The Emulsifying Properties, In Vitro Digestion Characteristics and Storage Stability of High-Pressure-Homogenization-Modified Dual-Protein-Based Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Protein Solution and HPH Treatment
2.3. Emulsion Preparation
2.4. Dual-Protein Emulsion Measurements
2.4.1. Droplet Size and Zeta Potential of Emulsion
2.4.2. Interfacial Protein Absorption Rate of Emulsions
2.4.3. Physical Stability of Emulsions
2.4.4. Micro-Rheological Properties of Emulsions
2.5. In Vitro Digestion Behavior Analysis of Dual-Protein Emulsions
2.5.1. In Vitro Digestion Treatment
2.5.2. The Droplet Size and Zeta Potential of the Emulsions during In Vitro Digestion
2.5.3. The Free Amino Groups of the Emulsions during In Vitro Digestion
2.5.4. In Vitro Digestibility of Emulsions
2.6. Storage Stability of Dual-Protein Emulsions
2.7. Statistical Analysis
3. Results and Discussion
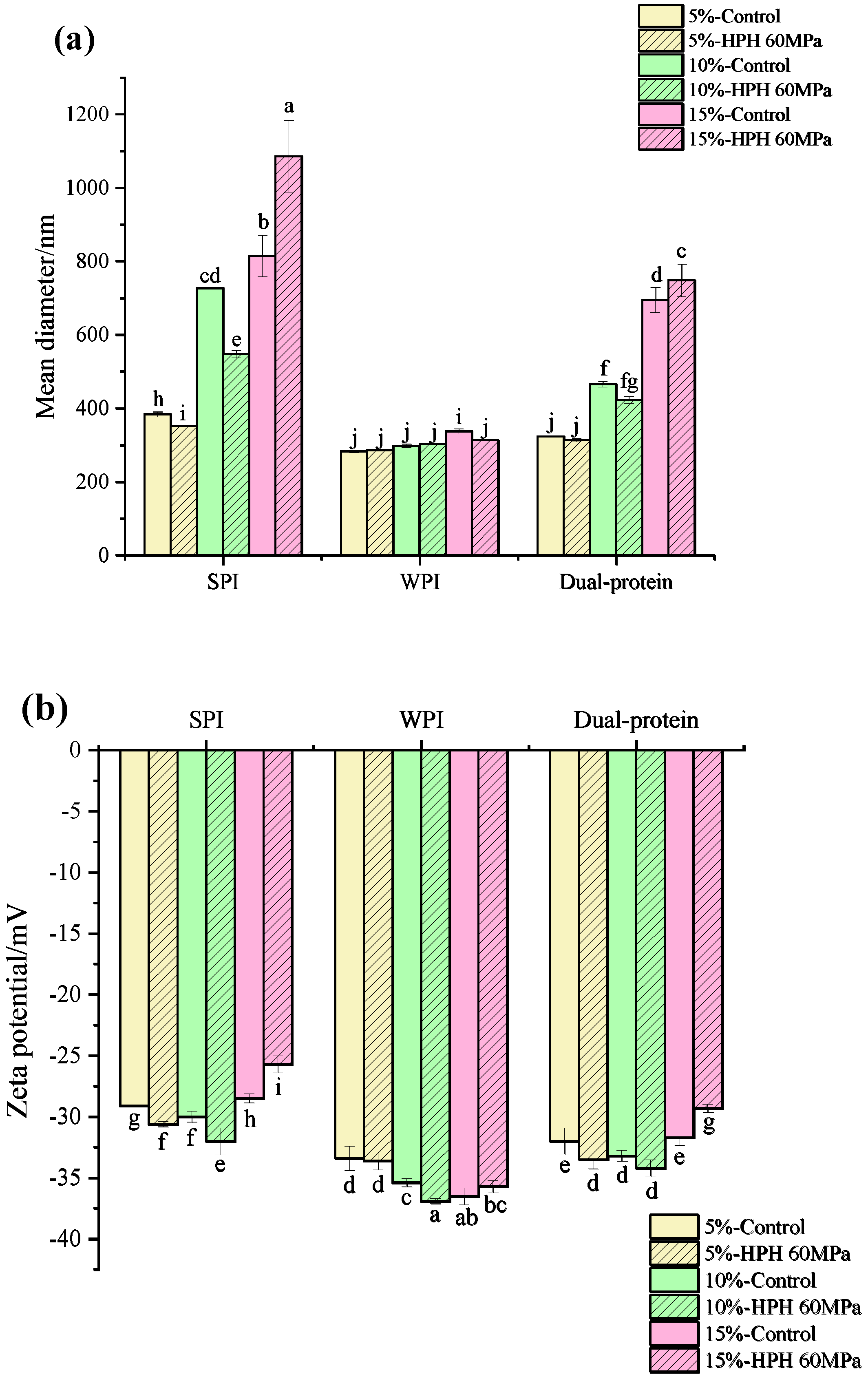
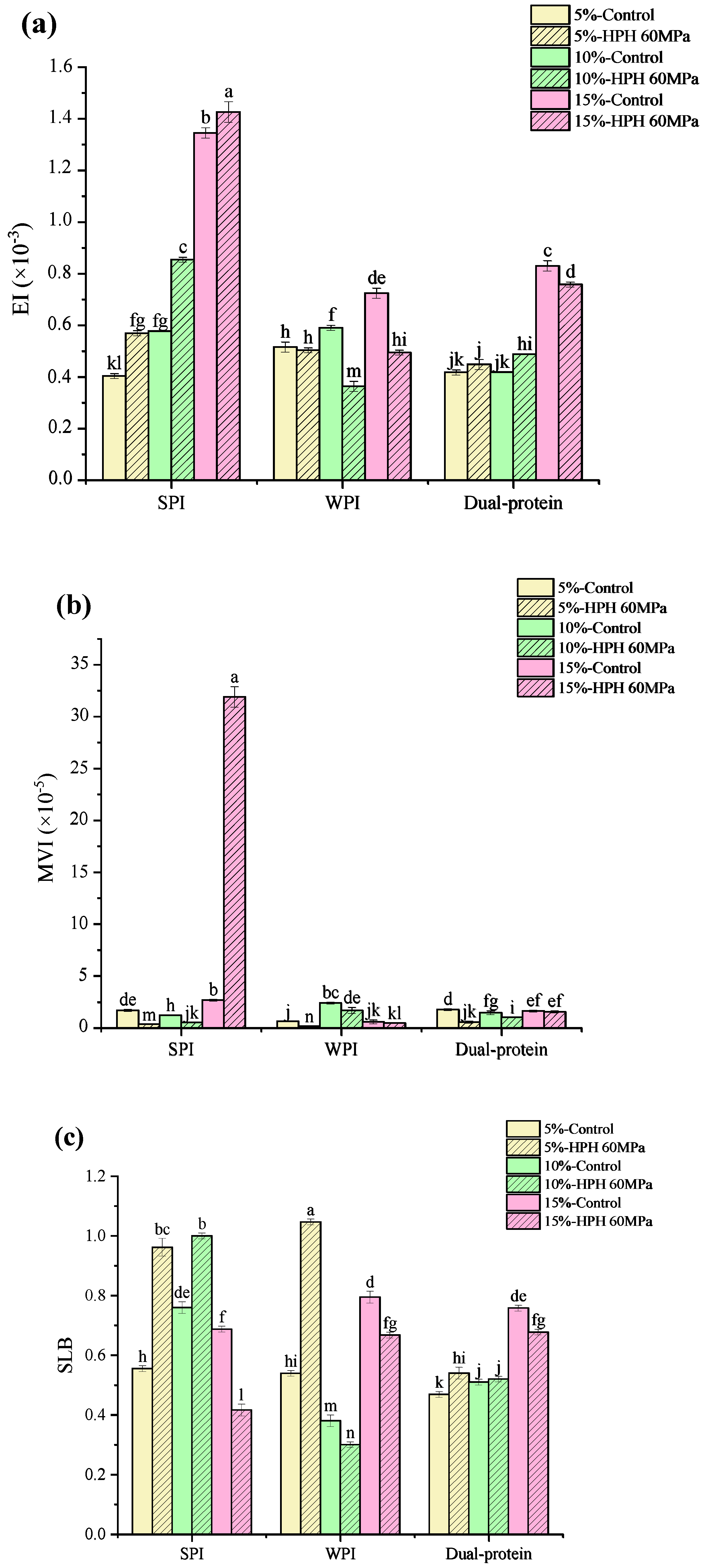
3.1. Droplet Size and Zeta Potential
3.2. Interfacial Protein Absorption Rate
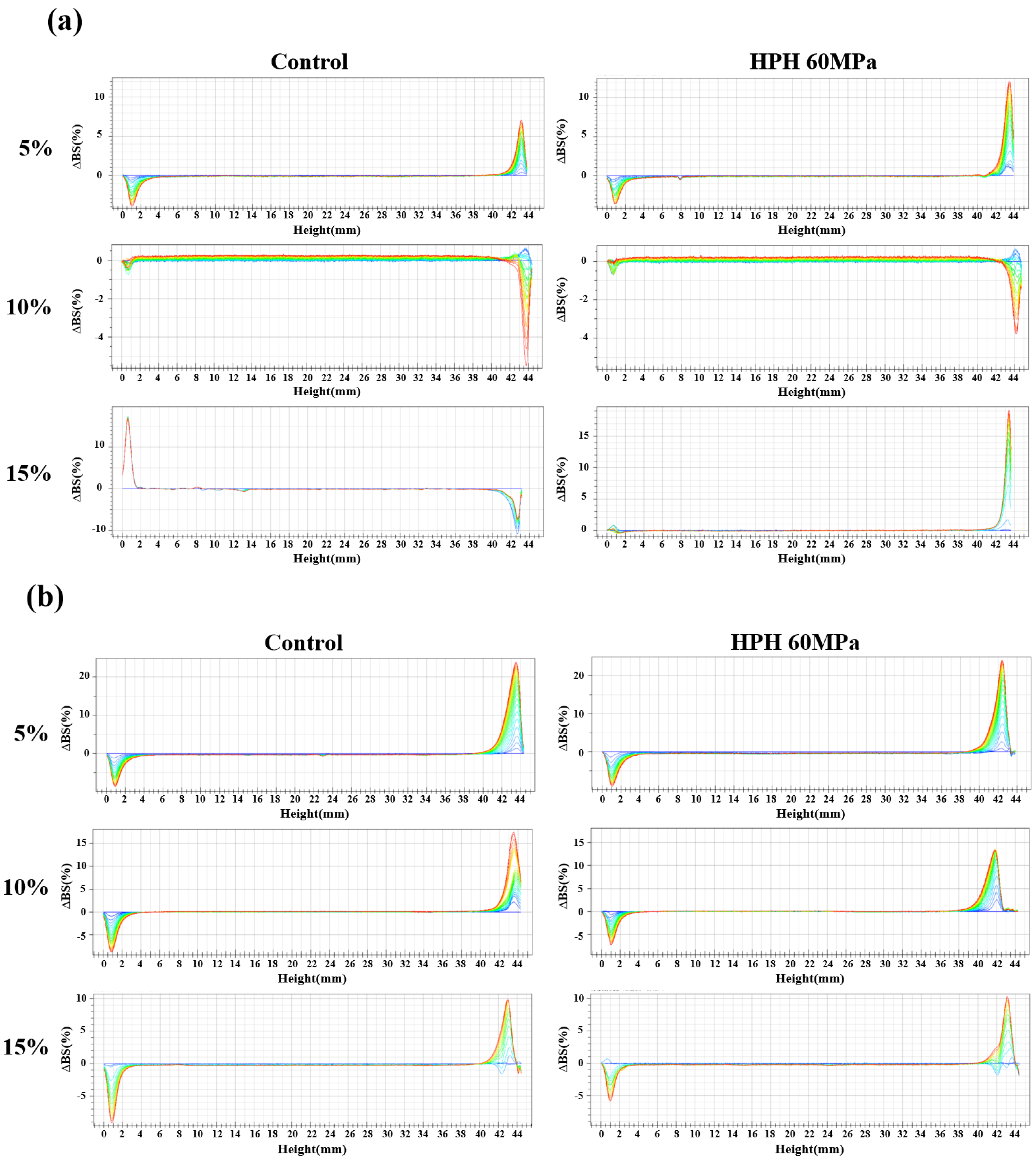
3.3. Physical Stability Properties
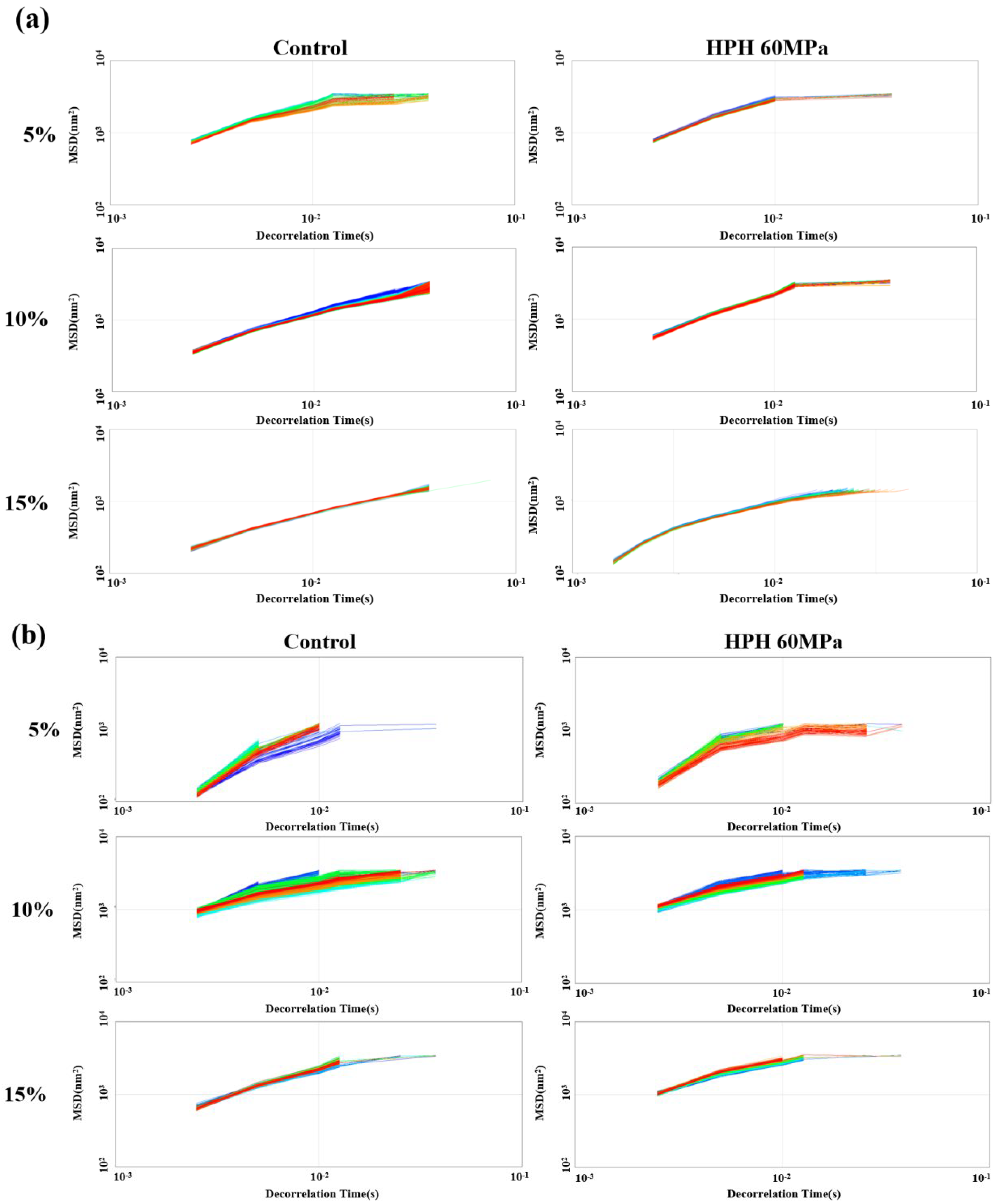
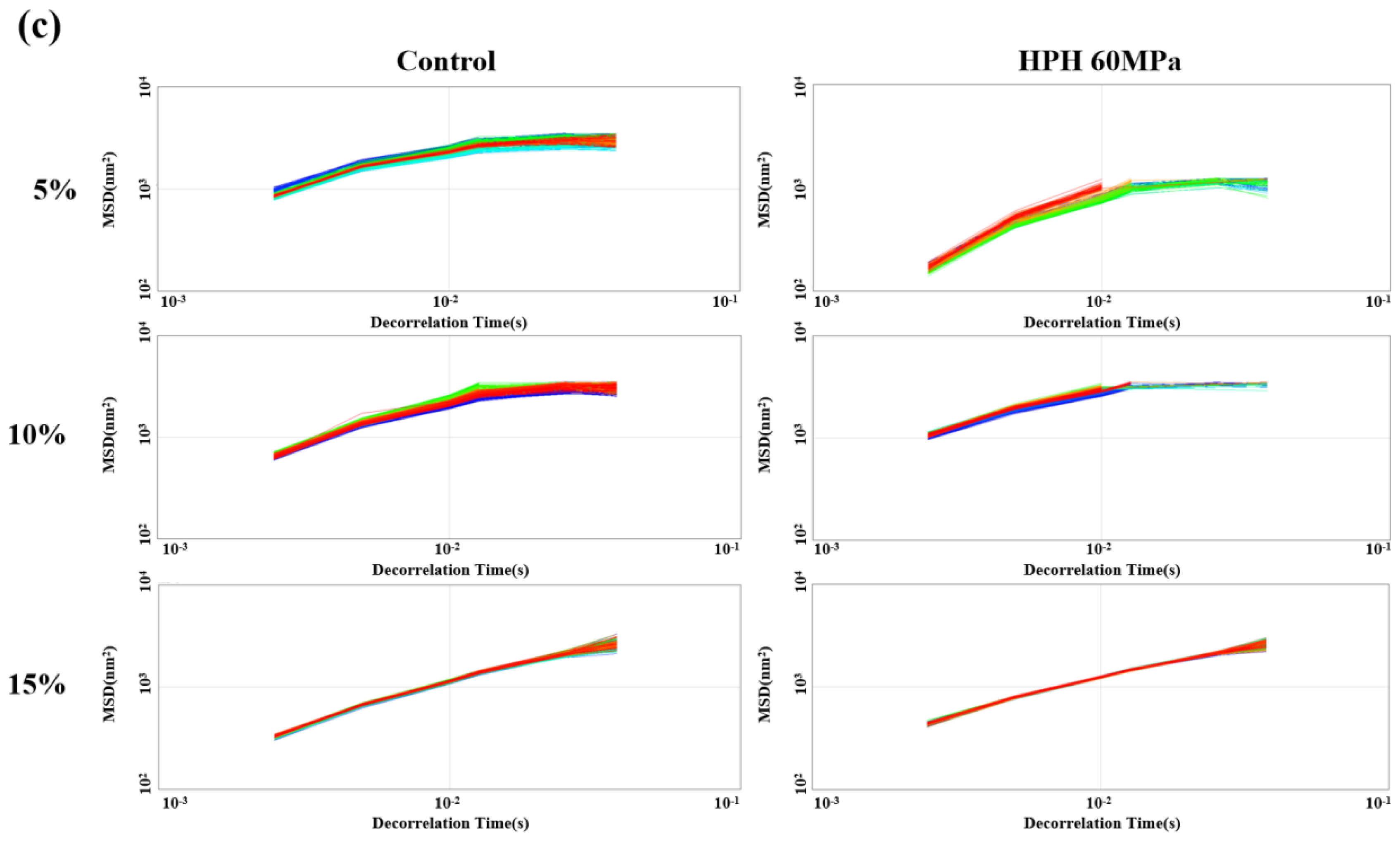
3.4. Micro-Rheology of Emulsions
3.5. In Vitro Digestion Properties of Emulsion
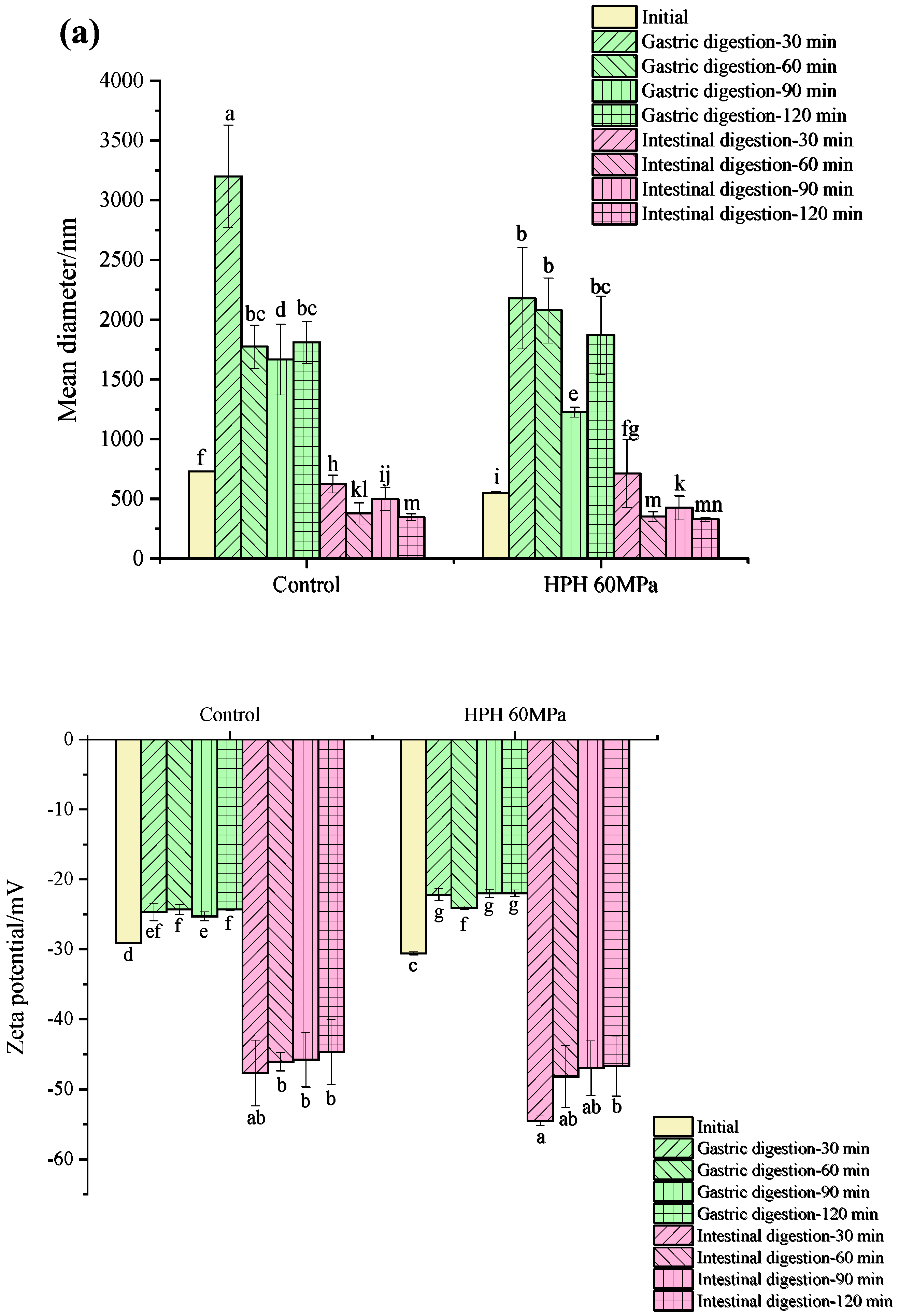
