Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products
Abstract
:1. Introduction
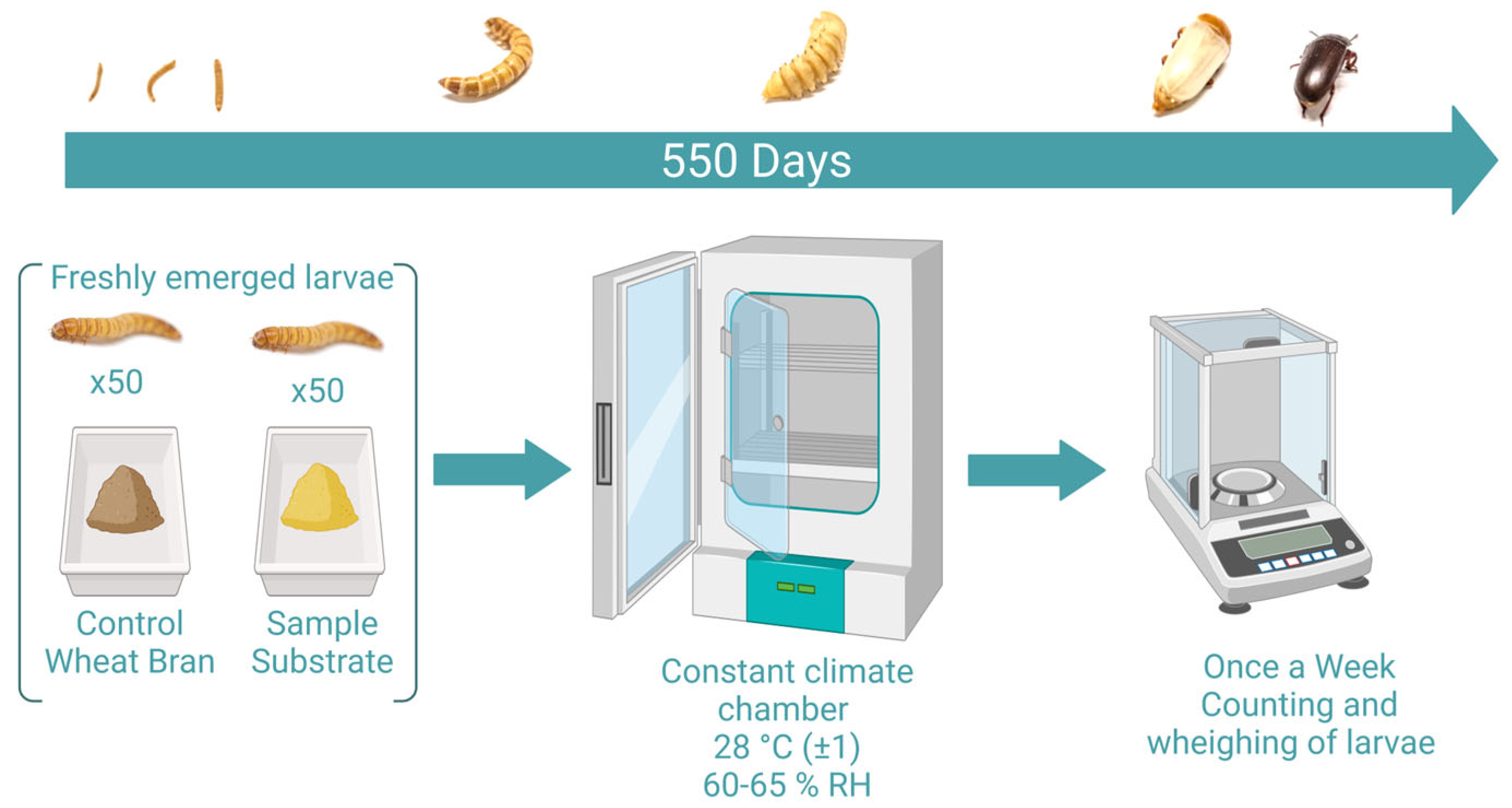
2. Materials and Methods
2.1. Diet Selection and Preparation
2.2. Data Analysis
- Survival rate of larvae;The survival rate was determined by daily counting the number of dead larvae for each treatment and calculating the rate in percentage based on the total number of larvae.
- 2.
- Development times (larvae -> pupae, pupae -> adults);Development time of larvae was recorded beginning with the first day of the experiment (with about three-week-old larvae) until the first pupa emerged.
- 3.
- Individual weights of the larvae, pupae, and adult beetles;Pupae and beetles were weighed shortly after eclosion. For boxes containing > 10 larvae, ten randomly chosen larvae per box were weighed individually once a week and placed back to the box. For boxes holding < 10 larvae, all specimens were weighed individually.
- 4.
- Total biomass of larvae, beetles, and pupae;
- 5.
- Percentage of emerged pupae (pupation rate) and emerged beetles;The emergence of pupae and beetles was calculated based on the total number of initially investigated larvae and is given as a rate.
- 6.
- Sex ratio [%];Sex was determined for pupae and was subsequently checked again for adult beetles to assure the correct determination.
- 7.
- Anomalies of beetles (deformed adults in %);Adult beetles were visually inspected for any abnormality or deformation (any defect in appearance or mobility).
- 8.
- Initial weight of feed and amount of remaining feed (= FC, “feed consumed”);FC = subtracting the remaining substrate from the total amount of the substrate provided (initial weight of feed) [7].
- 9.
