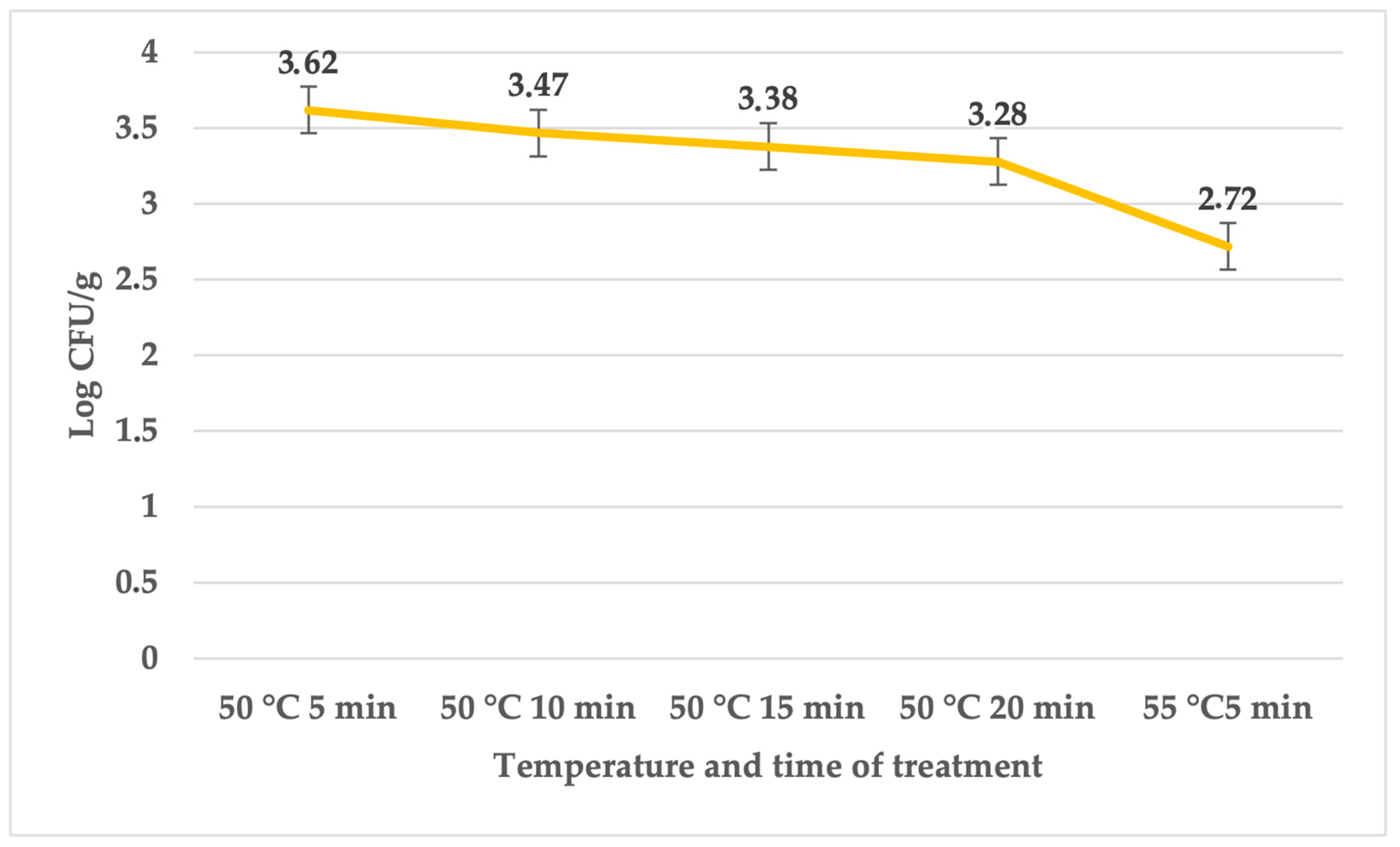

| Treatment | Temperature (°C) | Time (min) | Average | SD | p Value |
|---|---|---|---|---|---|
| BM | 50 | 5 | 3.76 | 0.08 | 4.114 × 10−3 |
| BMSEEO | 50 | 5 | 3.27 | 0.12 | |
| BM | 50 | 10 | 3.63 | 0.17 | 2.110 × 10−3 |
| BMSEEO | 50 | 10 | 2.90 | 0.06 | |
| BM | 50 | 15 | 3.35 | 0.09 | 3.302 × 10−4 |
| BMSEEO | 50 | 15 | 2.72 | 0.04 | |
| BM | 50 | 20 | 3.35 | 0.05 | 3.219 × 10−2 |
| BMSEEO | 50 | 20 | 2.58 | 0.05 | |
| BM | 55 | 5 | 3.28 | 0.05 | 1.814 × 10−2 |
| BMSEEO | 55 | 5 | 2.47 | 0.05 | |
| BM | 55 | 10 | 3.16 | 0.02 | 1.315 × 10−2 |
| BMSEEO | 55 | 10 | 2.22 | 0.05 | |
| BM | 55 | 15 | 3.08 | 0.04 | 1.230 × 10−2 |
| BMSEEO | 55 | 15 | 2.15 | 0.03 | |
| BM | 55 | 20 | 2.94 | 0.05 | 8.874 × 10−3 |
| BMSEEO | 55 | 20 | 1.94 | 0.05 | |
| BM | 60 | 5 | 2.68 | 0.07 | 5.179 × 10−2 |
| BMSEEO | 60 | 5 | 1.68 | 0.07 | |
| BM | 60 | 10 | 2.62 | 0.04 | 2.366 × 10−2 |
| BMSEEO | 60 | 10 | 1.62 | 0.04 | |
| BM | 60 | 15 | 2.35 | 0.06 | 1.631 × 10−2 |
| BMSEEO | 60 | 15 | 1.35 | 0.06 | |
| BM | 60 | 20 | 2.17 | 0.03 | 7.026 × 10−3 |
| BMSEEO | 60 | 20 | 1.17 | 0.03 | |
| BM | 65 | 10 | 2.03 | 0.006 | 1.527 × 10−3 |
| BMSEEO | 65 | 10 | 1.02 | 0.02 |
| Treatment | Temperature (°C) | Time (min) | Average | SD | p Value |
|---|---|---|---|---|---|
| BM | 50 | 5 | 4.41 | 0.14 | 3.003 × 10−1 * |
| BMSEEO | 50 | 5 | 4.52 | 0.06 | |
| BM | 50 | 10 | 4.36 | 0.05 | 4.214 × 10−2 |
| BMSEEO | 50 | 10 | 4.45 | 0.03 | |
| BM | 50 | 15 | 4.28 | 0.04 | 3.377 × 10−2 |
| BMSEEO | 50 | 15 | 4.40 | 0.06 | |
| BM | 50 | 20 | 4.17 | 0.04 | 2.279 × 10−2 |
| BMSEEO | 50 | 20 | 4.44 | 0.12 | |
| BM | 55 | 5 | 4.08 | 0.03 | 5.406 × 10−4 |
| BMSEEO | 55 | 5 | 4.31 | 0.02 | |
| BM | 55 | 10 | 3.95 | 0.03 | 1.253 × 10−4 |
| BMSEEO | 55 | 10 | 2.50 | 0.17 | |
| BM | 55 | 15 | 3.79 | 0.12 | 1.413 × 10−2 |
| BMSEEO | 55 | 15 | 2.16 | 0.01 | |
| BM | 55 | 20 | 3.63 | 0.06 | 7.191 × 10−3 |
| BMSEEO | 55 | 20 | 2.08 | 0.03 |
| Treatment | Temperature (°C) | Time (min) | Average | SD | p Value |
|---|---|---|---|---|---|
| BM | 50 | 5 | 2.81 | 0.05 | 5.500 × 10−2 |