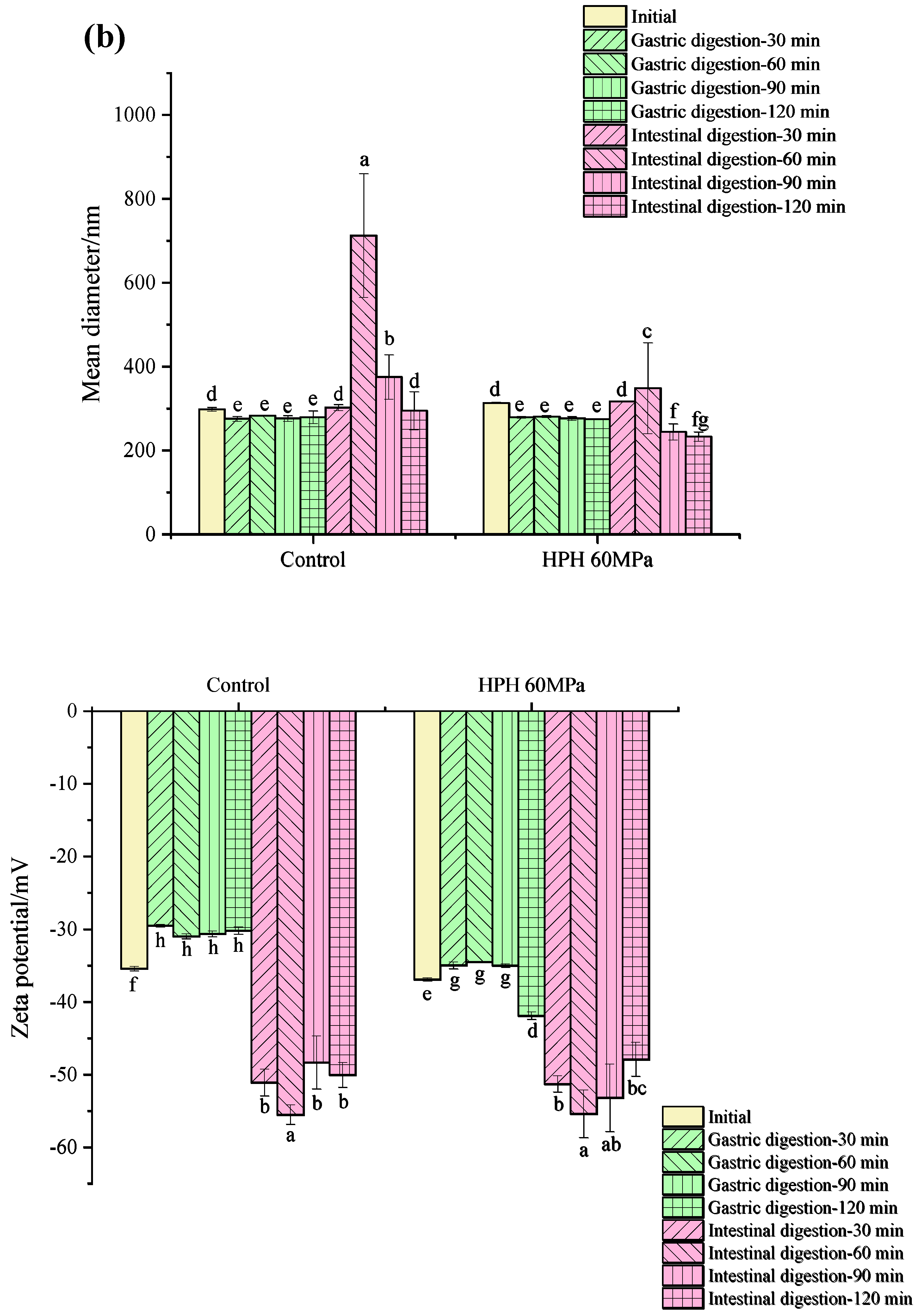
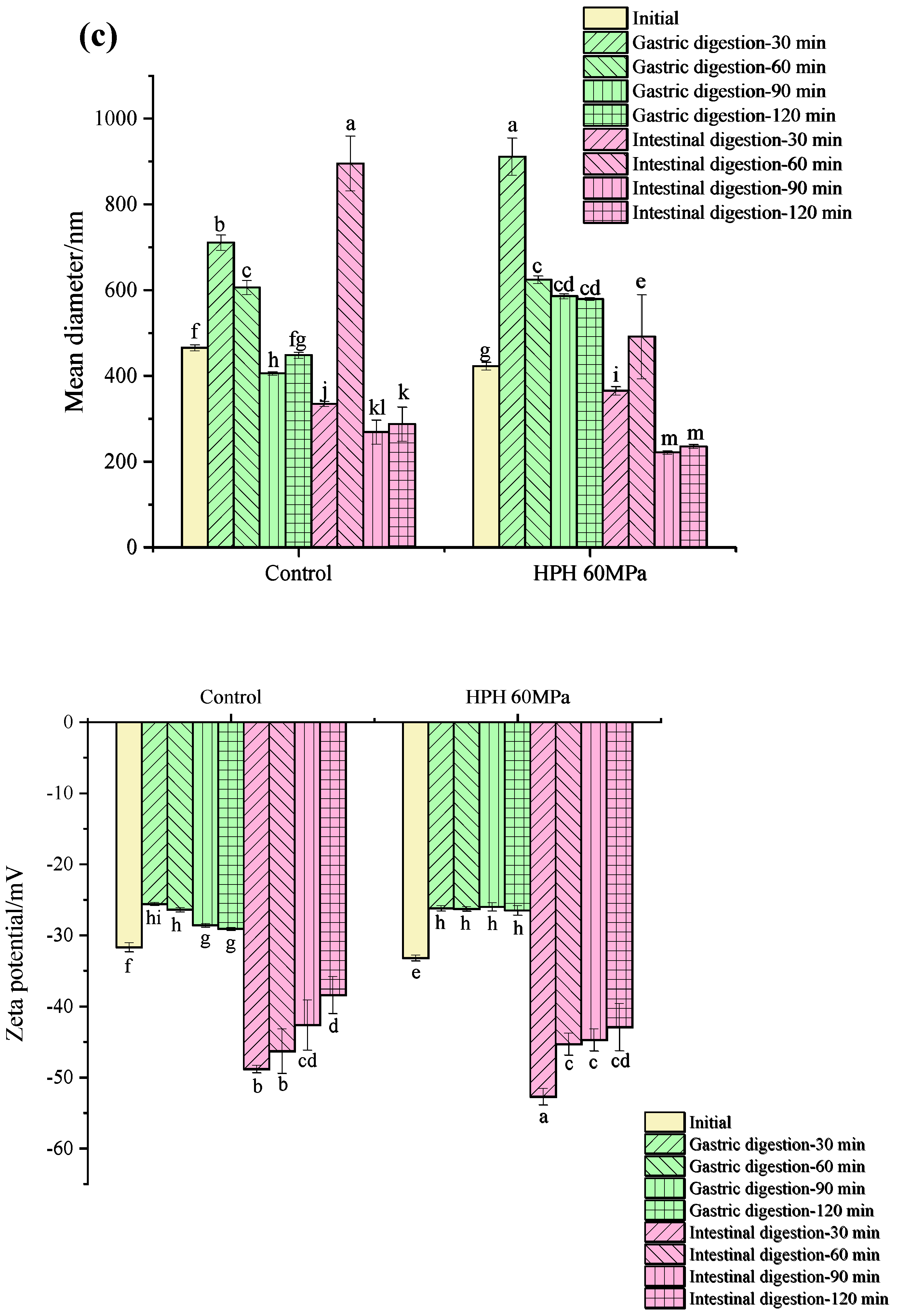
3.5.1. Droplet Size and Zeta Potential during In Vitro Digestion
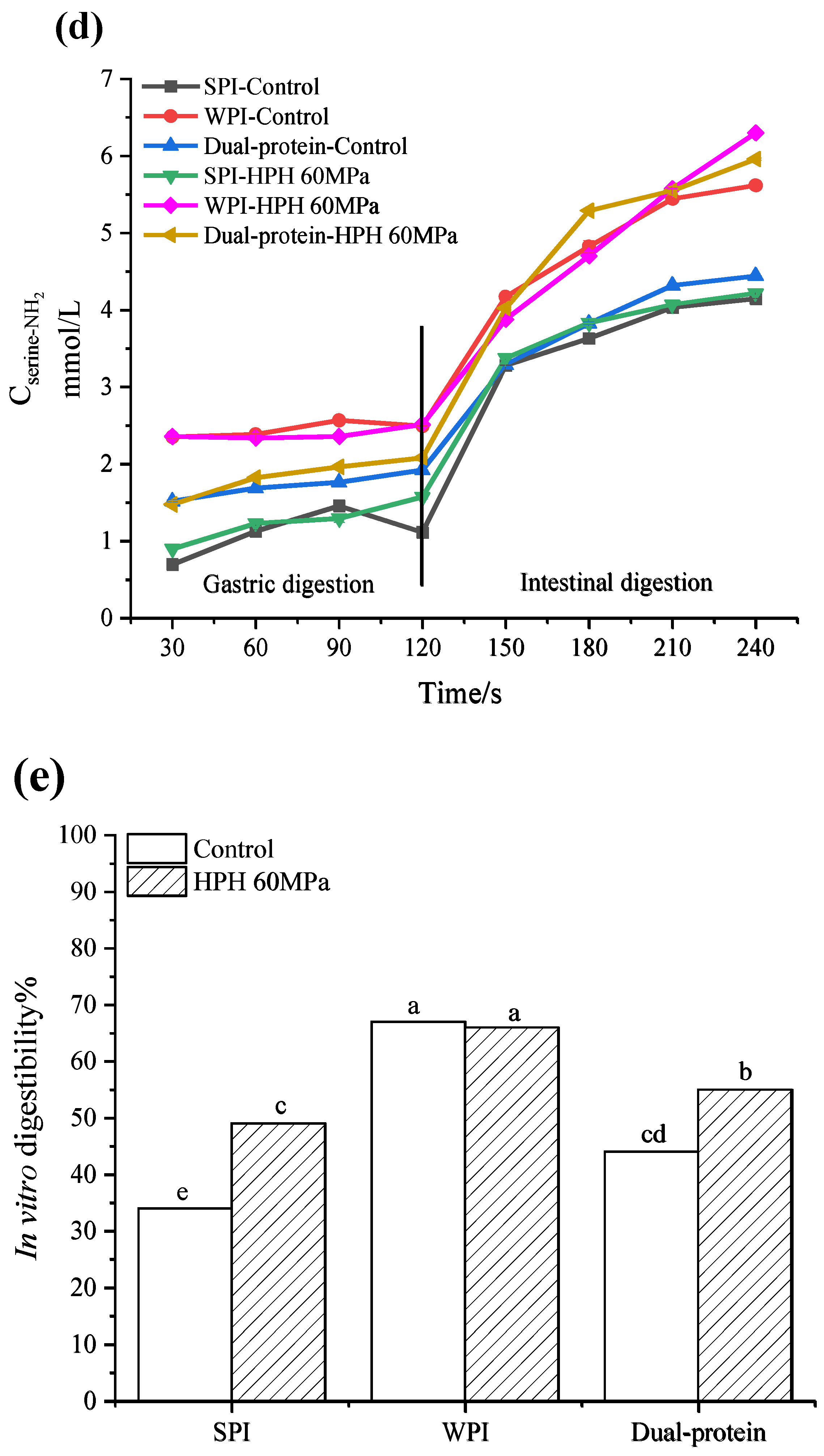
3.5.2. The Release of Free Amino Groups during In Vitro Digestion
3.5.3. In Vitro Digestibility
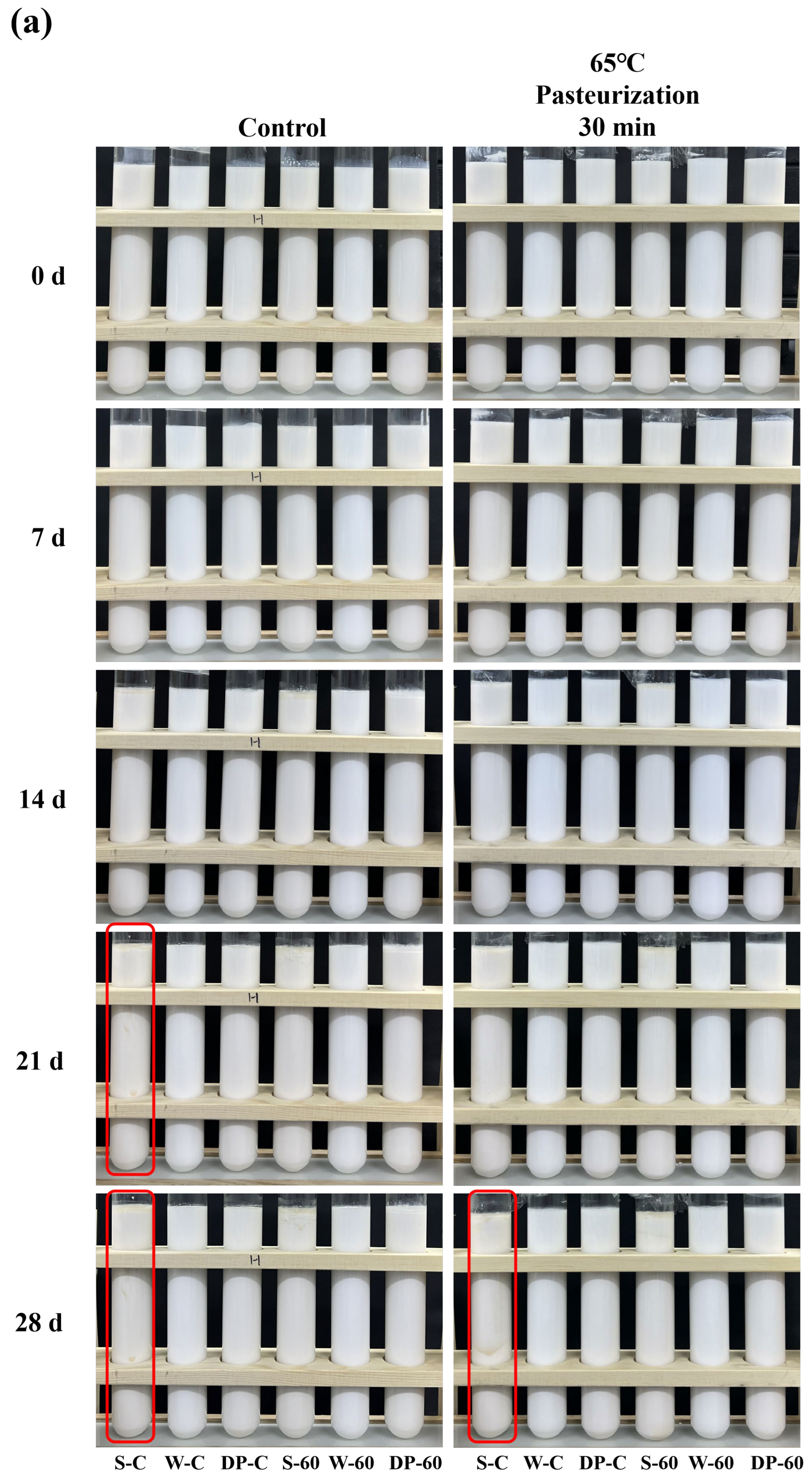
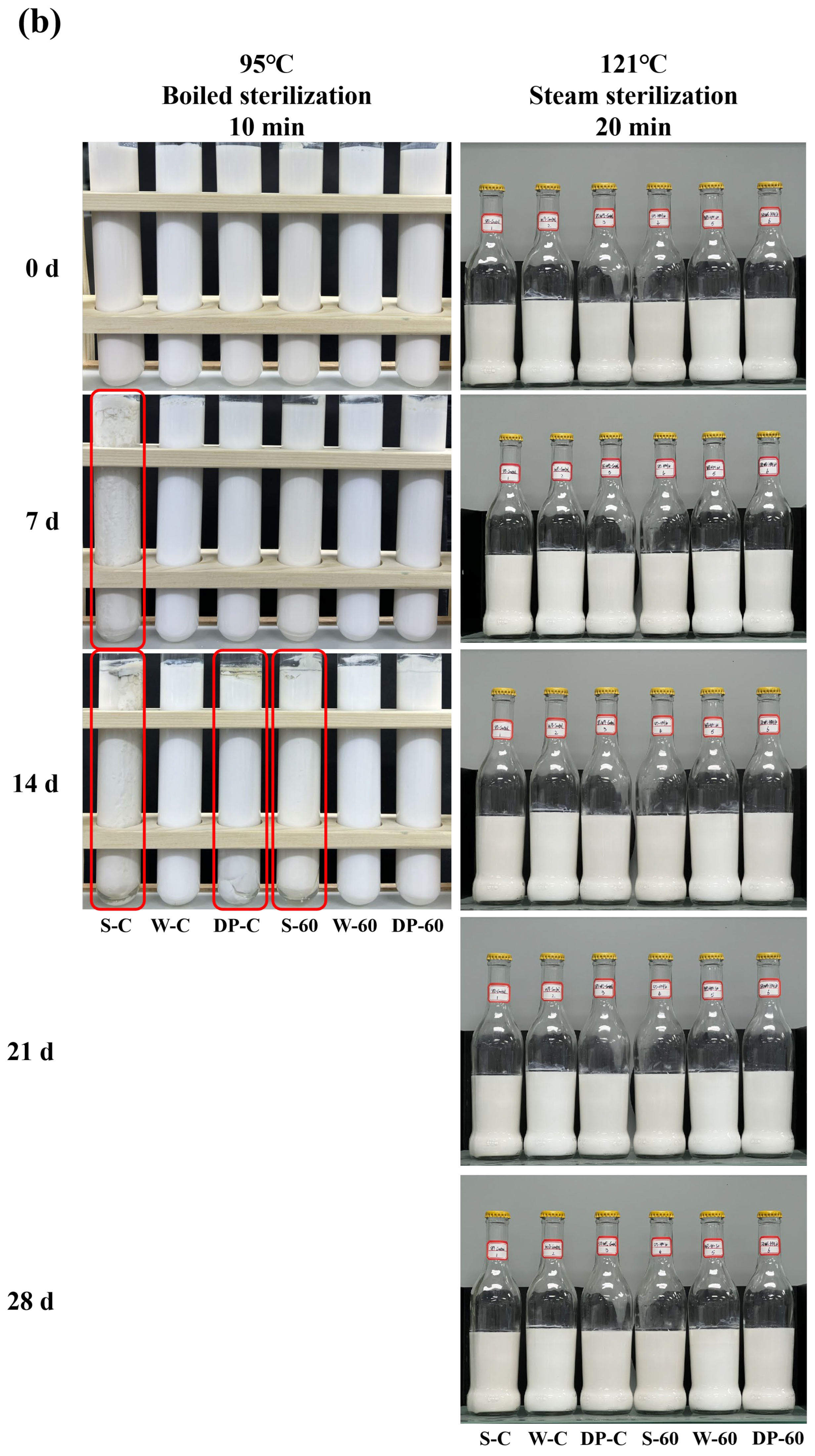
3.6. Storage Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J.; Rao, J.J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Sivapratha, S.; Sarkar, P. Multiple layers and conjugate materials for food emulsion stabilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein-polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Rutkevicius, M.; Allred, S.; Velev, O.D.; Velikov, K.P. Stabilization of oil continuous emulsions with colloidal particles from water-insoluble plant proteins. Food Hydrocoll. 2018, 82, 89–95. [Google Scholar] [CrossRef]
- Yan, X.J.; Ma, C.C.; Cui, F.Z.; McClements, D.J.; Liu, X.B.; Liu, F.G. Protein-stabilized pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Bos, M.A.; Van Vliet, T. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Ren, G.X.; Zhang, J.P.; Li, M.H.; Yi, S.Q.; Wang, J. Protein blend ingestion before allogeneic stem cell transplantation improves protein-energy malnutrition in patients with leukemia. Nutr. Res. 2017, 46, 68–77. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, Q.W.; Liu, H.; Liu, X.Y.; Yu, Y.H.; Han, D.; He, X.Y.; Zeng, P.; Wang, J. Soy-whey dual-protein alleviates osteoporosis of ovariectomized rats via regulating bone fat metabolism through gut-liver-bone axis. Nutrition 2022, 103–104, 111723. [Google Scholar] [CrossRef]
- Huang, Y.C.; Zhang, K.; Zhang, L.; Qiu, J.H.; Fu, L.; Ying, T.Y.; Wang, J.; Qin, R.; Zhang, J.J.; Dong, X.W.; et al. Dosage of dual-protein nutrition differentially impacts the formation of atherosclerosis in apoE-/-mice. Nutrients 2022, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Szczepanska, J.; Skapska, S.; Polaska, M.; Marszalek, K. High pressure homogenization with a cooling circulating system: The effect on physiochemical and rheological properties, enzymes, and carotenoid profile of carrot juice. Food Chem. 2022, 370, 131023. [Google Scholar] [CrossRef]
- Kruszewski, B.; Domian, E.; Nowacka, M. Influence of high-pressure homogenization on the physicochemical properties and betalain pigments of red beetroot (Beta vulgaris L.) juice. Molecules 2023, 28, 2018. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gracia-Julia, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Sahil; Madhunita, M.; Prabhakar, P.K.; Kumar, N. Dynamic high pressure treatments: Current advances on mechanistic-cumtransport phenomena approaches and plant protein functionalization. Crit. Rev. Food Sci. Nutr. 2022, 2022, 2125930. [Google Scholar] [CrossRef]