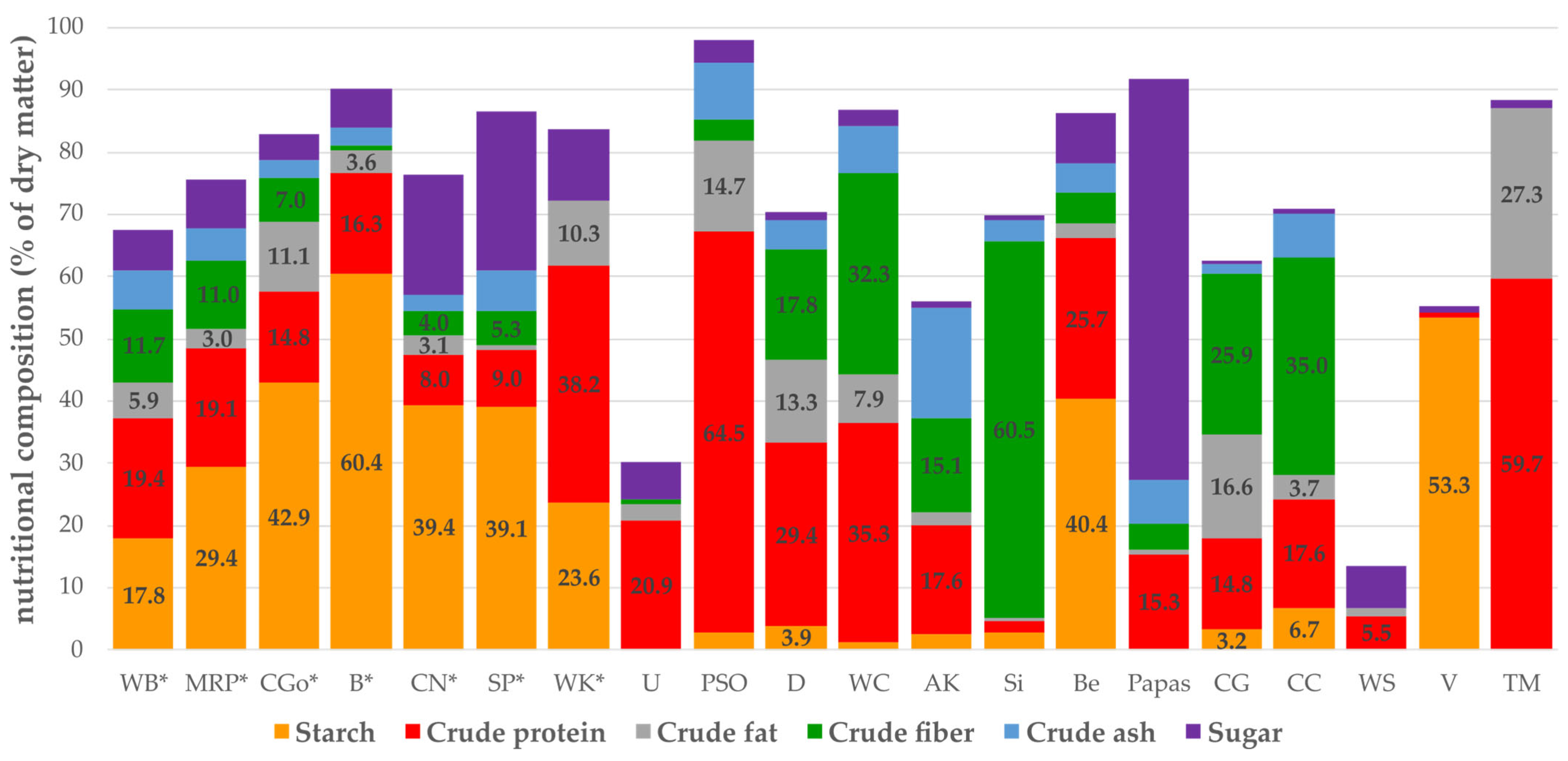
- Chemical composition of the offered diets;Substrates and larvae were analyzed by “Futtermittellabor Rosenau” (Futtermittellabor der Landwirtschaftskammer Niederösterreich, Lower Austria), following the VDLUFA regulations (Association of German Agricultural Analytic and Research Institutes, https://www.methodenbuch.de/produkt/methodenbuch-band-iii-futtermittel/, accessed on 10 November 2023). The total content of protein was determined using the Kjeldahl method. Depending on the substrate, the fat content was analyzed by two different methods. For all substrates, the extraction was performed with petroleum ether as solvent. Substrates of vegetable origins containing soy protein, yeast, or potato protein were treated with hydrochloric acid before the extraction. To determine the crude ash content, the samples were burned at 550 °C and weighed afterwards. To verify the crude fiber content, the defatted substrates were treated with sulfuric acid and potassium hydroxide solution, filtrated, washed, dried, weighed, burned, and the residue was weighed again. For the sugar content, samples were dissolved in ethanol, cleared with Carrez solutions, and the content was determined by the Luff Schoorl method. CGo (corn germ meal) was analyzed by BOKU (University of Natural Resources and Life Sciences, Vienna). Larvae for chemical analysis (“TM”, fed with WB and carrots) were fasted 24 h before being killed by freezing at −18 °C and were also analyzed by “Futtermittellabor Rosenau”.
2.3. Statistical Analysis
3. Results
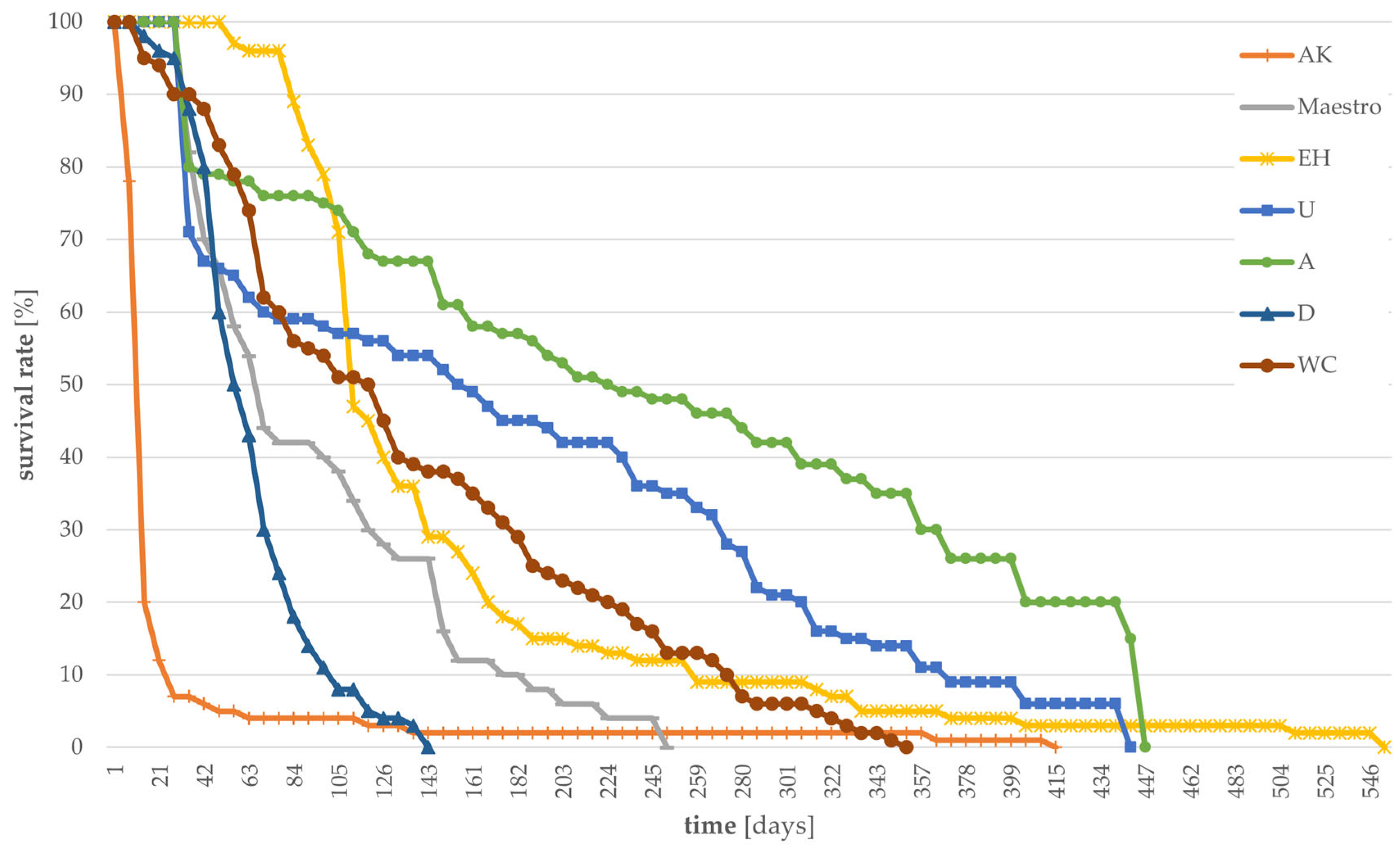
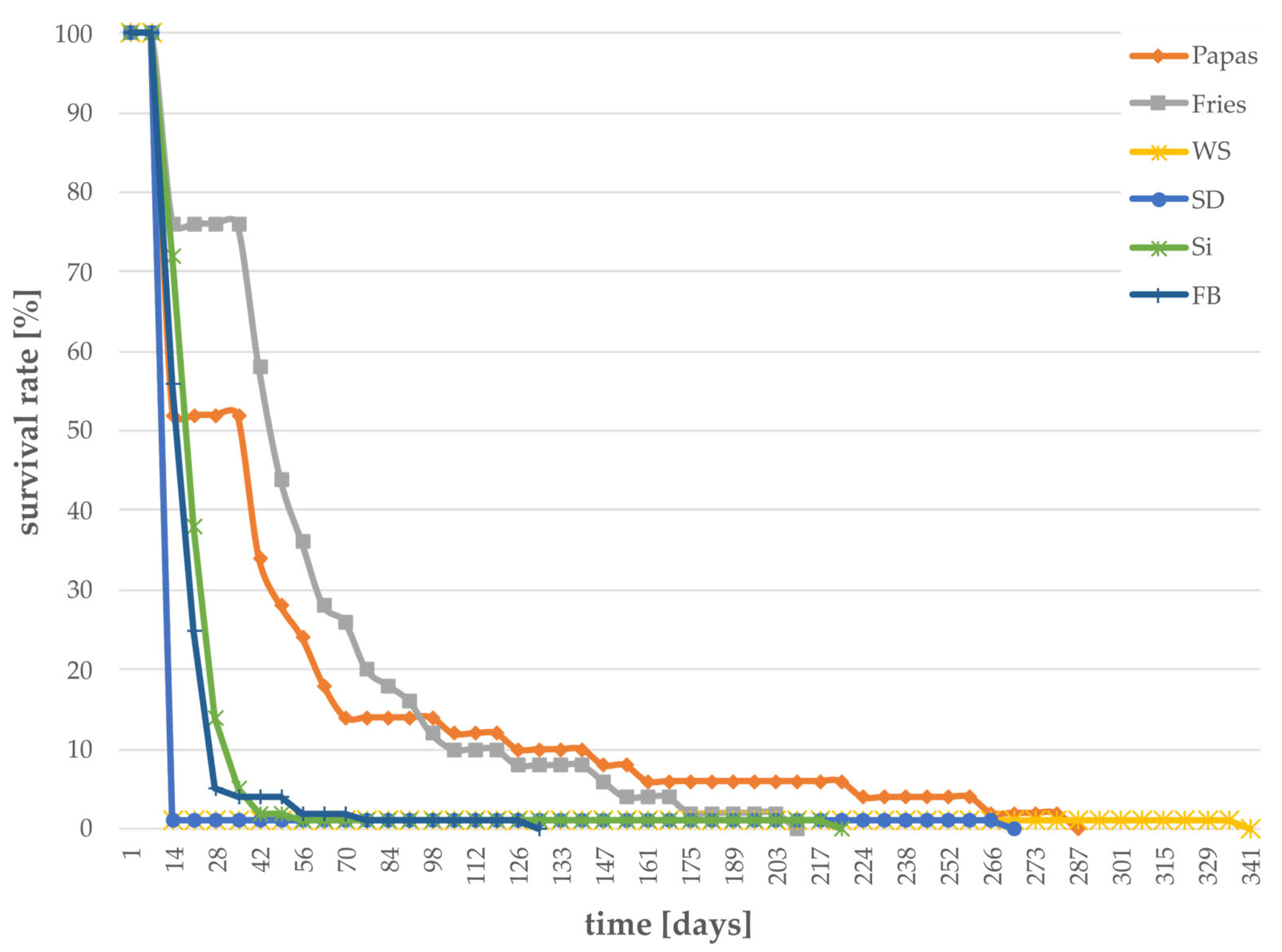
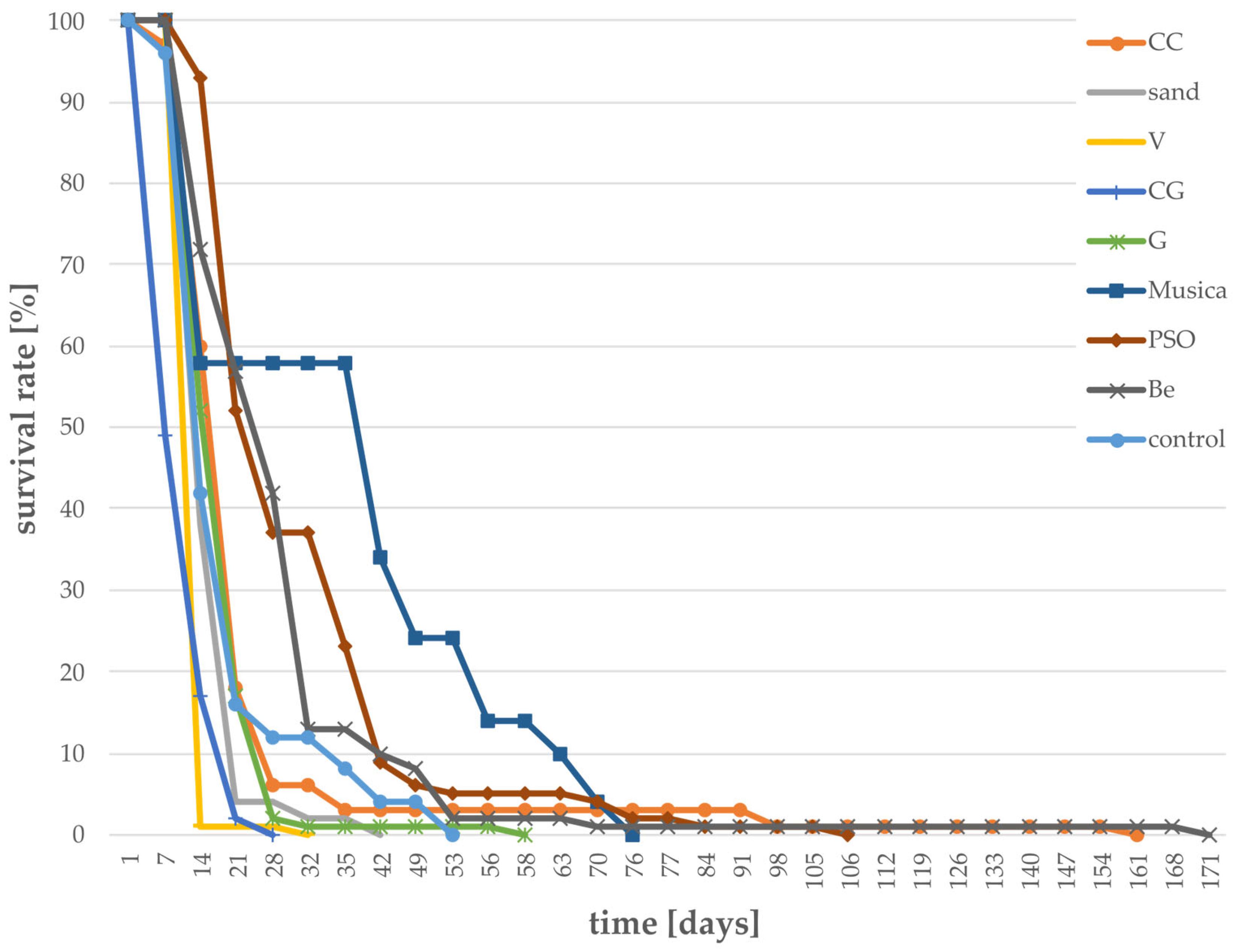