| BMSEEO | 50 | 5 | 3.35 | 0.03 | |
| BM | 50 | 10 | 2.49 | 0.06 | 2.833 × 10−2 |
| BMSEEO | 50 | 10 | 3.23 | 0.02 |
| Treatment | Temperature (°C) | Time (min) | Average | SD | p Value |
|---|---|---|---|---|---|
| BM | 50 | 5 | 5.20 | 0.04 | 2.519 × 10−4 |
| BMSEEO | 50 | 5 | 4.70 | 0.06 | |
| BM | 50 | 10 | 5.08 | 0.01 | 1.213 × 10−4 |
| BMSEEO | 50 | 10 | 4.59 | 0.06 | |
| BM | 50 | 15 | 4.93 | 0.06 | 5.822 × 10−4 |
| BMSEEO | 50 | 15 | 4.57 | 0.03 | |
| BM | 50 | 20 | 4.83 | 0.04 | 1.144 × 10−2 |
| BMSEEO | 50 | 20 | 4.54 | 0.11 | |
| BM | 55 | 5 | 4.72 | 0.04 | 3.776 × 10−4 |
| BMSEEO | 55 | 5 | 4.42 | 0.03 | |
| BM | 55 | 10 | 4.52 | 0.06 | 1.357 × 10−4 |
| BMSEEO | 55 | 10 | 3.55 | 0.10 | |
| BM | 55 | 15 | 4.36 | 0.04 | 1.004 × 10−4 |
| BMSEEO | 55 | 15 | 3.25 | 0.12 | |
| BM | 55 | 20 | 4.24 | 0.06 | 5.148 × 10−3 |
| BMSEEO | 55 | 20 | 2.66 | 0.03 |
| Treatment | Temperature (°C) | Time (min) | Average | SD | p Value |
|---|---|---|---|---|---|
| BM | 50 | 5 | 2.90 | 0.07 | 4.896 × 10−4 |
| BMSEEO | 50 | 5 | 3.47 | 0.07 | |
| BM | 50 | 10 | 2.67 | 0.10 | 3.394 × 10−4 |
| BMSEEO | 50 | 10 | 3.37 | 0.05 | |
| BM | 50 | 15 | 2.37 | 0.05 | 1.369 × 10−2 |
| BMSEEO | 50 | 15 | 3.28 | 0.04 | |
| BM | 50 | 20 | 2.19 | 0.02 | 1.707 × 10−2 |
| BMSEEO | 50 | 20 | 3.22 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, R.; Čmiková, N.; Kačániová, M.; Mokrejš, P. Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage. Foods 2023, 12, 4172. https://doi.org/10.3390/foods12224172
Gál R, Čmiková N, Kačániová M, Mokrejš P. Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage. Foods. 2023; 12(22):4172. https://doi.org/10.3390/foods12224172
Chicago/Turabian StyleGál, Robert, Natália Čmiková, Miroslava Kačániová, and Pavel Mokrejš. 2023. "Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage" Foods 12, no. 22: 4172. https://doi.org/10.3390/foods12224172
APA StyleGál, R., Čmiková, N., Kačániová, M., & Mokrejš, P. (2023). Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage. Foods, 12(22), 4172. https://doi.org/10.3390/foods12224172