- Ma, W.C.; Wang, J.M.; Wu, D.; Xu, X.B.; Wu, C.; Du, M. Physicochemical properties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocoll. 2019, 100, 105429. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Trujillo, A.J. Ultra-high pressure homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.; Moretton, M.; Calligaris, S.; Manzocco, L.; Nicoli, M.C. High pressure homogenization shapes the techno-functionalities and digestibility of pea proteins. Food Bioprod. Process. 2022, 131, 77–85. [Google Scholar] [CrossRef]
- Dybowska, B.E.; Krupa-Kozak, U. Stability of oil-in-water emulsions as influenced by thermal treatment of whey protein dispersions or emulsions. Int. J. Dairy Technol. 2020, 73, 513–520. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; Tommaso, J.D.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2020, 99, 627–639. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.H.; Yan, H.J.; Zou, H.N.; Yu, C.P. The conformation and physico-chemical properties of pH-treated golden pompano protein on the oil/water interfacial properties and emulsion stability. Int. J. Food Sci. Technol. 2022, 57, 5611–5620. [Google Scholar] [CrossRef]
- Shi, J.Y.; Xiao, J.X.; Liu, L.; Dong, X.Y. Ultrasonic assisted oil-in-water emulsions stabilized by flaxseed protein isolate: Influence of different oils. J. Dispers. Sci. Technol. 2021, 43, 1789–1800. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, S.; Zhang, H.; Cheng, Y.; Yin, L. Physical stability, microstructure and micro-rheological properties of water-in-oil-in-water (W/O/W) emulsions stabilized by porcine gelatin. Food Chem. 2018, 253, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Z.; Liu, L.J.; Zhang, T.; Tao, N.P.; Wang, X.C.; Zhong, J. Effect of interfacial layer number on the storage stability and in vitro digestion of fish oil-loaded multilayer emulsions consisting of gelatin particle and polysaccharides. Food Chem. 2021, 336, 127686. [Google Scholar] [CrossRef] [PubMed]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Gupta, A.; Badruddoza, A.Z.M.; Doyle, P.S. A general route for nanoemulsion synthesis using low-energy methods at constant temperature. Langmuir ACS J. Surf. Colloids 2017, 33, 7118–7123. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhou, C.J.; Fu, F.; Chen, Z.L.; Wu, Q.L. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy glycinin as food-grade pickering stabilizers: Part. I. structural characteristics, emulsifying properties and adsorption/arrangement at interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhou, F.B.; Shen, P.H.; Zhao, Q.Z.; Zhao, M.M. Influence of thermal treatment on oil-water interfacial properties and emulsion stabilization prepared by sono-assembled soy peptide nanoparticles. Food Hydrocoll. 2020, 103, 105646. [Google Scholar] [CrossRef]
- Christian, C.; Elena, T.; Donato, C.; Donatella, P.; Massimo, F. Turbiscan Lab® expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2019, 72, 155–160. [Google Scholar] [CrossRef]
- Hebishy, E.; Ferragut, V.; Blasco-Moreno, A.; Trujillo, A.J. Impact of oil phase concentration on physical and oxidative stability of oil-in-water emulsions stabilized by sodium caseinate and ultra-high pressure homogenization. J. Dispers. Sci. Technol. 2019, 42, 46–57. [Google Scholar] [CrossRef]
- Yang, J.Q.; Liu, G.Y.; Zeng, H.B.; Chen, L.Y. Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Meleties, M.; Martineau, R.L.; Gupta, M.K.; Montclare, J.K. Particle-based microrheology as a tool for characterizing protein based materials. ACS Biomater. Sci. Eng. 2022, 8, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, C.; Fleury, M.; Brunel, L.; Bru, P.; Meunier, G. Analytical series soft matter analysis by means of microrheology. JCT CoatingsTech 2012, 9, 46–51. [Google Scholar]