3.1. Survival Rates of Larvae Grown on Different Substrates
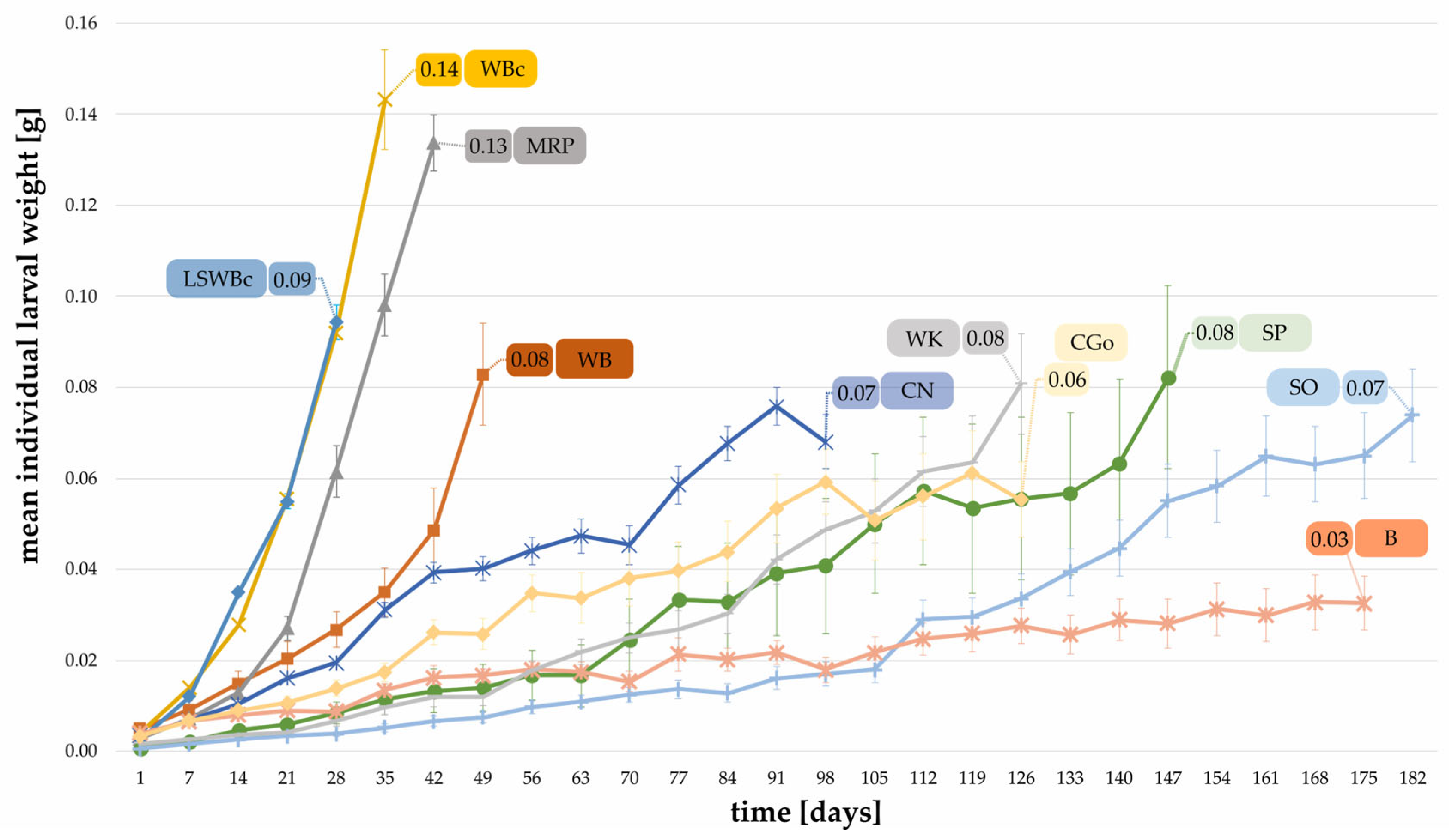
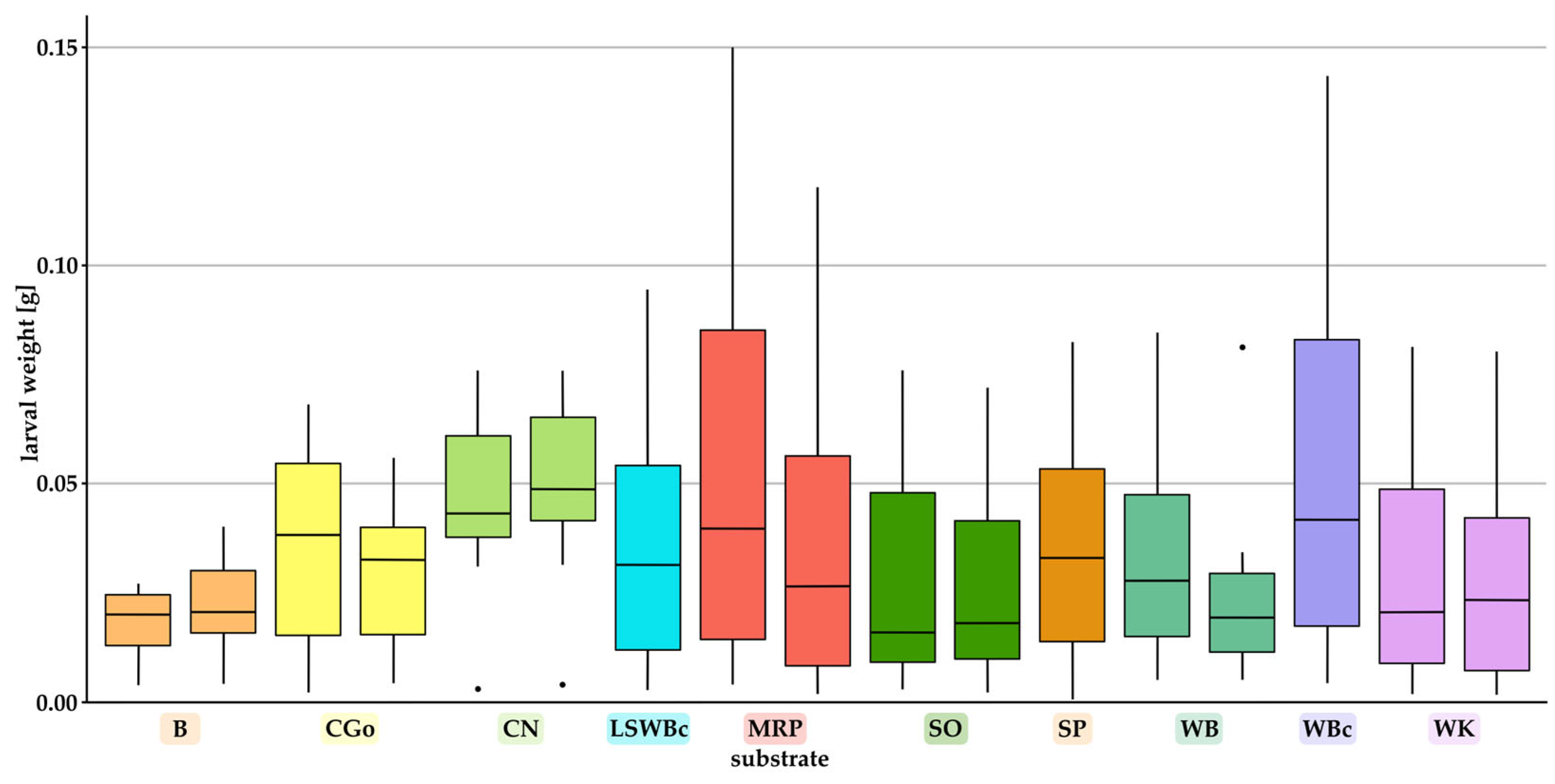
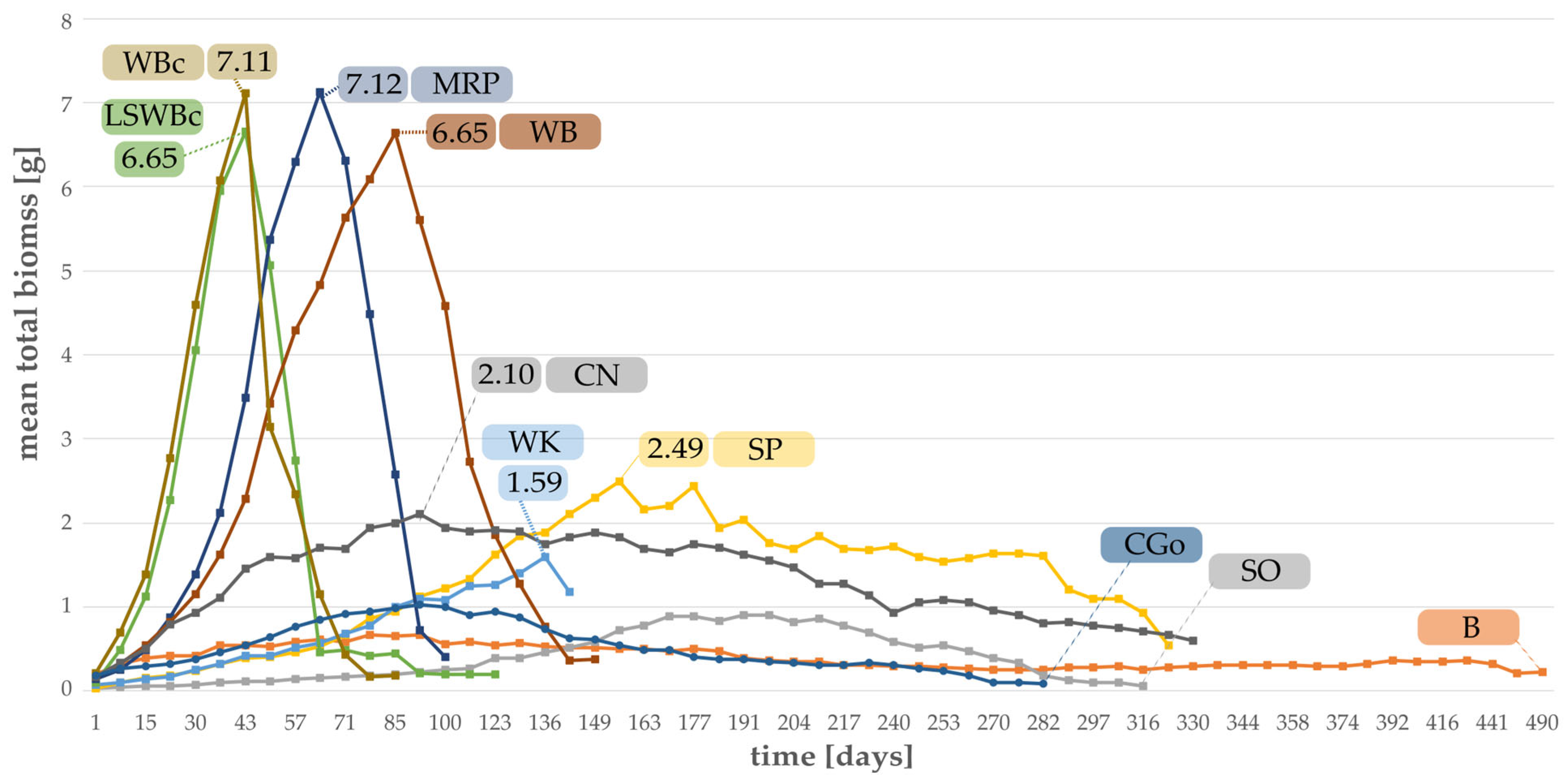
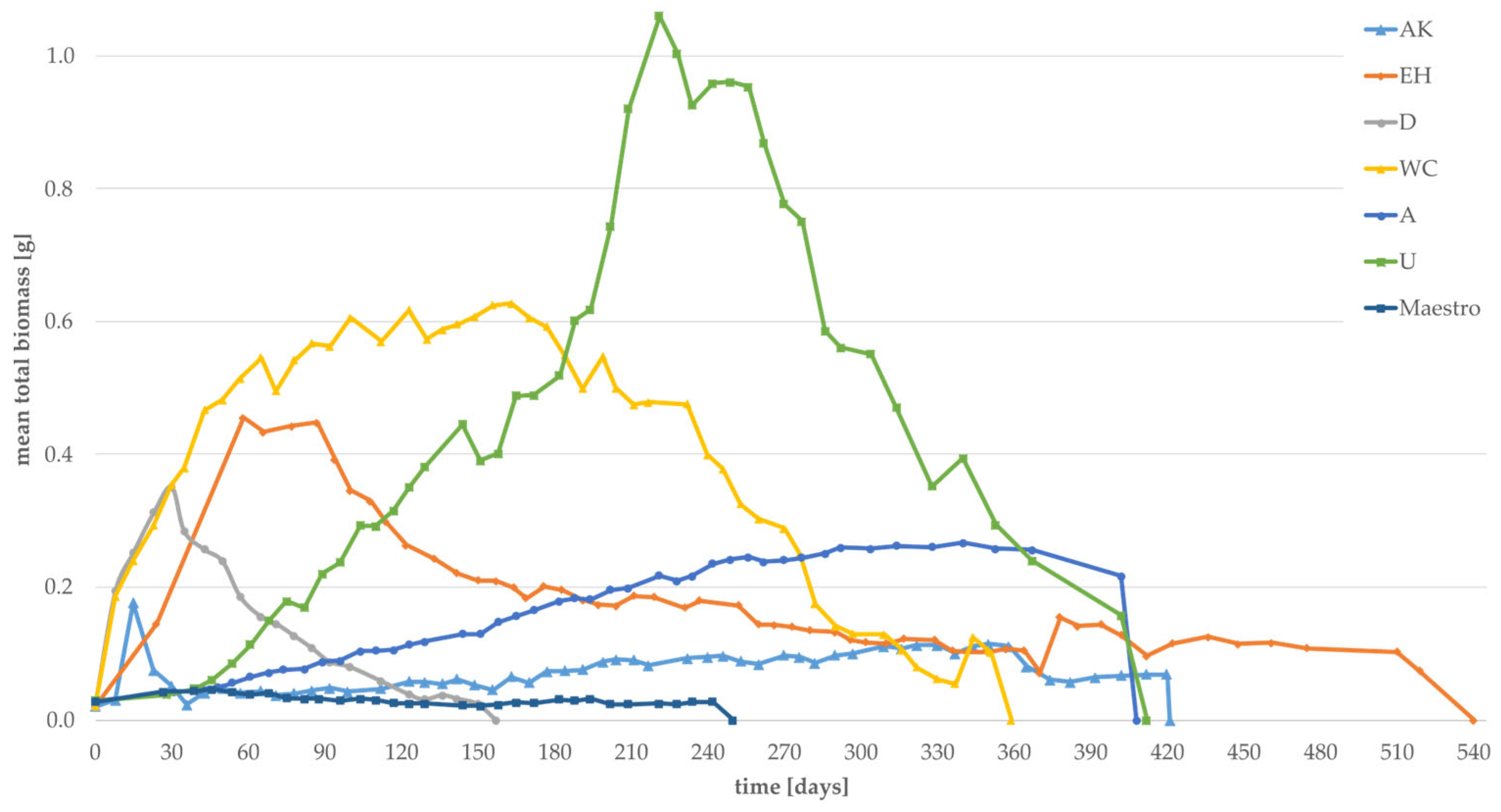
3.2. Effect of Substrates on the Individual Weight and the Total Biomass of Larvae
3.3. Growth Parameters and Development Times of Pupae and Beetles
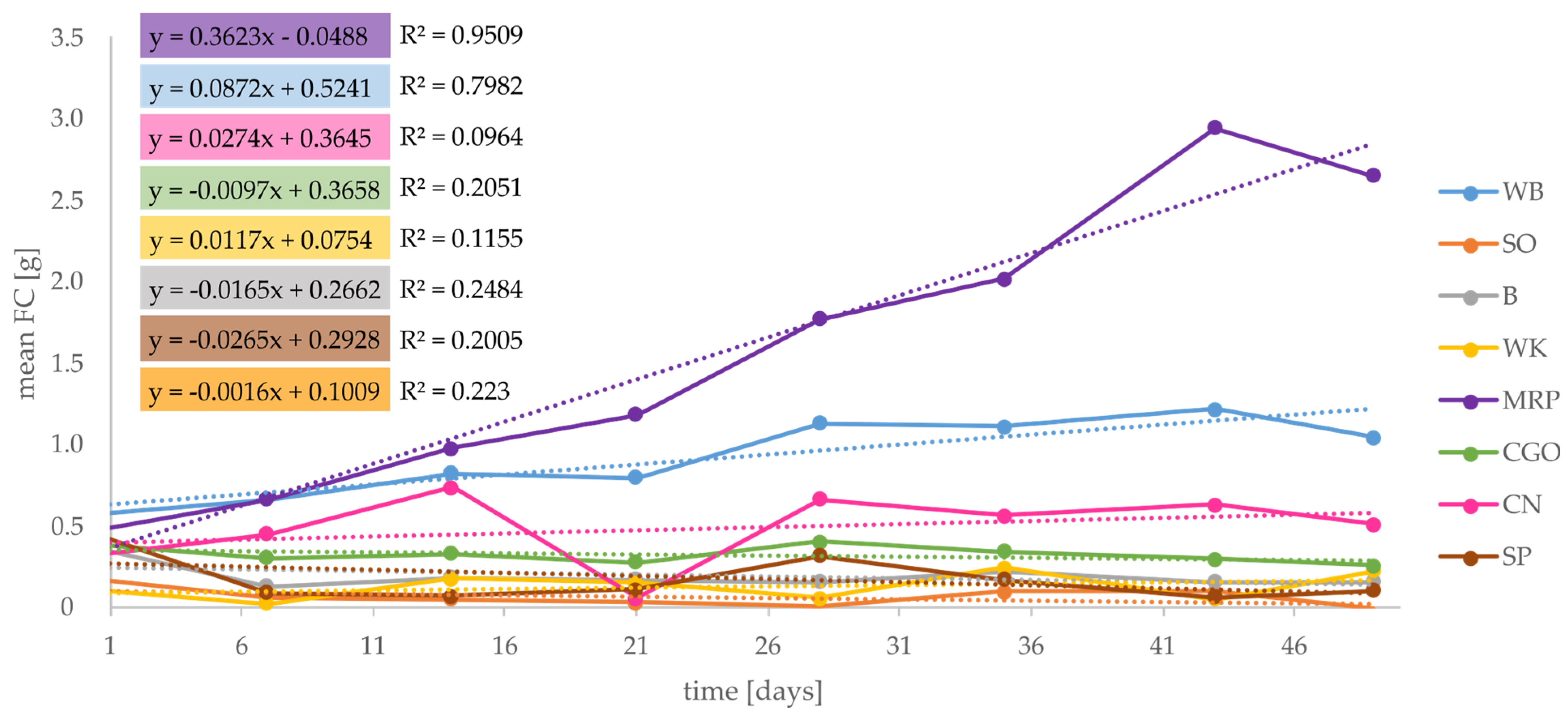
3.4. Feed Consumed
3.5. Nutrient Composition of Substrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.; Van Huis, A.; Van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Ribeiro, N.; Abelho, M.; Costa, R. A review of the scientific literature for optimal conditions for mass rearing Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2018, 53, 434–454. [Google Scholar]
- Oonincx, D.G.; De Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Weaver, D.K.; McFarlane, J.E. The effect of larval density on growth and development of Tenebrio molitor. J. Insect Physiol. 1990, 36, 531–536. [Google Scholar] [CrossRef]
- Cotton, R.T. Notes on the Biology of the Meal Worms, Tenebrio Molitor Linne and T. Obscurus Fab. Ann. Entomol. Soc. Am. 1927, 20, 81–86. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Alkoaik, F.N. The yellow mealworm as a novel source of protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.B.; Lee, Y.B.; Yoon, H.J.; Lee, K.Y.; Kim, N.J. Growth characteristics of mealworm Tenebrio molitor. J. Seric. Entomol. Sci. 2015, 53, 1–5. [Google Scholar]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Fraenkel, G. The nutrition of the mealworm, Tenebrio molitor L. (Tenebrionidae, Coleoptera). Physiol. Zool. 1950, 23, 92–108. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-llan, D.I.; Tedders, W.L. Use of nutrient self-selection as a diet refining tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Ribeiro, N.T.G.M. Tenebrio molitor for Food or Feed: Rearing Conditions and the Effects of Pesticides on its Performance. Ph.D. Thesis, Politécnico de Coimbra, Coimbra, Portugal, 2017. [Google Scholar]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, T.H.; Kim, S.H.; Song, S.; Kim, N. Recycling agricultural wastes as feed for mealworm (Tenebrio molitor). Korean J. Appl. Entomol. 2014, 53, 365–371. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Yoon, H.J.; Lee, K.Y.; Kim, N.J. Nutritional analysis of alternative feed ingredients and their effects on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Entomol. Res. 2017, 47, 194–202. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of agricultural byproducts, DDG and MSG, on the larval development of mealworms. Int. J. Ind. Entomol. 2016, 32, 69–79. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Eberle, S.; Schaden, L.M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of temperature and photoperiod on development, survival, and growth rate of mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Self-selection of feeding substrates by Tenebrio molitor larvae of different ages to determine optimal macronutrient intake and the influence on larval growth and protein content. Insects 2022, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Zsolt, I.V.; László, A.; Gyuricza, C. Evaluation of organic wastes as substrates for rearing Zophobas morio, Tenebrio molitor, and Acheta domesticus larvae as alternative feed supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Che, P.F.; Zhang, C.R.; Zhang, B.; Ali, A.; Zang, L.S. Recycling of spent mushroom substrate: Utilization as feed material for the larvae of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). PLoS ONE 2020, 15, e0237259. [Google Scholar] [CrossRef]
- Molnár, Á.; Abigeal, T.O.; Fehér, M. Investigation of the production parameters, nutrient and mineral composition of mealworm (Tenebrio molitor) larvae grown on different substrates. Acta Agrar. Debreceniensis 2022, 1, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-selection of agricultural by-products and food ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and impact on food utilization and nutrient intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.D.; Rivers, J.P.W.; Cowgill, U.M. Culturing mealworms as food for animals in captivity. Int. Zoo Yearb. 1976, 16, 63–70. [Google Scholar] [CrossRef]
- Derler, H.; Lienhard, A.; Berner, S.; Grasser, M.; Posch, A.; Rehorska, R. Use them for what they are good at: Mealworms in circular food systems. Insects 2021, 12, 40. [Google Scholar] [CrossRef]
- Pöllinger-Zierler, B.; Lienhard, A.; Mayer, C.; Berner, S.; Rehorska, R.; Schöpfer, A.; Grasser, M. Tenebrio molitor (Linnaeus, 1758): Microbiological Screening of Feed for a Safe Food Choice. Foods 2023, 12, 2139. [Google Scholar] [CrossRef]
- Birk, Y.; Harpaz, I.; Ishaaya, I.; Bondi, A. Studies on the proteolytic activity of the beetles Tenebrio and Tribolium. J. Insect Physiol. 1962, 8, 417–429. [Google Scholar] [CrossRef]
- Baek, S.; Perez, A.E.; Turcotte, R.M.; White, J.B.; Adedipe, F.; Park, Y.L. Response of Tenebrio molitor (Coleoptera: Tenebrionidae) adults to potato: Implications for monitoring and sampling. J. Stored Prod. Res. 2015, 60, 5–10. [Google Scholar] [CrossRef]
- Meireles, E.A.; Carneiro, C.N.; DaMatta, R.A.; Samuels, R.I.; Silva, C.P. Digestion of starch granules from maize, potato and wheat by larvae of the yellow mealworm, Tenebrio molitor and the Mexican bean weevil, Zabrotes subfasciatus. J. Insect Sci. 2009, 9, 43. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of Brewer’s spent grain (BSG) on larval growth of mealworms, Tenebrio molitor (Coleoptera: Tenebrionidae). Int. J. Ind. Entomol. 2016, 32, 41–48. [Google Scholar] [CrossRef]
- Nathanson, J.A. Caffeine and related methylxanthines: Possible naturally occurring pesticides. Science 1984, 226, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.V.; Sanjinez-Argandoña, E.J.; Linzmeier, A.M.; Cardoso, C.A.L.; Macedo, M.L.R. Food value of mealworm grown on Acrocomia aculeata pulp flour. PLoS ONE 2016, 11, e0151275. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.S.; Lee, K.P. Balanced intake of protein and carbohydrate maximizes lifetime reproductive success in the mealworm beetle, Tenebrio molitor (Coleoptera: Tenebrionidae). J. Insect Physiol. 2016, 91, 93–99. [Google Scholar] [CrossRef]
- Zaelor, J.; Kitthawee, S. Growth response to population density in larval stage of darkling beetles (Coleoptera; Tenebrionidae) Tenebrio molitor and Zophobas atratus. Agric. Nat. Resour. 2018, 52, 603–606. [Google Scholar] [CrossRef]
- Riaz, K.; Iqbal, T.; Khan, S.; Usman, A.; Al-Ghamdi, M.S.; Shami, A.; Mohamed, R.; Almadiy, A.A.; Galil, F.; Alfuhaid, N.; et al. Growth Optimization and Rearing of Mealworm (Tenebrio molitor L.) as a Sustainable Food Source. Foods 2023, 12, 1891. [Google Scholar] [CrossRef]