- Li, Q.; He, S.H.; Xu, W.L.; Peng, F.S.; Gu, C.; Wang, R.C.; Ma, Y. Formation, stability and in vitro digestion of beta-carotene in oil-in-water milk fat globule membrane protein emulsions. Food Biophys. 2018, 13, 198–207. [Google Scholar] [CrossRef]
- Ozel, B.; Zhang, Z.Y.; He, L.L.; McClements, D.J. Digestion of animal-and plant-based proteins encapsulated in κ-carrageenan/protein beads under simulated gastrointestinal conditions. Food Res. Int. 2020, 137, 1096642. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, Z.P.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of lipid type on gastrointestinal fate of oil-in-water emulsions: In vitro digestion study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef]
- Yao, M.F.; Xiao, H.; McClements, D.J. Delivery of lipophilic bioactives: Assembly, disassembly, and reassembly of lipid nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Sun, M.J.; Mu, T.H.; Sun, H.N.; Zhang, M. Digestibility and structural properties of thermal and high hydrostatic pressure treated sweet potato (Ipomoea batatas L.) protein. Plants Foods Hum. Nutr. 2014, 69, 270–275. [Google Scholar] [CrossRef] [PubMed]


| Oil Phase Concentration (%, w/w) | Control | HPH 60 MPa | ||||
|---|---|---|---|---|---|---|
| SPI | WPI | Dual Protein | SPI | WPI | Dual Protein | |
| 5 | 0.2 | 0.9 | 0.7 | 0.3 | 1.2 | 0.8 |
| 10 | 0.2 | 0.4 | 0.2 | 0.1 | 0.6 | 0.3 |