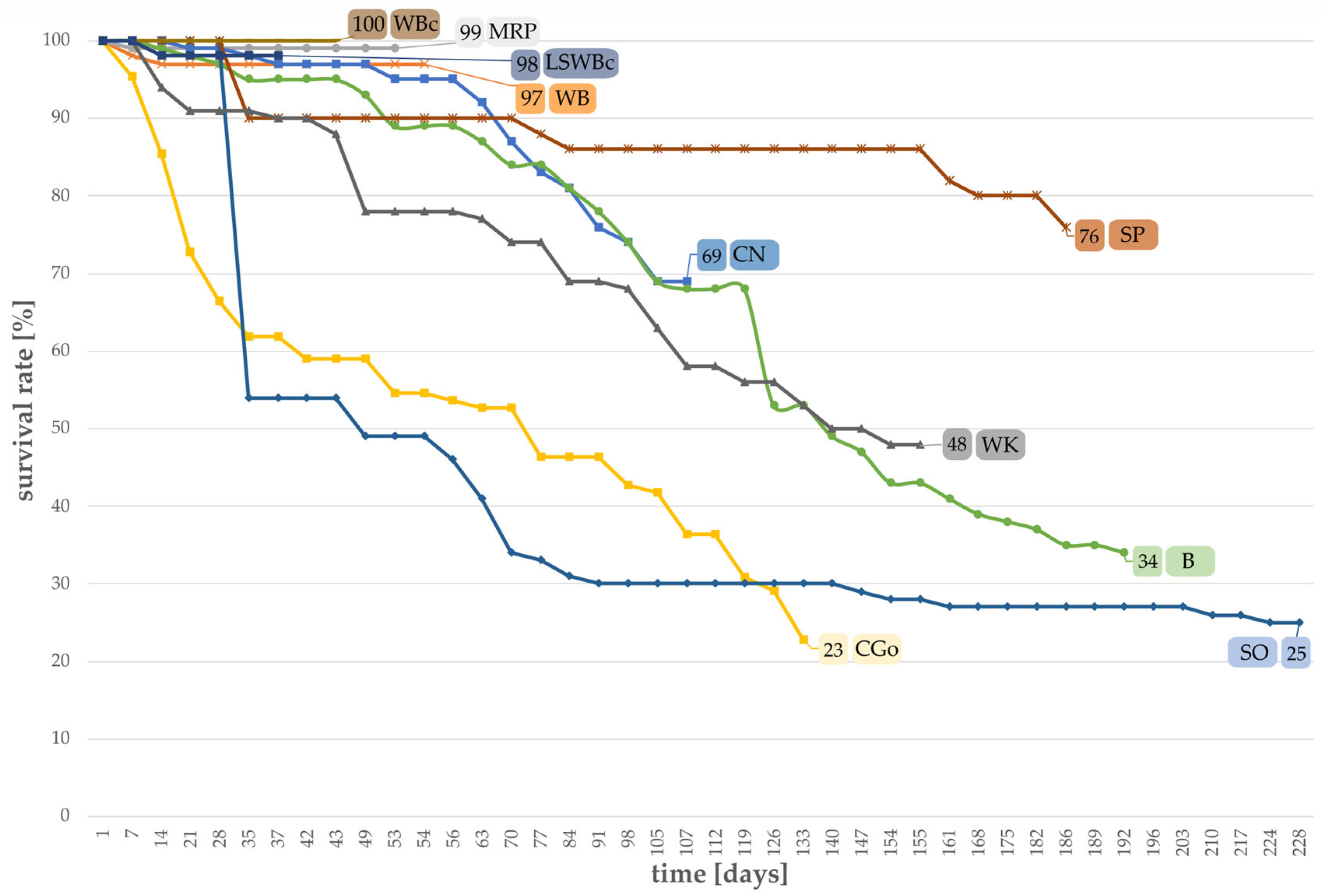
| Diet | 1st Pupa 1 [Days] | Pupation Rate 2 [%] | Mean Pupal Weight ±SD [g] | Min/Max Weight [g] | Sex Ratio m/f [%] | Emerged Beetles 3 [%] |
|---|---|---|---|---|---|---|
| CGo | 132 | 3 | 0.13 ± 0.02 | 0.11/0.15 | 33/67 | 3 |
| CN | 106 | 14 | 0.12 ± 0.04 | 0.04/0.19 | 43/57 | 12 |
| MRP | 52 | 93 | 0.16 ± 0.02 | 0.11/0.21 | 49/51 | 95 |
| SP | 186 | 20 | 0.11 ± 0.01 | 0.10/0.13 | 50/50 | 20 |
| WB | 53 | 79 | 0.15 ± 0.02 | 0.10/0.21 | 45/55 | 73 |
| LSWBc | 73 | 98 | 0.15 ± 0.02 | 0.10/0.23 | 53/47 | 98 |
| WBc | 42 | 96 | 0.17 ± 0.03 | 0.12/0.24 | 47/53 | 98 |
| WK | 154 | 34 | 0.11 ± 0.02 | 0.06/0.14 | 41/59 | 20 |
| B | 191 | 3 | 0.11 ± 0.00 | 0.11/0.12 | 67/33 | 0 |
| SO | 227 | 2 | 0.11 ± 0.01 | 0.10/0.12 | 50/50 | 0 |
| Diet | Mean Weight ±SD [g] | Min/Max Weight [g] | Duration of Pupal Stage [days] (Min/Max) | Morph. Abnormalities 1 [%] |
|---|---|---|---|---|
| CGo | 0.11 ± 0.02 | 0.08/0.14 | 7 (7/8) | 33 |
| CN | 0.10 ± 0.04 | 0.07/0.16 | 7 (4/10) | 83 |
| MRP | 0.14 ± 0.02 | 0.09/0.19 | 6 (5/8) | 22 |
| SP | 0.10 ± 0.01 | 0.07/0.11 | 6 (4/7) | 40 |
| WB | 0.13 ± 0.02 | 0.07/0.17 | 7 (4/10) | 32 |
| WK | 0.10 ± 0.01 | 0.09/0.13 | 6 (5/7) | 0 |
| LSWBc | 0.13 ± 0.02 | 0.08/0.21 | 6 (4/7) | 39 |
| WBc | 0.14 ± 0.02 | 0.10/0.20 | 7 (5/9) | 16 |
| Substrate | Abbreviation | Suitability | |
|---|---|---|---|
| wheat bran | WB | ✓ | main rearing substrate |
| malt residual pellets | MRP | ✓ | |
| corn germ meal | CGo | ✓ | |
| sweet chestnuts with peel | CN | ✓ | |
| sweet potatoes | SP | ✓ | |
| wheat germs (extracts) | WK | ✓ | |
| soybeans | SO | ✓~ | main feeding substrate 1 |
| bread remains | B | ✓~ | |
| brewer´s spent grain | D | ~ | supplement |
| hempseed cake | WC | ~ | |
| Urtica | U | ~ | |
| acron flour | EH | ~ | |
| pearl oyster mushroom | A | ~ | |
| potatoes | Maestro | ~ | |
| foam peanuts | V | ~ | |
| pearl oyster mushroom mycelia with coffee grounds and coffee chaff | AK | ~ | |
| coffee grounds | CG | ✕ | not suitable |
| coffee chaff | CC | ✕ | |
| pumpkin kernel cake | PSO | ✕ | |
| Fallopia x bohemica | FB | ✕ | |
| Sida | Si | ✕ | |
| garlic peel | G | ✕ | |
| runner beans | Be | ✕ | |
| Mur sand | Sand | ✕ | |
| sawdust | SD | ✕ | |
| wheat straw | WS | ✕ | |
| potatoes | Musica | ✕ | |
| potatoes | Fries | ✕ | |
| jerusalem artichoke | Papas | ✕ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lienhard, A.; Rehorska, R.; Pöllinger-Zierler, B.; Mayer, C.; Grasser, M.; Berner, S. Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products. Foods 2023, 12, 4092. https://doi.org/10.3390/foods12224092
Lienhard A, Rehorska R, Pöllinger-Zierler B, Mayer C, Grasser M, Berner S. Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products. Foods. 2023; 12(22):4092. https://doi.org/10.3390/foods12224092
Chicago/Turabian StyleLienhard, Andrea, René Rehorska, Barbara Pöllinger-Zierler, Chiara Mayer, Monika Grasser, and Simon Berner. 2023. "Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products" Foods 12, no. 22: 4092. https://doi.org/10.3390/foods12224092
APA StyleLienhard, A., Rehorska, R., Pöllinger-Zierler, B., Mayer, C., Grasser, M., & Berner, S. (2023). Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products. Foods, 12(22), 4092. https://doi.org/10.3390/foods12224092