| 15 | 0.5 | 0.6 | 0.4 | 0.8 | 0.7 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; He, X.; Feng, D.; Li, H.; Han, D.; Li, Q.; Zhao, B.; Li, N.; Liu, T.; Wang, J. The Emulsifying Properties, In Vitro Digestion Characteristics and Storage Stability of High-Pressure-Homogenization-Modified Dual-Protein-Based Emulsions. Foods 2023, 12, 4141. https://doi.org/10.3390/foods12224141
Wu M, He X, Feng D, Li H, Han D, Li Q, Zhao B, Li N, Liu T, Wang J. The Emulsifying Properties, In Vitro Digestion Characteristics and Storage Stability of High-Pressure-Homogenization-Modified Dual-Protein-Based Emulsions. Foods. 2023; 12(22):4141. https://doi.org/10.3390/foods12224141
Chicago/Turabian StyleWu, Meishan, Xiaoye He, Duo Feng, Hu Li, Di Han, Qingye Li, Boya Zhao, Na Li, Tianxin Liu, and Jing Wang. 2023. "The Emulsifying Properties, In Vitro Digestion Characteristics and Storage Stability of High-Pressure-Homogenization-Modified Dual-Protein-Based Emulsions" Foods 12, no. 22: 4141. https://doi.org/10.3390/foods12224141
APA StyleWu, M., He, X., Feng, D., Li, H., Han, D., Li, Q., Zhao, B., Li, N., Liu, T., & Wang, J. (2023). The Emulsifying Properties, In Vitro Digestion Characteristics and Storage Stability of High-Pressure-Homogenization-Modified Dual-Protein-Based Emulsions. Foods, 12(22), 4141. https://doi.org/10.3390/foods12224141






